- *Corresponding Author:
- A. K. Halve
School of Studies in Chemistry, Jiwaji University, Gwalior-474 011, India
E-mail: drhalve_chemjiwaji@rediffmail.com
| Date of Submission | 30 May 2005 |
| Date of Revision | 06 September 2005 |
| Date of Acceptance | 30 July 2006 |
| Indian J Pharm Sci, 2006, 68 (4): 510-514 |
Abstract
The title compounds were synthesized by the condensation of nitro-substituted 2-hydroxy-5- (nitro-substituted phenylazo) benzaldehyde (3) with different aromatic amines in presence of ethanol in good yield. The chemical structures were confirmed by IR, 1H NMR and elemental analysis. All the synthesized compounds (4a-j) have been evaluated for their in vitro antimicrobial activity against S. aureus , P. aeruginosa , E. coli , A. fumigatus , A. niger and C. neoformans .
Introduction
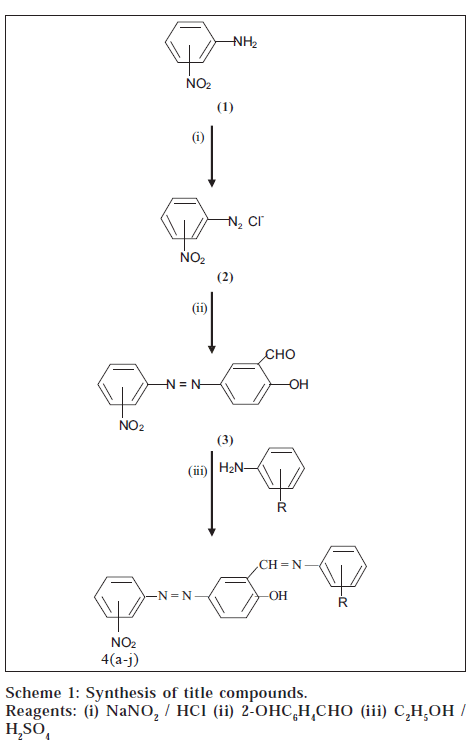
Schiff bases, a class of organic compounds [1], exhibit a variety of biological [2-6] and therapeutic properties [7-8]. These compounds are characterized by -N=CH- (imine), the toxophoric group, which imparts in elucidating the mechanism of transamination and racemization reaction in biological systems [9]. In mild acidic conditions, these compounds could be hydrolyzed selectively by tumour cells as these cells have lower pH than cells in normal tissue [10]. As a consequence, they have been evaluated for their antiproliferative properties against a variety of tumors [11-12]. In addition, these compounds have a wide range of biological activities, including antibacterial [13-15], antifungal [16], antitubercular [17], anticancer18 and anti-HIV [19] activities. In view of the pronounced biological activities of these compounds, we report herein a new synthesis of the title compounds (4a-j) by the reaction of 2-hydroxy-5(3'-nitrophenylazo)benzaldehyde and 2-hydroxy-5-(4'-nitro phenylazo)benzaldehyde (3) with nitro- and methoxysubstituted aromatic primary amines (Scheme 1). Elemental analysis, IR and 1H NMR spectra characterized the constitutions of synthesized compounds.
All the melting points were determined in open glass capillaries and are uncorrected. The purity of the compounds was ascertained by TLC on silica gel-G plates and spots were visualized by using iodine vapours. IR spectra were recorded on a Perkin Elmer spectrophotometer. 1H NMR spectra were recorded on a Varian EM-390 MHz NMR spectrometer in DMSO-d6 using TMS as internal reference and chemical shift values were expressed in ppm (δ). Elemental analysis was performed on Carlo Erba 1108 analyzer. Some of the physical characteristics of the synthesized compound have been presented in Table 1.
| Compound | -NO2 | R’ | m.p.(o) | Yield (%) | Molecular formula | Nitrogen % | |
|---|---|---|---|---|---|---|---|
| Found | Calcd. | ||||||
| 4a | 3' | H | 180 | 68 | C19H14N4O3 | 16.11 | 16.18 |
| 4b | 3' | NO2(m) | 172 | 65 | C19H13N5O5 | 17.87 | 17.9 |
| 4 c | 3' | NO2(p) | 178 | 72 | C19H13N5O5 | 17.84 | 17.9 |
| 4d | 3' | OCH3(m) | 162 | 64 | C20H16N4O4 | 14.82 | 14.89 |
| 4e | 3' | OCH3(p) | 182 | 67 | C20H16N4O4 | 14.85 | 14.89 |
| 4f | 4' | H | 185 | 61 | C19H14N4O3 | 16.10 | 16.18 |
| 4 g | 4' | NO2(m) | 160 | 75 | C19H13N5O5 | 17.81 | 17.9 |
| 4h | 4' | NO2(p) | 190 | 60 | C19H13N5O5 | 17.79 | 17.9 |
| 4i | 4' | OCH3(m) | 166 | 63 | C20H16N4O4 | 14.83 | 14.89 |
| 4j | 4' | OCH3(p) | 175 | 70 | C20H16N4O4 | 14.87 | 14.89 |
| All the compounds gave satisfactory elemental analysis within ±0.06% of the theoretical values. | |||||||
Table 1: Data Of Synthesized Compounds (4a-j)
2-hydroxy-5-(3'-nitrophenylazo)benzaldehyde and 2hydroxy-5-(4'-nitrophenylazo) benzaldehyde (3) were prepared by diazotization and coupling methods at 0 - 5°. 2-Hydroxy-5-(3'-nitrophenylazo) benzylidine aniline (4a) was synthesized by refluxing equimolar quantities of 2hydroxy-5-(3'-nitrophenylazo)benzaldehyde and aniline in absolute ethanol for 2 h. After completion of reaction, a drop of sulphuric acid was added. The product obtained was filtered under suction, washed and recrystallised from ethanol. m p, 172°, yield: 68%, IR (KBr) cm-1: 3540 (O-H), 1620 (C=N), 1583 (N=N), 1498 (N=O, Asym.), 1349 (N=O, Sym.): 1H NMR (DMSO-d6), δ, ppm: 4.2 (s, 1H, OH), 7.3 7.8 (m, 12H, ArH), 9.3 (s, 1H, CH=N).
2-Hydroxy-5-(3'-nitrophenylazo)-3'’-nitrobenzylidine aniline (4b): IR (KBr) cm-1: 3510 (O-H), 1624 (C=N), 1590 (N=N), 1489 (N=O, Asym.), 1351 (N=O, Sym.): 1H NMR (DMSO-d6) δ, ppm: 4.3 (s, 1H, OH), 7.2 - 7.5 (m, 11H, Ar-H), 9.4 (s, 1H, CH=N). 2-hydroxy-5- (3'-nitrophenylazo)4'’-nitrobenzylidine aniline (4c): IR (KBr) cm-1: 3522 (OH), 1650 (C=N), 1598 (N=N), 1493 (N=O, Asym.), 1353 (N=O, Sym.): 1H NMR (DMSO-d6) δ, ppm: 4.1 (s, 1H, OH), 7.1 - 7.6 (m, 11H, Ar-H), 9.7 (s, 1H, CH=N). 2hydroxy-5-(3'-nitrophenylazo)-3'’-methoxybenzylidine aniline (4d): IR (KBr) cm-1: 3512 (O-H), 1610 (C=N), 1588 (N=N), 1483 (N=O, Asym.), 1310 (N=O, Sym.), 1280 (OCH3, Asym.), 1010 (O-CH3), Sym.); 1H NMR (DMSO-d6) δ, ppm: 3.42 (s, 3H, OCH3), 4.5 (s, 1H, OH), 7.3-7.5 (m, 11H, Ar - H), 9.62 (s, 1H, CH=N).
2-Hydroxy-5-(3'-nitrophenylazo)-4'’-methoxybenzylidine aniline (4e): IR (KBr) cm-1: 3540 (O-H), 1613 (C=N), 1593 (N=N), 1498 (N=O, Asym.), 1321 (N=O, Sym.), 1281 (OCH3, Asym.), 1028 (O-CH3, Sym.): 1H NMR (DMSO-d6) δ, ppm: 3.41 (s, 3H, OCH3), 4.8 (s, 1H, OH), 7.1 - 7.5 (m, 11H, Ar-H), 9.42 (s, 1H, CH=N). 2-hydroxy-5- (4'nitrophenylazo) benzylidine aniline (4f): IR (KBr) cm-1: 3548 (O-H), 1680 (C=N), 1605 (N=N), 1491 (N=O, Asym.), 1373 (N=O, Sym.): 1H NMR (DMSO-d6) δ, ppm: 4.31 (s, 1H, OH), 7.4 - 7.8 (m, 12H, Ar-H), 9.2 (s, 1H, CH=N). 2hydroxy-5- (4' -nitrophenylazo)-3'’-nitrobenzylidine aniline (4g): IR (KBr) cm-1: 3534 (O-H), 1630 (C=N), 1601 (N=N), 1485 (N=O, Asym.), 1348 (N=O, Sym.): 1H NMR (DMSOd6), δ, ppm: 4.48 (s, 1H, OH), 7.1 - 7.6 (m, 11H, ArH), 9.79 (s, 1H, CH=N).
2-Hydroxy-5-(4'-nitrophenylazo)-4'’-nitrobenzylidine aniline (4h): IR (KBr) cm-1: 3538 (O-H), 1641 (C=N), 1596 (N=N), 1490 (N=O, Asym.), 1352 (N=O, Sym.): 1H NMR (DMSO-d6), δ, ppm: 4.53 (s, 1H, OH), 7.3 - 7.7 (m, 11H, Ar-H), 9.75 (S, 1H, CH=N).2-hydroxy-5- (4'nitrophenylazo)-3'’-methoxybenzylidine aniline (4i): IR (KBr) cm-1: 3522 (O-H), 1628 (C=N), 1587 (N=N), 1487 (N=O, Asym.), 1343 (N=O, Sym.), 1283 (O-CH3, Asym.), 1042 (O-CH3 Sym.): 1H NMR (DMSO-d6), δ, ppm: 3.38 (s, 3H, O-CH3), 4.83 (s, 1H, OH), 7.5 - 7.7 (m, 11H, Ar-H), 9.54(s, 1H, CH=N). 2-hydroxy-5-(4'-nitrophenylazo)4'’-methoxybenzylidine aniline (4j): IR (KBr) cm1: 3529 (O-H), 1635 (C=N), 1581 (N=N), 1494 (N=O, Asym.), 1356 (N=O, Sym.), 1288 (O-CH3, Asym.), 1048 (O-CH3, Sym.): 1H NMR (DMSO-d6), δ, ppm: 3.41 (s, 3H, OCH), 4.87 (s, 1H, OH), 7.0 - 7.4 (m, 12H, Ar-H), 9.48 (s, 3 1H, CH= N).
All the compounds were screened for their in vitro antimicrobial activity against 24 h old cultures of bacterial and fungal pathogens. Antibacterial activity was determined against Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli by the cup plate method. For this, sterile filter paper disks (6 mm) impregnated with fixed doses (100 μg/ml) of synthesized compounds under investigation were placed upon the seeded Petri dishes. Similar disks were prepared for the standard drug, Chloromycetin and solvent control, dimethylformamide. The plates were allowed to stay for 24 h at 37°. The zone of inhibition, observed around the disks after incubation, was measured and percent inhibition of the compounds was calculated. The results were presented in Table 2.
| Compound | S. aureus | P. aeruginosa | E. coli | |||
|---|---|---|---|---|---|---|
| Zone of inhibition (mm) | % inhibition | Zone of inhibition (mm) | % inhibition | Zone of inhibition (mm) | % inhibition | |
| 4a | 22 | 88 | 15 | 66.6 | 18 | 81.8 |
| 4b | 24 | 96 | 18 | 75 | 20 | 90.9 |
| 4 c | 10 | 40 | 12 | 50 | 12 | 54.5 |
| 4d | 14 | 56 | 8 | 33.3 | - | - |
| 4e | 18 | 72 | 14 | 58.3 | 10 | 45.4 |
| 4f | 14 | 56 | 8 | 33.3 | 8 | 36.3 |
| 4 g | 20 | 80 | 18 | 75 | 16 | 72.7 |
| 4h | - | - | - | - | - | - |
| 4i | - | - | 12 | 50 | 10 | 45.4 |
| 4j | 10 | 40 | 8 | 33.3 | 8 | 36.3 |
| Chloramphenicol | 25 | 100 | 24 | 100 | 22 | 100 |
| Control (DMF) = No activity. Both, test compounds and standard were tested at 100 mg/ml. | ||||||
Table 2: Results Of In Vitro Antibacterial Activity Of The Newly Synthesized Compounds (4a-j)
Antifungal activity was carried out against three fungal pathogens, namely, A. fumigatus, A. niger and C. neoformans, by using serial-dilution tube technique. Concentrations varying from 15.62 to 8,000 μg/ml of each compound were prepared by dissolving the compounds in DMF using Sabouraud’s Dextrose media and results were compared with that of standard drug fluconazole (Table 3).
| Compound | A.fumigatus (μg) |
C.neoformans (μg) |
A.niger (μg) |
|---|---|---|---|
| 4a | 2000 | 250 | 2000 |
| (4000) | (500) | (4000) | |
| 4b | 1000 | 62.5 | 2000 |
| (2000) | (125) | (4000) | |
| 4c | 1000 | 1000 | 4000 |
| (2000) | (2000) | (8000) | |
| 4d | 4000 | 2000 | 4000 |
| (8000) | (4000) | (8000) | |
| 4e | 4000 | 62.5 | 2000 |
| (8000) | (125) | (4000) | |
| 4f | 4000 | 2000 | 4000 |
| (8000) | (4000) | (8000) | |
| 4g | 2000 | 1000 | 4000 |
| (4000) | (2000) | (8000) | |
| 4h | 4000 | 4000 | 4000 |
| (8000) | (8000) | (8000) | |
| 4i | 4000 | 1000 | 4000 |
| (8000) | (2000) | (8000) | |
| 4j | 2000 | 2000 | 2000 |
| (4000) | (4000) | (4000) | |
| Fluconazole | 2000 | 250 | 2000 |
| (4000) | (500) | (4000) |
Table 3: Results Of In Vitro Antifungal Activity Of The Newly Synthesized Compounds (4a-j)
The results of antibacterial screening reveal that compound (4b) with m-nitro substitution showed highest activity (87.3%) against all the bacterial pathogens and it follows the order: S. aureus >E. coli >P. aeruginosa. Compound (4a), having unsubstituted benzylideneamino group, showed more activity (78.8%) in comparison to compounds (4c), (4d) and (4e) bearing p-nitro (48.16%), m-methoxy (29.76%) and p-methoxy (58.56%), substitution at benzylideneamino group. It was also observed that p-nitro substitution at phenylazo moiety reduces the antibacterial activity (27.55%) in compounds (4f), (4h), (4i) and (4j). However, compound 4g, constituting p-nitro phenylazo moiety with meta nitro substitution at benzylideneamino group, showed promising antibacterial activity (75.9%). Thus, it has been concluded that among all the synthesized compounds, antibacterial activity decreases when there is p-nitro substitution and it enhances with m-nitro substitution, showing maximum activity when attached to the 3rd position of benzylideneamino group (87.3%).
From the antifungal screening results, it has been observed that compounds (4a), (4b) and (4e) showed better activity as compared to the standard drug. In case of A. fumigatus, compounds (4a) and (4c) showed good antifungal activity, while compounds (4a), (4g) and (4j) exhibited comparable activity to fluconazole. Among the tested compounds, (4a), (4b) and (4e) showed maximum activity against C. neoformans. However, compounds (4a), (4b), (4e) and (4j) are more active against A. niger. Apart from this, compound (4h) showed cidal activity at higher concentrations. Thus, it has been observed that m-nitro substitution exhibits prominent antifungal activity against the tested fungal pathogens.
Acknowledgements
Sincere thanks are due to the Dean, Birla Institute of Medical Research and College of Life Sciences, Gwalior, for providing facilities; and to the UGC, New Delhi, for financial assistance.
References
- Dutta, M.M., Goswami, B.N. and Katkay, J.C.S., J. Indian Chem. Soc., 1986, 23, 793.
- Banik, J. and Banik, B.N., J. Med. Chem., 2003, 46, 12.
- Verdegem, P.J., Luhman, H. and Levit, M.H., J. Amer. Chem. Soc., 2004, 126, 3948.
- Kandori, H., Belenky, M. and Herzfeld, J. Biochem., 2002, 41, 6026.
- Pandeya, S.N. and Reddy, V.M., Indian J. Pharm. Sci., 1994, 56, 33.
- Sarangapani, M. and Reddy, V.M., Indian J. Pharm. Sci., 1994, 56, 174.
- Frere, F., Schubert, W.D., Stauffer, F., Neier, R., Jahn, D. and Heinz, D.W., J. Mol. Biol., 2002, 320, 237.
- Stoyanova, R., Kaloyanov, N., Traldi, P. and Bliznakov, M., Arzneim. Forsch- Drug Res.,. 2001, 51, 991.
- Lau, K.Y., Mayr, A. and Cheung, K.K., Inorg. Chim. Acta., 1999, 285, 223.Ashby, B.S., Lancet, 1966, 2, 312.
- Finch, A.R., ChiuMao, L., Grill, S.P., Rose, W.C., Lomis, R., Vasquez, K.M., Cheng, C.Y. and Sartorelli, A.C., Biochem. Pharmacol., 2000, 59, 983.
- LiMou, Z., King, T., Doyle, T.W. and ShuHui, C., Curr. Med. Chem., 2001, 8, 121.
- Chohan, Z.H., Rau, A. and Noreen, S., J. Enz. Inhib. Med. Chem., 2002, 17, 101.
- Sridhar, S.K., Saravanan, M. and Ramesh, A., Eur. J. Med. Chem., 2001, 36, 615.
- Pandeya, S.N., Sriram, D., Nath, G. and De Clerq, E., Indian J. Pharm. Sci., 1999, 61, 358.
- Vicini, P., Geronikaki, A., Incerti, M., Busonera, B., Poni, G., Cabras,
- C.A. and Colla, L., Bioorg. Med. Chem., 2003, 11, 4785.
- Hearn, M.J. and Cynamon, M.H., J. Antimicrob. Chemother., 2004, 53, 185.
- Kuzmin, V., Lozitsky, V.P., Kamalov, G.L., Lozitaskaya, R.N., Zheltvay, A.I., Fedtchouk, A.S. and Kryzhanovsky, D.N., Acta. Biochim. Pol., 2000, 47, 867.
- AlAbed, Y., Dubrovsky, L., Ruzsieska, B., Seepersaud, M. and Bukrinsky, M., Bioorg. Med. Chem. Lett, 2002, 12, 3117.