- *Corresponding Author:
- G. Nagalakshmi
Department of Pharmaceutical Chemistry, The Erode College of Pharmacy, Vallipurathanpalayam post, Erode - 638 1124-31, Karnam thottam, Gudalur Post, (Salem district) - 637 103, India
E-mail: gnl78@yahoo.com
| Date of Submission | 19 October 2006 |
| Date of Revision | 24 November 2007 |
| Date of Acceptance | 16 January 2008 |
| Indian J Pharm Sci, 2008, 70 (1): 49-55 |
Abstract
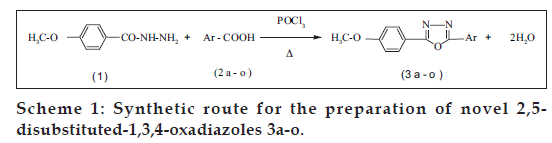
In the present study, 2,5-disubstituted-1,3,4-oxadiazoles (3a-o) have been synthesized by the condensation of 4-methoxybenzohydrazide (1) with different aromatic acids (2a-o) in presence of phosphoryl chloride. The structural assignment of this compound (3a-o) has been made on the basis of elemental analysis, UV, IR, 1 H NMR and mass spectral data. The synthesized compounds were screened for their in vitro growth inhibiting activity against different strains of bacteria and fungi viz., Staphylococcus aureus , Bacillus subtilis , Bacillus megaterium , Escherichia coli , Pseudomonas aeruginosa , Shigella dysenteriae , Candida albicans , Aspergillus niger and Aspergillus flavus were compared with the standard antibiotics such as chloramphenicol (50 µg/ml) and griseofulvin (50 µg/ml) using well agar diffusion technique. Compounds 3e, 3g, 3h and 3m exhibits highest antibacterial activity and compounds 3d, 3g and 3h showed better antifungal activity. The synthesized compounds (3a-o) were screened for their in vitro antiinflammatory activity against carrageenan-induced rat paw oedema. Compounds 3f and 3i were found to be most active compound of this series, which shows 46.42% and 50% inflammation inhibitory activity, whereas standard drug phenylbutazone exhibit 53.57% antiinflammatory activity at a dose of 50 mg/kg po.
Keywords
1,3,4-oxadiazole, 2,5-disubstituted-1,3,4-oxadiazole, antimicrobial agents, 4-methoxybenzohydrazide, antiinflammatory activity
2,5-Disubstituted-1,3,4-oxadiazoles have been found to exhibit diverse biological activities such as antibacterial [1], antiHIV [1], antifungal [2], genotoxic [2], antitubercular [3], virucidal [4], antimalarial [5], insecticidal [6], herbicidal [7], analgesic [8], antiinflammatory [9], muscle relaxants [10], anticonvulsant [11], sedative, hypnotic [12], anticancer [13] and lipid peroxidation inhibitor [14]. Aryl alkanoic acids provide one of the fascinating classes of compounds recognized for various pharmacological activities like antipyretic, analgesic and anti-in ß ammatory [15], used extensively in the symptomatic treatment of rheumatic fever, arthritis [16] (rheumatoid, osteo and jaundice arthritis), myocardial infarctions and management of primary dysmenorrhea [17].
The major side effects in the use of aryl alkanoic acids is their gastric irritancy, which is partly due to the corrosive nature of carboxylic acid group present in them. In order to reduce or mask the side effects of carboxylic moiety we planned to synthesize various 2,5-disubstituted-1,3,4-oxadiazole via the condensation of 4-methoxybenzohydrazide with various aromatic acids in presence of phosphoryl chloride respectively in the hope of getting potent biodynamic agents and evaluate their antimicrobial and antiinß ammatory activity.
Materials and Methods
The identification and purity of the products were checked by TLC (Merck Silica-60F254) with Ethyl acetate: acetone (9:1) using iodine vapours and UV light as detecting agents and the Rf value were given below. Melting points were measured on open capillaries in a liquid paraffin bath and are uncorrected. The absorbance maxima (λmax) were determined on a Systronics UV-Vis double beam spectrophotometer (2201) in ethanol. IR Spectra were taken on a Perkin Elmer Spectrum RX I, FTIR Spectrophotometer using potassium bromide pellets. 1H NMR spectra were recorded in DMSO-d6 on AMX-400, NMR spectrometer using TMS as an internal standard (chemical shift in δ ppm). FAB mass spectra were taken out on a JEOL SX102/ DA-6600 mass spectrometer using Argon/Xenon (6 kV, 10 mA) as the FAB gas. Elemental analysis was obtained on a Carlo Erba 1108 Heraeus elemental analyzer. All the chemicals used were of synthetic and AR grade and were procured from Alfa Aesar (4-methoxybenzohydrazide), USA, S.D. Fine Chem. Ltd and Merck, Mumbai, India.
General procedure for the synthesis of 2,5- disubstituted-1,3,4-oxadiazoles (3a-o)
A mixture of different aromatic acid(s) (0.01 mol) with 4-methoxybenzohydrazide (1.6617 g, 0.01 mol) in phosphoryl chloride (15 ml) was refluxed over a steam bath for 5-6 h. The progress of the reaction was monitored by TLC (Merck Silica-60F254) using ethyl acetate: acetone (9:1) as eluent. The reaction mixture was cooled and poured on to crushed ice (∼200 g) with continuous stirring. The solid mass separated was neutralized with sodium bicarbonate solution (10% w/v). The resulting solid thus obtained was collected by filtration, washed well with cold water, dried in vacuum and recrystallized from absolute ethanol (95%) and analyzed. Adopting the above procedure fifteen different 2,5-disubstituted-1,3,4-oxadiazoles (3a-o) were synthesized and their characterization data are presented in Table 1. Yield and melting point of the product(s) were determined and summarized below.
| Compounds | R | Mol. FormulaMol.weight | Elemental analysis found (calcd)% | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 3a | C6H5 | C15H12N2O2/252.26 | 71.37(71.42) | 4.77(4.79) | 11.06(11.10) |
| 3b | 4-CH3C6H4 | C16H14N2O2/266.29 | 72.13(72.16) | 5.26(5.30) | 10.49(10.52) |
| 3c | CH = CH-C6H5 | C17H14N2O2/278.30 | 73.32(73.37) | 5.04(5.07) | 10.02(10.07) |
| 3d | 4-NH4C6H4 | C15H13N3O2/267.28 | 67.36(67.40) | 4.86(4.90) | 15.70(15.72) |
| 3e | 4-NO2C6H4 | C15H11N3O4/297.26 | 60.58(60.61) | 3.70(3.73) | 14.10(14.14) |
| 3f | 3,5-(NO2)2C6H3 | C15H10N4O6/342.26 | 52.61(52.64) | 2.90(2.94) | 16.34(16.37) |
| 3g | 2,4-(NO2)2C6H3NH6C4 H | C21H15N5O6/433.37 | 58.18(58.20) | 3.45(3.49) | 16.12(16.16) |
| 3h | 2-NO2C6H4NHC6H4 | C21H16N4O4/388.37 | 64.91(64.94) | 4.12(4.15) | 14.38(14.43) |
| 3i | C6H5CONHC6H4 | C22H17N3O3/371.38 | 71.14(71.15) | 4.58(4.61) | 10.42(10.44) |
| 3j | 4-OHC6H4 | C15H12N2O3/268.26 | 67.13(67.16) | 4.49(4.51) | 11.28(11.31) |
| 3k | 3,4,5-(OCH3)3C6H2 | C18H18N2O5/342.34 | 63.12(63.15) | 5.28(5.30) | 8.18(8.18) |
| 3l | C5H4N | C14H11N3O2/253.25 | 66.38(66.40) | 4.37(4.38) | 16.56(16.59) |
| 3m | 2,4-(OH)2C6H3 | C15H12N2O4/284.26 | 63.35(63.38) | 4.24(4.25) | 9.84(9.85) |
| 3n | 3-NH2C6H4 | C15H13N3O2/267.28 | 67.40(67.40) | 4.89(4.90) | 15.70(15.72) |
| 3o | 2-OH3-CH3C6H3 | C16H14N2O3/282.29 | 68.04(68.07) | 4.98(5.00) | 9.89(9.92) |
Table 1: Physical And Analytical Data Of 2,5-Disubstituted-1,3,4- Oxadiazoles (3a-O)
2-(4-methoxyphenyl)-5-phenyl-1,3,4-oxadiazole (3a)
Yield: 69.37% (1.75 g); mp: 221°; Rf value: 0.69; UV (λmax, nm): 348.3; IR (KBr, cm−1): 3027 (aromatic C-H), 1602, 1493, 1456 (aromatic C = C), 1650 (C = N), 1210 (asymmetric C-O-C), 1028 (symmetric C-O-C), 2962 (methyl C-H, γas CH3), 2872 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.87-7.0 (m, 4H, Ar-H), 7.21-7.38 (m, 5H, Ar-H), 3.74 (s, 3H, Ar-OCH3); MS (FAB) m/z: 252 (M+), 253 (M+ + 1, 100%) for C15H12N2O2.
2-(4-methoxyphenyl)-5-(4-methylphenyl)-1,3,4- oxadiazole (3b)
Yield: 78.86% (2.1 g); mp: 229°; Rf value: 0.84; UV (λmax, nm): 276.2; IR (KBr, cm−1): 3040 (aromatic C-H), 1595, 1499, 1472 (aromatic C = C), 1640 (C = N), 1250 (asymmetric C-O-C), 1032 (symmetric C-O-C), 2950 (methyl C-H, γas CH3), 2859 (methyl C-H, γs CH3), 750 (out-of-plane aromatic C-H bend); 1H NMR (DMSO-d6, δ ppm): 6.87-7.10 (m, 4H, Ar-H), 7.27-7.48 (m, 4H, Ar-H), 2.37 (s, 3H, Ar-CH3), 3.76 (s, 3H, Ar-OCH3); MS (FAB) m/z: 266 (M+), 267 (M+ + 1, 100%) for C16H14N2O2.
2-(4-methoxyphenyl)-5-(2-phenylvinyl)-1,3,4- oxadiazole (3c)
Yield: 70.09% (1.95 g); mp: 231°; Rf value: 0.78; UV (λmax, nm): 267.2; IR (KBr, cm−1): 3055 (aromatic C-H), 1604, 1493, 1464 (aromatic C = C), 1668 (C = N), 2972 (methyl C-H, γas CH3), 2840 (methyl C-H, γs CH3), 1249 (asymmetric C-O-C), 1040 (symmetric C-O-C), 3048 (alkene C-H), 1640 (C = C, alkene), 991 (out-of-plane alkene C-H bend); 1H NMR (DMSO-d6, δ ppm): 6.82-7.12 (m, 4H, Ar-H), 7.21- 7.38 (m, 5H, Ar-H), 3.9 (s, 3H, Ar-OCH3), 4.82-5.92 (d, 2H, CH = CH); MS (FAB) m/z: 278 (M+), 279 (M+ + 1, 100%) for C17H14N2O2.
4-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]aniline (3d)
Yield: 80.44% (2.15 g); mp: 202°; Rf value: 0.82; UV (λmax, nm): 278.2; IR (KBr, cm−1): 3048 (aromatic C-H), 1598, 1494, 1474 (aromatic C = C), 1654 (C = N), 1252 (asymmetric C-O-C), 1047 (symmetric C-O-C), 3510 (N-H, aromatic primary amine, asymmetric), 3421 (N-H, aromatic primary amine, symmetric), 1292 (C-N, aromatic primary amine), 2965 (methyl C-H, γas CH3), 2874 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.85-7.07 (m, 4H, Ar-H), 7.29-7.48 (m, 4H, Ar-H), 3.82 (s, 3H, Ar-OCH3), 4.48 (s, 2H, NH2); MS (FAB) m/z: 267 (M+), 268 (M+ + 1, 100%) for C15H13N3O2.
2-(4-methoxyphenyl)-5-(4-nitrophenyl)-1,3,4- oxadiazole (3e)
Yield: 65.60% (1.95 g); mp: 235°; Rf value: 0.69; UV (λmax, nm): 273.2; IR (KBr, cm−1): 3054 (aromatic C-H), 1603, 1498, 1470 (aromatic C = C), 1646 (C = N), 1247 (asymmetric C-O-C), 1046 (symmetric C-O-C), 1523 (asymmetric ArNO2, NO2), 1347 (symmetric ArNO2, NO2), 852 (C-N, ArNO2), 2958 (methyl C-H, γas CH3), 2843 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.83-7.0 (m, 4H, Ar-H), 7.44-7.48 (m, 4H, Ar-H), 3.81 (s, 3H, Ar-OCH3); MS (FAB) m/z: 297 (M+, 100%) for C15H11N3O4.
2-(3,5-dinitrophenyl)-5-(4-methoxyphenyl)-1,3,4- oxadiazole (3f)
Yield: 82.39% (2.82 g); mp: 258°; Rf value: 0.78; UV (λmax, nm): 282.0; IR (KBr, cm−1): 3048 (aromatic C-H), 1609, 1492, 1462 (aromatic C = C ring), 1648 (C = N), 1258 (asymmetric C-O-C), 1052 (symmetric C-O-C), 1538 (asymmetric ArNO2, NO2), 1350 (symmetric ArNO2, NO2), 856 (C-N, ArNO2), 2963 (methyl C-H, γas CH3), 2874 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.85-7.17 (m, 4H, Ar-H), 7.31-7.52 (m, 3H, Ar-H), 3.78 (s, 3H, Ar-OCH3); MS (FAB) m/z: 342 (M+, 100%) for C15H10N4O6.
N-{4-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2- yl]phenyl}-2,4-dinitroaniline (3g)
Yield: 87.68% (3.8 g); mp: 249°; Rf value: 0.69; UV (λmax, nm): 268.4; IR (KBr, cm−1): 3045 (aromatic C-H), 1596, 1496, 1472 (aromatic C = C), 1672 (C = N), 1260 (asymmetric C-O-C), 1050 (symmetric C-O-C), 1530 (asymmetric ArNO2, NO2), 1348 (symmetric ArNO2, NO2), 854 (C-N, ArNO2), 3332 (N-H, aromatic secondary amine), 1310 (C-N, secondary aromatic amine), 2950 (methyl C-H, γas CH3), 2836 (methyl C-H, γs CH3); 1H NMR (DMSOd 6, δ ppm): 6.86-7.0 (m, 4H, Ar-H), 7.21-7.48 (m, 7H, Ar-H), 3.80 (s, 3H, Ar-OCH3), 2.18 (s, 1H, NH); MS (FAB) m/z: 433 (M+), 434 (M+ + 1, 100%) for C21H15N5O6.
N-{4-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2- yl]phenyl}-2-nitroaniline (3h)
Yield: 75.18% (2.92 g); mp: 224°; Rf value: 0.75; UV (λmax, nm): 285.2; IR (KBr, cm−1): 3038 (aromatic C-H), 1596, 1492, 1458 (aromatic C = C), 1636 (C = N), 1246 (asymmetric C-O-C), 1046 (symmetric C-O-C), 1520 (asymmetric ArNO2, NO2), 1342 (symmetric ArNO2, NO2), 854 (C-N, ArNO2), 3332 (N-H, aromatic secondary amine), 1298 (C-N, secondary aromatic amine), 2964 (methyl C-H, γas CH3), 2870 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.89-7.14 (m, 4H, Ar-H), 7.20-7.52 (m, 8H, Ar-H), 3.78 (s, 3H, Ar-OCH3), 2.21 (s, 1H, NH); MS (FAB) m/z: 388 (M+, 100%) for C21H16N4O4.
N-{4-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2- yl]phenyl}benzamide (3i)
Yield: 71.35% (2.65 g); mp: 242°; Rf value: 0.68; UV (λmax, nm): 288.0; IR (KBr, cm−1): 3065 (aromatic C-H), 1607, 1496, 1465 (aromatic C = C), 1654 (C = N), 1245 (asymmetric C-O-C), 1040 (symmetric C-O-C), 3430 (N-H, secondary amide), 1644 (C = O, secondary amide), 2968 (methyl C-H, γas CH3), 2875 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.87-7.10 (m, 4H, Ar-H), 7.18-7.48 (m, 9H, Ar-H), 3.86 (s, 3H, Ar-OCH3), 8.51 (s, 1H, CONH); MS (FAB) m/z: 371 (M+), 372 (M+ + 1, 100%) for C22H17N3O3.
4-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]phenol (3j)
Yield: 75.27% (2.125 g); mp: 279°; Rf value: 0.79; UV (λmax, nm): 270.0; IR (KBr, cm−1): 3030 (aromatic C-H), 3598 (O-H), 1224 (C-O), 1605, 1495, 1465 (aromatic C = C), 1645 (C = N), 1252 (asymmetric C-O-C), 1049 (symmetric C-O-C), 2960 (methyl C-H, γas CH3), 2869 (methyl C-H, γs CH3), 750 (out-of-plane aromatic C-H bend), 1361 (in-plane O-H bend); 1H NMR (DMSO-d6, δ ppm): 6.87-7.13 (m, 4H, Ar-H), 3.52 (s, 3H, OCH3), 7.17-7.42 (m, 4H, Ar-H), 10.44 (s, 1H, Ar-OH); MS (FAB) m/z: 268 (M+), 269 (M+ + 1, 100%) for C15H12N2O3.
2-(4-methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)- 1,3,4-oxadiazole (3k)
Yield: 77.48% (2.65 g); mp: 252°; Rf value: 0.81; UV (λmax, nm): 310.2; IR (KBr, cm−1): 3045 (aromatic C-H), 1219 (C-O), 1608, 1492, 1459 (aromatic C = C), 1634 (C = N), 1242 (asymmetric C-O-C), 1021 (symmetric C-O-C), 2963 (methyl C-H, γas CH3), 2872 (methyl C-H, γs CH3); 1H NMR (DMSO-d6, δ ppm): 6.37-6.92 (m, 2H, Ar-H), 7.18-7.42 (m, 4H, Ar-H), 3.9 (s, 9H, OCH3); MS (FAB) m/z: 342 (M+), 343 (M+ + 1, 100%) for C18H18N2O5.
3-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]pyridine (3l)
Yield: 73.05% (1.85 g); mp: 240°; Rf value: 0.79; UV (λmax, nm): 285.4; IR (KBr, cm−1): 3040 (aromatic C-H), 1601, 1492, 1473 (aromatic C = C), 1642 (C = N), 1251 (asymmetric C-O-C), 1045 (symmetric C-O-C), 2960 (methyl C-H, γasy CH3), 2869 (methyl C-H, γsy CH3), 748 (out-of-plane aromatic C-H bend), 690 (out-of-plane ring C = C bend); 1H NMR (DMSO-d6, δ ppm): 6.92-7.12 (m, 4H, Ar-H), 7.70-7.75 (d, 4H, pyridyl), 3.85 (s, 3H, OCH3); MS (FAB) m/z: 239 (M+, 100%), 240 (M+ 1)+ for C14H11N3O2.
4-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2- yl]benzene-1,3-diol (3m)
Yield: 68.59% (1.95 g); mp: 287°; Rf value: 0.80; UV (λmax, nm): 281.6; IR (KBr, cm−1): 3038 (aromatic C-H), 3602 (O-H), 1224 (C-O), 1606, 1446, 1466 (aromatic C = C), 1645 (C = N), 1249 (asymmetric C-O-C), 1035 (symmetric C-O-C), 2965 (C-H, γas CH3), 2876 (C-H, γs CH3), 1224 (C-O), 780 (outof- plane aromatic C-H bend), 689 (out-of-plane ring C = C bend); 1H NMR (DMSO-d6, δ ppm): 6.87-7.11 (m, 4H, Ar-H), 7.17-7.38 (m, 3H, Ar-H), 10.7 (s, 2H, OH), 3.86 (s, 3H, OCH3); MS (FAB) m/z: 284 (M+), 285 (M+ + 1, 100%) for C15H12N2O4.
3-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]aniline (3n)
Yield: 71.08% (1.90 g); mp: 217°; Rf value: 0.81; UV (λmax, nm): 292.0; IR (KBr, cm−1): 3045 (aromatic C-H), 1602, 1497, 1470 (aromatic C = C), 1647 (C = N), 1250 (asymmetric C-O-C), 1041 (symmetric C-O-C), 3522 (N-H, aromatic primary amine, asymmetric), 3416 (N-H, primary amine, symmetric), 2968 (C-H, γas CH3), 2877 (C-H, γs CH3), 1294 (C-N str, aromatic primary amine); 1H NMR (DMSO-d6, δ ppm): 6.92-7.21 (m, 4H, Ar-H), 7.27-7.52 (m, 4H, Ar-H), 3.81 (s, 3H, OCH3), 4.41 (s, 2H, NH2); MS (FAB) m/z: 267 (M+), 268 (M+ + 1, 100%) for C15H13N3O2.
2-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]-3- methylphenol (3o)
Yield: 74.39% (2.1 g); mp: 261°; Rf value: 0.72; UV (λmax, nm): 291.0; IR (KBr, cm−1): 3050 (aromatic C-H), 3606 (O-H), 1226 (C-O), 1595, 1499, 1470 (aromatic C = C), 1652 (C = N), 1247 (asymmetric C-O-C), 1040 (symmetric C-O-C), 2962 (methyl C-H, γas CH3), 2872 (methyl C-H, γs CH3), 1378 (C-H bend, δs CH3), 1362 (in-plane O-H bend), 692 (out-of-plane ring C = C bend); 1H NMR (DMSO-d6, δ ppm): 6.42-6.92 (m, 3H, Ar-H), 6.97-7.21 (m, 4H, Ar-H), 10.28 (s, 1H, OH), 2.42 (s, 3H, Ar-CH3) 3.82 (s, 3H, OCH3); MS (FAB) m/z: 282 (M+), 283 (M+ + 1, 100%) for C16H14N2O3.
Screening for antimicrobial activity
The antimicrobial activity of all the newly synthesized compounds were determined by well plate method18 in nutrient agar (Hi-Media) was used for antibacterial activity and Sabouraud dextrose agar (SDA) (Hi- Media) was used for antifungal activity. The bacterial strain used were Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923) and Bacillus megaterium (ATCC 1327) for gram positive and Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Shigella dysenteriae (ATCC 13313) for gram negative and for fungal strain viz., Candida albicans (ATCC 10231), Aspergillus niger (ATCC 16404) and Aspergillus flavus (ATCC 22547).
The compounds were tested at a concentration of 100 μg/ml (or) 0.00001 nanomoles were prepared in dimethylformamide (DMF). The petridishes used for antibacterial screening were incubated at 37 ± 1º for 24 h, while those used for antifungal activity were incubated at 28º for 48-72 h. The diameters of zone of inhibition (mm) surrounding each of the wells were recorded.
The results were compared to chloramphenicol (50 μg/ ml (or) 0.000005 nanomoles) and griseofulvin (50 μg/ ml (or) 0.000005 nanomoles) for antibacterial and antifungal activity. The antibacterial and antifungal screening results were presented in Table 2 and Table 3.
| Compounds | Antibacterial activity | |||||
|---|---|---|---|---|---|---|
| Zone of inhibition (mm) | ||||||
| B. subtilis | Staph. aureus | B. megaterium | E. coli | P. aeruginosa | S. dysenteriae | |
| 3a | 10 | 12 | 13 | 14 | 12 | 12 |
| 3b | 20 | 20 | 17 | 19 | 16 | 15 |
| 3c | 11 | 15 | 13 | 13 | 13 | 10 |
| 3d | 16 | 16 | 15 | 20 | 15 | 14 |
| 3e | 23 | 19 | 21 | 22 | 17 | 15 |
| 3f | 14 | 14 | 15 | 14 | 13 | 12 |
| 3g | 22 | 17 | 18 | 24 | 17 | 20 |
| 3h | 21 | 14 | 22 | 22 | 20 | 16 |
| 3i | 16 | 14 | 15 | 17 | 14 | 14 |
| 3j | 24 | 15 | 16 | 20 | 20 | 12 |
| 3k | 15 | 12 | 15 | 17 | 12 | 14 |
| 3l | 12 | 13 | 12 | 13 | 14 | 11 |
| 3m | 21 | 16 | 17 | 22 | 19 | 18 |
| 3n | 20 | 20 | 15 | 20 | 15 | 12 |
| 3o | 17 | 16 | 16 | 14 | 14 | 15 |
| Chloramphenicol | 23 | 22 | 20 | 26 | 24 | 22 |
Table 2: Antibacterial Screening Results Of Compounds (3a-O)
| Compounds | Antifungal activity | ||
|---|---|---|---|
| Zone of inhibition(mm) | |||
| Candida albicans | Aspergillus niger | Aspergillus flavus | |
| 3a | 17 | 16 | 13 |
| 3b | 14 | 14 | 15 |
| 3c | 13 | 11 | 16 |
| 3d | 18 | 19 | 18 |
| 3e | 14 | 16 | 14 |
| 3f | 15 | 12 | 11 |
| 3g | 21 | 19 | 19 |
| 3h | 20 | 19 | 18 |
| 3i | 15 | 16 | 18 |
| 3j | 17 | 17 | 14 |
| 3k | 16 | 15 | 13 |
| 3l | 13 | 14 | 12 |
| 3m | 18 | 17 | 15 |
| 3n | 14 | 14 | 12 |
| 3o | 18 | 16 | 18 |
| Griseofulvin (50µg/ml) | 21 | 21 | 19 |
Table 3: Antifungal Screening Results Of Compounds(3a-O)
Acute toxicity study
Acute toxicity study was carried out by “Stair case” method [19]. Swiss mice of either sex were injected with a particular dose, say 100 mg/kg and observed for a period of 24 h for any mortality. The subsequent doses are then increased by a factor 1.5 if the dose was tolerated, and decreased by a factor 0.7 if it was lethal. The LD50 of the drug was found to be 500 mg/kg body wt. One tenth of this dose was selected as the therapeutic dose for evaluation (i.e. 50 mg/kg).
Antiinflammatory activity against carrageenan-induced rats paws oedema
Antiinflammatory activity was determined by carrageenan-induced rat paw method of winter et al. [20]. Male Wistar rats (120-150 g) was used for the experiment. They were fed with standard pellet diet and water was given ad libitum. The animals were acclimatized for one week under laboratory conditions before performing the test. They were housed in polypropylene cages under standard conditions (30 ± 1º, 12/12 h light/dark cycles on 60-70% RH). The standard groups received phenylbutazone 50 mg/kg body weight po, suspended in 1% w/v of carboxymethylcellulose (CMC) in distilled water. The test group received synthesized compounds (3a-o) (50 mg/kg body weight po, suspended in 1% w/v of CMC in water). The control group received corresponding amount of vehicle (1% w/v of CMC). All the test compounds and standard drug were administered 30 min prior to carrageenan injection. The antiinß ammatory activity of synthesized compounds (3a-o) was carried out in Periyar College of Pharmaceutical Sciences for Girls, Tiruchirappalli 21, Tamilnadu. Before performing these experiments, ethical clearance was obtained from Institutional Animal Ethics Committee and conducted according to the Indian National Science Academy guidelines for the use and care of experimental animals (CPCSEA Reg No. 418).
Acute edema was induced in the right hind paw of rats by injecting 0.1 ml of freshly prepared 1% w/v of aqueous solution of carrageenan (Sigma, USA) in the subplanter region of right hind paw. After the carrageenan injection the paw volume was measured before and after 1, 2 and 3 h by plethysmometer (UGO-Basile, Italy). The difference between the left and right paw was taken as a measure of oedema. Any significant reduction in the volume of the paw compared to the control group was considered as anti-inflammatory response [21]. Percent inhibition of inflammation after 3 h was calculated by applying Newbould formula. % inhibition = 100 [1 − a − x/ b − y], where, x = mean paw volume of rats before the administration of carrageenan injection in the test and the standard groups, y = mean paw volume of rats before the administration of carrageenan injection in the control group, a = mean paw volume of rats after the administration of carrageenan and test compound or standard compound, b = mean paw volume of rats after the administration of carrageenan injection in control group. The results are presented in Table 4.
| Compounds | Normal paw volume (x) | Paw oedema 3 h after Carrageenan injected (a) | %inhibition of oedema (1 -a -x/ b -y)×100 |
|---|---|---|---|
| 3a | 0.71 ±0.04 | 0.93 ±0.05 | 21.42 |
| 3b | 0.68 ±0.02 | 0.85 ±0.02 | 39.28 |
| 3c | 0.69 ±0.02 | 0.87 ±0.02 | 35.71 |
| 3d | 0.66 ±0.04 | 0.82 ±0.03 | 42.86 |
| 3e | 0.72 ±0.03 | 0.95 ±0.03 | 17.85 |
| 3f | 0.68 ±0.02 | 0.83 ±0.04 | 46.42 |
| 3g | 0.70 ±0.03 | 0.86 ±0.03 | 42.86 |
| 3h | 0.69 ±0.02 | 0.89 ±0.03 | 28.57 |
| 3i | 0.66 ±0.04 | 0.80 ±0.03 | 50.00 |
| 3j | 0.675 ±0.02 | 0.832 ±0.04 | 43.96 |
| 3k | 0.712 ±0.04 | 0.940 ±0.03 | 18.46 |
| 3l | 0.660 ±0.04 | 0.836 ±0.04 | 37.21 |
| 3m | 0.670 ±0.02 | 0.841 ±0.02 | 39.10 |
| 3n | 0.710 ±0.04 | 0.92 ±0.04 | 25.00 |
| 3o | 0.694 ±0.02 | 0.852 ±0.02 | 43.46 |
| Control | 0.69 ±0.02 (y) | 0.97 ±0.03 (b) | - |
| Phenyl-butazone | 0.70 ±0.04 | 0.83 ±0.03 | 53.57 |
***P<0.001, P vs. standard mean ± SEM
Table 4: Antiinflammatory Activity Of 2,5- Disubstituted-1,3,4-Oxadiazoles(3a-O)
Results and Discussion
2,5-Disubstituted-1,3,4-oxadiazole (3a-o) was synthesized by the condensation of 4-methoxybenzohydrazide with various aromatic acids in presence of phosphoryl chloride (Scheme 1). The physical and analytical data of the compounds (3a-o) were collected and presented in Table 1. The yields of 3a-o fall in the range of 66-88%. The spectral (IR, 1H NMR and MS) and analytical data are in good agreement with their structures.
In the toxicity study, LD50 of the drug was found to be 500 mg/kg body wt. The therapeutic dose of the drug is considered as 1/10th of the LD50 value. Screening results of antimicrobial activity reveal (Table 2) that the known standard antibiotics chloramphenicol (50 μg/ml (or) 0.000005 nanomoles) and griseofulvin (50 μg/ml (or) 0.000005 nanomoles) showed zone of inhibition at 20-26 mm and 19-21 mm against bacterial and fungal strains. Compound 3e and 3j displayed better activity against Bacillus subtilis, while the compound 3b, 3e and 3n showed maximum activity against Staphylococcus aureus. Compound 3e and 3h exhibited significant activity against Bacillus megaterium. Compound 3e, 3g, 3h and 3m were highly active against Escherichia coli whereas compound 3g, 3h, 3j and 3m displayed moderate activity against Pseudomonas aeruginosa and Shigella dysenteriae. Compound 3g showed better antifungal activity against Candida albicans and compound 3d, 3g, 3h, 3i and 3o displayed moderate activity against Aspergillus niger and Aspergillus flavus when compared to standard.
The results in Table 4 indicate that the compounds 3f and 3i are active (p < 0.001) with the standard. Moreover, compounds 3d, 3g, 3j and 3o show less significant anti-inflammatory activity (p < 0.01). Carrageenan-induced paw edema was taken as a prototype of exudative phase of inflammation. The development of edema has been described as biphasic. The initial phase is due to the release of histamine, serotonins, 5-hydroxy tryptamine and kinins in the first hour after injection of carrageenan. More pronounced second phase is related to the release of prostaglandin22-24 like substances in 2-3 h. Hence, the significant anti-inß ammatory effect may be due to an inhibitory effect exerted predominantly on the mediators of inflammation induced by phlogogenic stimuli.
Acknowledgements
The author is thankful to the Principal, Periyar college of Pharmaceutical Sciences for Girls, Tiruchirappalli for providing the facilities for screening of anti-inflammatory activity and to the Chairman, Indian Institute of Sciences (IIS), Bangalore and to the Head, Regional Sophisticated Instrumentation Centre (RSIC), Central Drug Research Institute (CDRI), Lucknow for providing spectral and analytical data.
References
- El-Emam AA, Al-Deeb OA, Al-Omar M. Synthesis, antibacterial and anti-HIV activity of certain 5-(l-adamantyl)-2-substituted thio-l,3,4- oxadiazoles and 5-(l-adamantyl)-3-substituted aminomethyl-l,3,4- oxadiazolin-2-thiones. Bioorg Med Chem 2OO4;l2:5lO7-l3.
- Maslat AO, Abussaud M, Tashtoush H, Al-Talib M. Synthesis, antibacterial, antifungal and genotoxic activity of bis-l,3,4-oxadiazole derivatives. Pol J Pharmacol 2OO2;54:55-9.
- Kucukguzel SG, Oruc EE, Rollas S, Sahin F, Ozbek A. Synthesis, characterization and biological activity of novel 4-thiazolidinones, l,3,4-oxadiazoles and some related compounds. Eur J Med Chem 2OO2;37:l97-2O6.
- Chauhan D, Chauhan JS, Singh J, Bajpai SK, Joshi MN. Synthesis and bioevaluation of some novel nucleosides as antiherptic agents. Indian J Chem 2OO3;42B:2l5-8.
- Kagthara PR, Shah NS, Doshi RK, Parekh HH. Synthesis of 2,5- disubstituted-l,3,4-oxadiazoles as biologically active heterocycles. Indian J Chem l999;38B:572-6.
- Mohan TP, Vishalakshi B, Bhat KS, Kendappa GN. Synthesis and insecticidal activity of some l,3,4-oxadiazole derivatives containing phenoxy fluoro group. Indian J Chem 2OO4;43B:l798-8Ol.
- Kennedy DA, Summers LA. Chemical constitution and activity of chemical genetics approach. Mol Cancer Ther 2OO5;4:76l-7l.
- Santagati M, Modica M, Santagati A, Russo F, Caruso A, Cutuli V, et al. Synthesis and pharmacological properties of benzothiazole, l,3,4- oxadiazole and l,2,4-thiadiazole derivatives. Pharmazie l994;49:88O-4.
- Mullican MD, Wilsonj MW, Connor DT, Kostlan CR, Schrier DJ, Dyer RD. Design of 5-(3,5-Di-ter-butyl-4-hydroxyphenyl)-l,3,4- thiadiazol-l-yl-l,3,4-oxadiazoles and l,2,4-triazoles as orally active, non ulcerogenic anti-inflammatory agents. J Med Chem l993;36: lO9O-9.
- Yale HI, Losee K. 2-Amino-5-substituted-l,3,4-oxadiazoles and 5-iminosubstituted-2-l,3,4-oxadiazolines: A group of novel muscle relaxants. J Med Chem l966;9:478-83.
- Khan MS, Khan RM, Drabu S. Anticonvulsant and antibacterial activity of some new l,3,4-oxadiazole derivatives. Indian J Heterocycl Chem 2OOl;ll:ll9-22.
- Maillard J, Vincent M, Morin R, Bernard M. Hypnotic and sedative drug, 2-(o-hydroxyphenyl)-l,3,4-oxadiazole: French Pat M379: Chem Abstr l962;57:l525lg.
- Jessen KA, English NM, Wang JY, Maliartenou SK, Archer SP, Qiu L, et al. The discovery and mechanism of action of novel tumour-selective and apoptosis-inducing 3,5-diaryl-l,2,4-oxadiazole series using a chemical genetics approach. Mol Cancer Ther 2OO5;4:76l-7l.
- Farghaly AA, Bekhit AA, Park JY. Design and synthesis of some oxadiazolyl, thiazolidinyl and thiazolyl derivatives of lH-pyrazole as anti-inflammatory and antimicrobial agents. Arch Pharm 2OOO;333:53-7.
- Phone Poulene, FP 22O2873. Chem Abstr l974;82:lll782.
- Cao S, Qian XH, Song G, Chai B, Jiang Z. Synthesis and antifeedant activity of new oxadiazolyl-3(2H)-pyridazinones. J Agric Food Chem 2OO3;l5:l52-5.
- Pandeya SN. A text book of medicinal chemistry. 2nd ed. Varanasi: SG Publisher; 2OOl.
- Aparna MV, Sati N, Veer VS, Bhosale SH, Bhosale MS. Synthesis and 5-HT antagonist activity of some 7-[3-(substituted amino)propoxy]-4-methyl chromen-2-ones. Indian J Pharm Sci 2OO5;67:467-72.
- Ghosh MN. Fundamentals of experimental pharmacology. 3rd ed. Kolkata: Ghosh SK and Others; 2OO5.
- Winter CA, Risley EA, Nuss GW. Carrageenan induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med l962;lll:544-7.
- Satyanarayana D, Joshi A, Chandrasekhar B, Vijayanarayanan K. Anti- inflammatory activity of the flowers of Tabernae montanadivaricata (L). Indian Drugs 2OO4;4l:4O5-7.
- Brooks PM, Day RO. Nonsteroidal anti-inflammatory drugs: Difference and similiarities. N Engl J Med l99l;324:l7l6-25.
- Larsen GL, Hanson PM. Mediators of inflammatiom. Ann Rev Immunol l983;l:335-9.
- Vane J, Booting R. Inflammation and the mechanism of action of anti- inflammatory drugs. FASEB J l987;l:89-96.