N. Kaushik*, N. Kumar1,2 and A. Kumar3
School of Pharmaceutical Sciences, IFTM University, Moradabad–244 102
1School of Medical and Allied Sciences, Department of Pharmacy, Galgotias University, Greater Noida, Gautam Budh Nagar–201 306
2Oxford College of Pharmacy, UPSIDC, M-G Road, Ghaziabad–201 001
3Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, Meerut–250 005, India
- *Corresponding Author:
- N. Kaushik
School of Pharmaceutical Sciences, IFTM University, Moradabad–244 102
E-mail: niranjankaushik79@gmail.com
Received date: 12 Sep 2016; Revised date: 31 May 2016; Accepted date: 06 Jun 2016
Abstract
A series of 1-[(5-substituted phenyl)-4, 5-dihydro-1H-pyrazol-3-yl]-5-phenyl-1H-tetrazole derivatives (4a-j) have been synthesized by the reaction of 1-(5-substitutedphenyl-1H-tetrazol-1-yl) prop-2-en-1-one (3a-j) with hydrazine hydrate in acetic acid under reflux for 5 h. Structure of the synthesized compounds was established by their spectral data. As a new strategy for alleviating the oxidative damage in Diabetes mellitus, a growing interest has been observed in the usage of antioxidants. The synthesized compounds were screened for their in vitro antioxidant activity by DPPH free radical scavenging method and in vivo antidiabetic activity by streptozotocin-induced model. Compounds 4b, 4g, 4h and 4j were exhibit prominent antioxidant as well as antidiabetic activity. Compound 4i was exhibit moderate antidiabetic activity.
Keywords
Tetrazole, pyrazole, antioxidant activity, antidiabetic activity
Diabetes Mellitus (DM) is a multifactroial disease which is characterized by hypoglycemia[1], lipoprotein abnormalities[2], raised basal metabolic rate[3], defect in reactive oxygen species scavenging enzymes[4] and high oxidative stress-induced damage to pancreatic beta cells[5].
Although the mechanism of diabetic complications remains unclear, much attention has been focused to the role of oxidative stress. Oxidative stress may contribute to the pathogenesis of different diabetic complications[6]. Furthermore, with diabetes, several features appear including an increase in lipid peroxidation[7], a decrease in the antioxidant enzyme activities. These changes indicate an oxidative stress caused by hyperglycemia[8].
Oxidative stress is characterized by an increased concentration of intracellular oxidizing species, such as reactive oxygen species (ROS) and is often accompanied by the loss of antioxidant defense capacity[9]. It is well known that excess ROS attack many organs, and induce oxidative damage directly to critical biological molecules, such as lipoproteins, proteins and nucleotides, causing lipid peroxidation and protein oxidation. Metabolic oxidative stress has been implicated, directly or indirectly, in the development of diseases and degenerative processes, including inflammation, cancer, dementia and physiological aging[10]. Moreover, oxidative stress also plays a central role in liver pathologies[11]. Antioxidant pharmacotherapy in various forms has emerged as a mean to minimize the bimolecular damage caused by attack of ROS on vital constituents of living organisms[12].
In DM, oxidative stress seems mainly to be due to an increased production of free radicals and a sharp reduction of antioxidant defense[13]. Therefore, there is a great interest in looking for new tetrazole containing pyrazole moiety, which could represent a good pharmacological alternative to counteract oxidative stress.
Development of tetrazole chemistry offers wide scale of applications in the medicine, biochemistry and agriculture fields[14]. The chemistry of tetrazoles as well as their medicinal applications has been covered in literature. They have found use in pharmaceuticals as lipophilic spacer and carboxylic acid surrogates[15]. Tetrazole and its derivatives have attracted much interest in medicinal chemistry owing to unique structure. The tetrazole functionality plays an important role in medicinal chemistry primarily due to its ability to serve as bioisostere of the carboxylic acid group[16]. One of the well-known methods for synthesis of 5-substituted- 1H-tetrazoles is by a [3+2] cycloaddition between hydrazoic acid and cyanides[17]. Their derivatives have been shown antibacterial, antifungal[18], anticancer[19], analgesic[20], antiinflammatory[21], antidiabetic, antihyperlipidimic[22-23] and antitubercular activities[24]. A large number of medicinally important drugs containing tetrazole moiety has been approved by USFDA[25].
In addition the pyrazole moiety is a versatile lead molecule in pharmaceutical development and has a wide range of biological activities. Literature survey reveals that pyrazole derivatives are well known to have antibacterial[26], antifungal[27], antiinflammatory[ 28], antitubercular[29], antidepressant, anticonvulsant[30], antioxidant[31], anticancer[32] and antiviral[33] activities. The pyrazolotetrazole nucleus has been studied by other researchers with different functional groups[34]. No detailed study has been carried out on the pyrazolotetrazole, in moderating oxidative stress associated with diabetes mellitus in experimental animals. Hence, the aim of present study is to synthesize various derivatives of tetrazole containing pyrazole moiety and evaluate their antioxidant and antidiabetic potential.
Materials And Methods
All of the reagents were purchased from commercial sources. Melting point was determined by open capillary method and was uncorrected. All the reactions were measured by thin layer chromatography (TLC). The compounds were analyzed for elemental analysis. Infrared (IR) spectra were recorded by using KBr disk on Shimadzu FTIR-8400S. 1H NMR spectra was recorded on a JEOL AL300 FTNMR 300 MHz spectrophotometer by using tetramethylsilane as internal standard. The values of chemical shift (δ) are given in ppm. Mass spectra were carried out utilizing Waters Micromass Q-Tof Micro Mass spectrometer equipped with electrospray ionization (ESI).
General procedure for the synthesis of 5-phenyl tetrazole (1):
The equimolar quantities of sodium azide, ammonium chloride and benzonitrile were refluxed with dimethyl formamide at 125° for 7-8 h to obtain the 5-phenyl tetrazole (1). The residue was dissolved in 100 ml of water and acidified with concentrated hydrochloric acid. The solution was cooled, filtered, dried and recrystallized using ethanol.
Brown solid; % yield: 80; MP: 208-210°, IR (KBr) v (cm-1): 3348 (NH), 3062 (Ar-CH), 1628 (C=N), 1292 (N-N=N-); 1H NMR (CDCl3) δ (ppm): 7.18-7.56 (m, 5H, Ar-H), 8.72 (s, 1H, NH). Anal. Calcd. (%) for C7H6N4: C, 57.53; H, 4.14; N, 38.34. Found: C, 57.74; H, 4.18; N, 38.47.
General procedure for the synthesis of 5-phenyl-1- acetyl tetrazole (2):
5-phenyl tetrazole (1, 10 mmol) was mixed with acetic anhydride (10 mmol), to this solution 2-3 drops of concentrated sulphuric acid was added. The reaction mixture was warmed at 60-70° for 15-20 min on water bath. The content was cooled to room temperature and then poured into ice cold water to obtain a white colored precipitate. The precipitate was filtered, washed, dried and recrystallized using ethanol.
White solid; % yield: 78; MP: 218-220°, IR (KBr) v (cm-1): 3058 (Ar-CH), 1730 (C=O), 1638 (C=N), 1285 (N-N=N-); 1H NMR (CDCl3) δ (ppm): 2.28 (s, 3H, CH3), 7.28-7.51 (m, 5H, Ar-H). Anal. Calcd. (%) for C9H8N4O: C, 57.44; H, 4.28; N, 29.77; O, 8.50. Found: C, 57.62; H, 4.31; N, 29.92.
General procedure for the synthesis of 1-(5-substitutedphenyl-1H-tetrazol-1-yl) prop-2- en-1-one (3a-j):
A solution of 5-phenyl-1-acetyl tetrazole (2, 10 mmol) and different substituted aromatic aldehyde (10 mmol) in ethanol (20 ml) was cooled to 5 to 10° in an ice bath. The cooled solution was treated with drop wise addition of 40% sodium hydroxide solution. The reaction mixture was magnetically stirred for 30 min and then left over night in the refrigerator. The resulting dark solution was diluted with ice water and acidified using hydrochloric acid. The solution was filtered, washed with water and recrystallized with ethanol.
3-(3-bromo-4-nitrophenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3a):
Brown solid; % yield: 86; MP: 260-262°, IR (KBr) v (cm-1): 3034 (Ar-CH), 1736 (C=O), 1618 (C=C), 1593 (C=N), 1570 (NO2), 1284 (N-N=N-), 646 (C-Br). 1H NMR (CDCl3) δ (ppm): 6.80 (d, 1H, ethylenic), 7.23 (d, 1H, ethylenic), 7.28-7.40 (m, 5H, Ar-H), 7.47 (d, 1H, Ar-H), 7.53 (s, 1H, Ar-H), 7.94 (d, 1H, Ar-H). Anal. Calcd. (%) for C16H10BrN5O3: C, 48.02; H, 2.52; Br, 19.97; N, 17.50; O, 11.99. Found: C, 48.18; H, 2.54; N, 17.58.
3-(3, 4, 5-trimethoxyphenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3b):
Yellowish brown solid; % yield: 82, MP: 230-232°, IR (KBr) v (cm-1): 3054 (Ar-CH), 1740 (C=O), 1630 (C=C), 1600 (C=N), 1248 (N-N=N), 1221 (OCH3); 1H NMR (CDCl3) δ (ppm): 3.66 (s, 9H, -OCH3), 6.40 (d, 1H, ethylenic), 6.56 (s, 2H, Ar-H), 7.28-7.45 (m, 5H, Ar-H), 7.47 (d, 1H, ethylenic). Anal. Calcd. (%) for: C19H18N4O4: C, 62.29; H, 4.95; N, 15.29; O, 17.47. Found: C, 62.34; H, 4.98; N, 15.37.
3-(2, 4-difluorophenyl)-1-(5-phenyl-1H-tetrazol-1- yl) prop-2-en-1-one (3c):
Creamy solid; % yield: 79; MP: 234-236°, IR (KBr) v (cm-1): 3059 (Ar-CH), 1712 (C=O), 1614 (C=C), 1608 (C=N), 1268 (N-N=N-), 1163 (C-F); 1H NMR (CDCl3) δ (ppm): 6.43 (d, 1H, ethylenic), 6.58 (s, 1H, Ar-H), 6.77 (s, 1H, Ar-H), 7.18 (d, 1H, Ar-H), 7.34-7.51 (m, 5H, Ar-H), 7.68 (d, 1H, ethylenic). Anal. Calcd. (%) for C16H10F2N4O: C, 61.54; H, 3.23; F, 12.17; N, 17.94; O, 5.12. Found: C, 61.72; H, 3.26; N, 18.12.
3-(4-fluorophenyl)-1-(5-phenyl-1H-tetrazol-1-yl) prop-2-en-1-one (3d):
Brown solid; % yield: 68; MP: 224-226°, IR (KBr) v (cm-1): 3060 (Ar-CH), 1734 (C=O), 1620 (C=C), 1606 (C=N), 1310 (N-N=N-), 1166 (C-F); 1H NMR (CDCl3) δ (ppm): 6.56 (d, 1H, ethylenic), 7.04 (d, 2H, Ar-H), 7.21 (d, 2H, Ar-H), 7.29-7.42 (m, 5H, Ar-H), 7.48 (d, 1H, ethylenic). Anal. Calcd. (%) for C16H11FN4O: C, 65.30; H, 3.77; F, 6.46; N, 19.04; O, 5.44. Found: C, 65.57; H, 3.80; N, 19.12.
3-(2-hydroxy-5-nitrophenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3e):
Brown solid; % yield: 63; MP: 252-254°, IR (KBr) v (cm-1): 3583 (OH), 3055 (Ar-CH), 1680 (C=O), 1630 (C=C), 1610 (C=N), 1564 (NO2), 1344 (N-N=N-); 1H NMR (CDCl3) δ (ppm): 4.92 (s, 1H, -OH), 6.76 (d, 1H, ethylenic), 6.81 (d, 1H, Ar-H), 7.25-7.48 (m, 5H, Ar- H), 7.54 (d, 1H, Ar-H), 7.57 (d, 1H, ethylenic), 7.76 (s, 1H, Ar-H). Anal. Calcd. (%) for C16H11N5O4: C, 56.98; H, 3.29; N, 20.76; O, 18.97. Found: C, 57.16; H, 3.31; N, 20.84.
3-(2-chloro-4-fluorophenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3f):
Reddish brown solid; % yield: 74; MP: 220-222°, IR (KBr) v (cm-1): 3054 (Ar-CH), 1735 (C=O), 1630 (C=C), 1608 (C=N), 1285 (N-N=N-), 1178 (C-F), 786 (C-Cl); 1H NMR (CDCl3) δ (ppm): 6.37 (d, 1H, ethylenic), 6.74 (d, 1H, Ar-H), 7.06 (s, 1H, Ar-H), 7.17 (d, 1H, Ar-H), 7.28-7.44 (m, 5H, Ar-H), 7.72 (d, 1H, ethylenic). Anal. Calcd. (%) for C16H10ClFN4O: C, 58.46; H, 3.07; Cl, 10.78; F, 5.78; N, 17.04; O, 4.87. Found: C, 58.62; H, 3.11; N, 17.18.
3-(2, 4-dimethoxyphenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3g):
Brown solid; % yield: 78; MP: 246-248°, IR (KBr) v (cm-1): 3054 (Ar-CH), 1666 (C=O), 1640 (C=C), 1606 (C=N), 1275 (N-N=N-), 1248 (OCH3); 1H NMR (CDCl3) δ (ppm): 3.68 (s, 6H, -OCH3), 6.47 (d, 1H, Ar-H), 6.53 (d, 1H, Ar-H), 6.71 (d, 1H, ethylenic), 7.16 (d, 1H, Ar-H), 7.30-7.41 (m, 5H, Ar-H), 7.62 (d, 1H, ethylenic). Anal. Calcd. for C18H16N4O3: C, 64.28; H, 4.79; N, 16.66; O, 14.27. Found: C, 64.36; H, 4.81; N, 16.78.
3-(2, 4, 6-trimethoxyphenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3h):
Yellowish brown solid; % yield: 82; MP: 212-214º, IR (KBr) v (cm-1): 3064 (Ar-CH), 1666 (C=O), 1652 (C=C), 1606 (C=N), 1290 (N-N=N-), 1186 (OCH3); 1H NMR (CDCl3) δ (ppm): 3.66 (s, 9H, OCH3), 6.63 (d, 1H, ethylenic), 6.67 (s, 2H, Ar-H), 7.30-7.45 (m, 5H, Ar-H), 7.63 (d, 1H, ethylenic). Anal. Calcd. (%) for C19H18N4O4; C, 62.29; H, 4.95; N, 15.29; O, 17.47. Found: C, 62.44; H, 4.97; N, 15.36.
3-(2-nitrophenyl)-1-(5-phenyl-1H-tetrazol-1-yl) prop-2-en-1-one (3i):
Yellow solid; % yield: 87; MP: 228-230°, IR (KBr) v (cm-1): 3074 (Ar-CH), 1726 (C=O), 1620 (C=C), 1608 (C=N), 1578 (NO2), 1248 (N-N=N-); 1H NMR (CDCl3) δ (ppm): 6.65 (d, 1H, ethylenic), 7.28 (t, 1H, Ar-H), 7.32-7.53 (m, 5H, Ar-H), 7.58 (t, 1H, Ar-H), 7.61 (d, 1H, Ar-H), 7.78 (d, 1H, Ar-H), 7.93 (d, 1H, ethylenic). Anal. Calcd. (%) for C16H11N5O3: C, 59.81; H, 3.45; N, 21.80; O, 14.94. Found: C, 59.93; H, 3.47; N, 21.88.
3-(3-bromo-4-methoxyphenyl)-1-(5-phenyl-1H-tetrazol- 1-yl) prop-2-en-1-one (3j):
Yellowish brown solid; % yield: 78; MP: 164-166º, IR (KBr) v (cm-1): 3034 (Ar-CH), 1660 (C=O), 1615 (C=C), 1608 (C=N), 1255 (N-N=N-), 1170 (OCH3), 640 (C-Br); 1H NMR (CDCl3) δ (ppm): 3.77 (s, 3H, OCH3), 6.58 (d, 1H, ethylenic), 6.64 (d, 1H, Ar-H), 7.16 (d, 1H, Ar-H), 7.27 (s, 1H, Ar-H), 7.29-7.41 (m, 5H, Ar-H), 7.47 (d, 1H, ethylenic). Anal. Calcd. (%) for C17H13BrN4O2: C, 53.00; H, 3.40; Br, 20.74; N, 14.54; O, 8.31. Found: C, 53.21; H, 3.44; N, 14.61.
General procedure for the synthesis of 1-[(5-substituted phenyl)-4, 5-dihydro-1H-pyrazol- 3-yl]-5-phenyl-1H-tetrazole (4a-j):
A mixture of 1-(5-substitutedphenyl-1H-tetrazol-1-yl) prop-2-en-1-one (3a-j, 10 mmol), hydrazine hydrate (10 mmol) and acetic acid (40 ml) was refluxed for 5 h and then poured into ice cold water. The precipitate was separated by filtration, dried and recrystallized from ethanol.
1-(5-(3-bromo-4-nitrophenyl))-4, 5-dihydro-1H-pyrazol- 3-yl)-5-phenyl-1H-tetarzole (4a):
Brown solid, % yield: 76; MP: 148-150°, IR (KBr) v (cm-1): 3352 (NH), 3073 (Ar-CH), 2990 (CH), 1622 (C=N), 1580 (NO2), 1270 (N-N=N-), 615 (C-Br); 1H NMR (CDCl3) δ (ppm): 2.10 (d, 2H, CH2), 3.74 (t, 1H, CH), 6.85 (s, 1H, NH), 7.23 (d, 1H, Ar-H), 7.32 (s, 1H, Ar-H), 7.37-7.53 (m, 5H, Ar-H), 7.87 (d, 1H, Ar-H). MS m/z: 413 [M+], Anal. Calcd. (%) for C16H12BrN7O2: C, 46.39; H, 2.92; N, 23.67; O, 7.73; Br, 19.29. Found: C, 46.52; H, 2.94; N, 23.74.
1- (4, 5-dihydro-5-(3, 4, 5-trimethoxypheny) -1H-pyrazol-3-yl)-5-phenyl-1H-tetarzole (4b):
Brown solid, % yield: 72; MP: 145-147°, IR (KBr) v (cm-1): 3340 (NH), 3052 (Ar-CH), 2930 (CH), 1640 (C=N), 1292 (N-N=N-), 1218 (OCH3); 1H NMR (CDCl3) δ (ppm): 1.83 (d, 2H, CH2), 3.62 (s, 9H, OCH3), 3.78 (t, 1H, CH), 6.71 (s, 2H, Ar-H), 7.18 (s, 1H, NH), 7.28-7.47 (m, 5H, Ar-H). MS m/z: 381 [M+], MS m/z: 380 [M+], Anal. Calcd. (%) for C19H20N6O3: C, 59.99; H, 5.30; N, 22.09; O, 12.62. Found: C, 61.23; H, 5.34; N, 22.18.
1-(5-(2, 4-difluorophenyl)-4, 5-dihydro-1H-pyrazol- 3-yl)-5-phenyl-1H-tetarzole (4c):
Yellowish brown solid, % yield: 78; MP: 150-152°, IR (KBr) v (cm-1): 3356 (NH), 3064 (Ar-CH), 2942 (CH), 1652 (C=N), 1289 (N-N=N-), 1158 (C-F); 1H NMR (CDCl3) δ (ppm): 1.93 (d, 2H, CH2), 3.79 (t, 1H, CH), 6.69 (s, 1H, Ar-H), 6.73 (d, 1H, Ar-H), 6.78 (d, 1H, NH), 7.14 (d, 1H, Ar-H), 7.30-7.51(m, 5H, Ar-H). MS m/z: 326 [M+], Anal. Calcd. (%) for C16H12F2N6: C, 58.89; H, 3.37; F, 11.64; N, 25.76. Found: C, 58.72; H, 3.39; N, 25.85.
1-(5-(4-fluorophenyl)-4, 5-dihydro-1H-pyrazol-3- yl)-5-phenyl-1H-tetarzole (4d):
Brown solid, % yield: 72; MP: 159-161º, IR (KBr) v (cm-1): 3312 (NH), 3094 (Ar-CH), 2963 (CH), 1682 (C=N), 1308 (N-N=N-), 1163 (C-F); 1H NMR (CDCl3) δ (ppm): 1.77 (d, 2H, CH2), 3.86 (t, 1H, CH), 6.81 (d, 2H, Ar-H), 6.88 (d, 1H, NH), 7.22 (d, 2H, Ar-H), 7.33- 7.47 (m, 5H, Ar-H). MS m/z: 309 [M+], Anal. Calcd. (%) for C16H13FN6: C, 62.33; H, 4.25; F, 06.16; N, 27.26. Found: C, 62.51; H, 4.27; N, 27.34.
2- (4, 5-dihydro-3-(5-phenyl-1H-tetrazol-1-yl)-1H-pyrazol- 5-yl)-4-nitrophenol (4e):
Reddish brown solid, % yield: 84; MP: 130-132°, IR (KBr) v (cm-1): 3540 (OH), 3345 (NH), 3062 (Ar-CH), 2902 (CH), 1638 (C=N), 1588 (NO2), 1292 (N-N=N-); 1H NMR (CDCl3) δ (ppm): 1.74 (d, 2H, CH2), 3.75 (t, 1H, CH), 4.88 (s, 1H, OH), 6.85 (d, 1H, Ar-H), 6.93 (d, 1H, NH), 7.28-7.54 (m, 5H, Ar-H), 7.88 (d, 1H, Ar-H), 7.94 (s, 1H, Ar-H). MS m/z: 352 [M+], Anal. Calcd. (%) for C16H13N7O3: C, 54.70; H, 3.73; N, 27.91; O, 13.66. Found: C, 54.87; H, 3.76; N, 27.98.
1-(5-(2-chloro-4-fluorophenyl)-4, 5-dihydro-1-H-pyrazol- 3-yl)-5-phenyl-1H-tetrazol (4f):
Yellowish brown solid, % yield: 71; MP: 162-164°, IR (KBr) v (cm-1): 3360 (NH), 3052 (Ar-CH), 2942 (CH), 1680 (C=N), 1306 (N-N=N-), 1165 (C-F), 782 (C-Cl); 1H NMR (CDCl3) δ (ppm): 2.07 (d, 2H, CH2), 3.78 (t, 1H, CH), 6.75 (d, 1H, Ar-H), 6.84 (d, 1H, NH), 7.08 (s, 1H, Ar-H), 7.17 (d, 1H, Ar-H), 7.25-7.41 (m, 5H, Ar-H). MS m/z: 343 [M+], Anal. Calcd. (%) for C16H12ClFN6: C, 56.07; H, 3.53; Cl, 10.34; F, 5.54; N, 24.52. Found: C, 56.26; H, 3.55; N, 24.61.
1-(4, 5-dihydro-5-(2, 4-dimethoxyphenyl)-1H-pyrazol- 3-yl)-5-phenyl-1H-tetrazol (4g):
Brown solid, % yield: 65; MP: 168-170°, IR (KBr) v (cm-1): 3325 (NH), 3086 (Ar-CH), 2980 (CH), 1644 (C=N), 1275 (N-N=N-), 1266 (OCH3); 1H NMR (CDCl3) δ (ppm): 2.10 (d, 2H, CH2), 3.82 (s, 6H, OCH3), 3.87 (t, 1H, CH), 6.68 (d, 1H, Ar-H), 6.74 (d, 1H, NH), 6.76 (d, 1H, Ar-H), 7.08 (d, 1H, Ar-H), 7.30- 7.54 (m, 5H, Ar-H). MS m/z: 350 [M+], Anal. Calcd. (%) for C18H18N6O2: C, 61.70; H, 5.18; N, 23.99; O, 9.13. Found: C, 61.88; H, 5.23; N, 24.12;
1-(4, 5-dihydro-5-(2, 4, 6-trimethoxyphenyl) -1H-pyrazol-3-yl)-5-phenyl-1H-tetarzole (4h):
Yellow solid, % yield: 68; MP: 141-143°, IR (KBr) v (cm-1): 3358 (NH), 3092 (Ar-CH), 2960 (CH), 1653 (C=N), 1263 (N-N=N-), 1236 (OCH3); 1H NMR (CDCl3) δ (ppm): 2.05 (d, 2H, CH2), 3.63 (s, 9H, OCH3), 3.72 (t, 1H, CH), 6.77 (d, 1H, NH), 6.82 (s, 2H, Ar-H), 7.32-7.46 (m, 5H, Ar-H). MS m/z: 380 [M+], Anal. Calcd. (%) for C19H20N6O3: C, 59.99; H, 5.30; N, 22.09; O, 12.62. Found: C, 60.15; H, 5.35; N, 22.01.
1- (4, 5-dihydro-5-(2-nitrophenyl)-1H-pyrazol-3- yl)-5-phenyl-1H-tetarzole (4i):
Reddish brown solid; % yield: 69; MP: 165-167°, IR (KBr) v (cm-1): 3308 (NH), 3098 (Ar-CH), 2936 (CH), 1682 (C=N), 1592 (NO2), 1276 (N-N=N-); 1H NMR (CDCl3) δ (ppm): 1.93 (d, 2H, CH2), 3.76 (t, 1H, CH), 6.88 (d, 1H, NH), 7.26-7.49 (m, 8H, Ar-H), 7.86 (d, 1H, Ar-H). MS m/z: 335 [M+], Anal. Calcd. (%) for C16H13N7O2: C, 57.31; H, 3.91; N, 29.24; O, 09.54. Found: C, 57.53; H, 3.96; N, 29.37.
1-(5-(3-bromo-4-methoxyphenyl)-4, 5-dihydro-1H-pyrazol- 3-yl)-5-phenyl-1H-tetarzole (4j):
Brown solid, % yield: 71; MP: 138-140°, IR (KBr) v (cm-1): 3348 (NH), 3060 (Ar-CH), 2964 (CH), 1632 (C=N), 1282 (N-N=N-), 1250 (OCH3), 618 (C-Br); 1H NMR (CDCl3) δ (ppm): 1.92 (d, 2H, CH2), 3.72 (t, 1H, CH), 3.78 (s, 3H, OCH3); 6.79 (d, 1H, Ar-H), 6.91 (d, 1H, NH), 7.13 (d, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 7.29- 7.48 (m, 5H, Ar-H). MS m/z: 399 [M+], Anal. Calcd. (%) for C17H15BrN6O: C, 51.14; H, 3.79; Br, 20.01; N, 27.44; O, 04.01. Found: C, 51.32; H, 3.83; N, 27.56.
Antioxidant activity:
All the synthesized compounds were screened for their in vitro antioxidant activity by scavenging of DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical. A stock solution of 100 μg/ml was prepared for all the test compounds as well as of standard, ascorbic acid. Different concentrations were made of 10, 20, 30, 40 and 50 μg/ml from stock solutions using methanol. DPPH in methanol (0.1 mM) was prepared in a volumetric flask and was completely kept away from light. One milliliter of all concentration of test and standards were mixed with 1.0 ml of DPPH solution. This solution was kept for 30 min in dark place. Methanol with DPPH was used as control. Absorbance of all the samples was taken on UV-spectrophotometer at a λmax of 517 nm. The free radical scavenging was expressed as the percentage inhibition and was calculated using the formula: percentage inhibition = [(Ao-A)/Ao]×100, where, Ao: Absorbance of control. A: Absorbance of test or standard.
The percent inhibition was plotted against the sample or the standard concentration to obtain the amount of antioxidant necessary to decrease the initial concentration of DPPH to 50% (IC50). IC50 values were calculated from calibration curve, and defined as the concentration of test compound required to achieve half maximal inhibition[35-37].
Antidiabetic activity:
All the synthesized compounds were screened for in vivo antidiabetic activity by streptozotocin induced model in albino rat[38-41].
Acute toxicity studies:
Group of 6 rats, weighing 180-200 g were fasted overnight and treated per orally with the test compounds at the dose of 200, 500, 1000 and 2000 mg/kg of body weight. The animals were observed for 72 h for any signs of acute toxicity such as increased or decreased motor activity, tremors, convulsion, sedation etc. It was observed that more than 50% of animals were died at the dose of 2000 mg/kg of body weight. Thus for the screening of antidiabetic activity, the dose selected was 200 mg/kg (i.e. 1/10 of the 2000 mg/kg of body weight) as per OECD guidelines. All the animal experiments were performed by the approval of Institutional Animal Ethics Committee (IAEC), Meerut Institute of Engineering and Technology, Meerut, India. During the study period, guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Institutional Animals Ethics Committee were followed for the maintenance of animals. The research work was approved by IAEC No: 711/02/a/CPCSEA/3/2013.
Streptozotocin-induced experimental diabetes:
The rats were injected intraperitoneally with streptozotocin dissolved in freshly prepared citrate buffer at a dose of 60 mg/kg body weight. A blood glucose range of 200-300 mg/dl was used for the experiment. Hyperglycemia was con?rmed in animals after 72 h of streptozotocin injection.
Experimental design:
Animals were divided into 13 groups of 6 animals (n=6). Group 1 treated as normal control (vehicle) received 1.0 ml of 0.5% CMC. Group 2 treated as diabetic control. Group 3 treated as diabetic animals received rosiglitazone 100 mg/kg. Groups 4-13 diabetic animals received compounds 4a-j in a single dose of 200 mg/kg body weight per oral respectively for 7 days continuously. Blood was withdrawn from the tail vain each time. Blood glucose was measured at 0, 7th, 14th, 21st, and 28th day by Blood Glucose Monitoring System.
Statistical analysis:
Values are represented as mean±SEM. Data were analyzed using analysis of variance and group means was compared with Bonferroni Post ANOVA test.
Results And Discussion
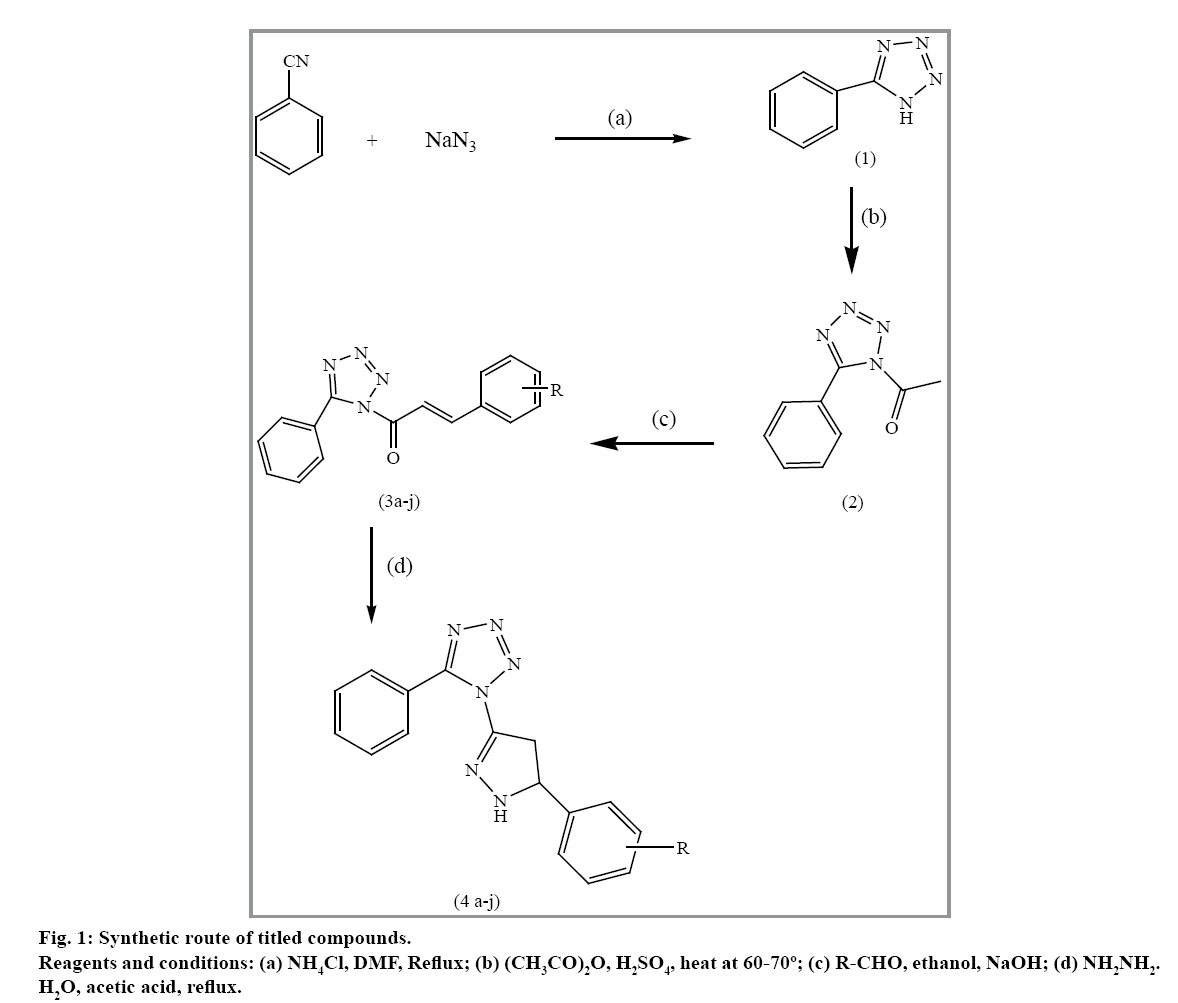
The reaction sequences used for the synthesis of titled compounds are shown in fig. 1. Several pyrazolotetrazole derivatives have been synthesized by Bhaskar et al. as target structures with different groups and evaluated for their biological activities like anticancer, antiinflammatory and antimicrobial activity. They have found that pyrazolotetrazole nucleus could be a lead compound for drug development[42-43]. In the present study, an attempt was made to explore antioxidant and antidiabetic activity of synthesized compounds.
The compound, 5-phenyltetrazole (1) was synthesized by the reaction of sodium azide, benzonitrile, ammonium chloride and dimethylformamide, which after acetylation produce 5-phenyl-1-acetyl tetrazole (2). The compound 2 and substituted aromatic aldehyde in ethanol was cooled to 5 to 10º and further treated with aqueous sodium hydroxide solution. The resulting solution was diluted with ice cooled water and acidified to form 1-(5-substitutedphenyl-1H-tetrazol- 1-yl) prop-2-en-1-one 3a-j. The compounds 3a-j were refluxed with hydrazine hydrate and acetic acid to form 1-[(5-substituted phenyl)-4, 5-dihydro-1H-pyrazol- 3-yl]-5-phenyl-1H-tetrazole (4a-j). Structures of synthesized compounds were confirmed by their spectral data interpretation.
The IR spectra of compound 3a-j showed characteristic C=O stretching at 1740-1666 cm-1 and C=C stretching frequencies at 1652-1614 cm-1, characteristic absorption band at 1610-1593 cm-1 and 1344-1248 cm-1 due to the presence of C=N and N-N=N- group confirm the formation of tetrazole ring. In 1H NMR spectra, the protons of α, β-unsaturated carbon atoms have displayed two doublets in the range of δ 6.40-6.80 and δ 7.23-7.93. Compounds 4a-j displayed characteristic absorption band in IR spectra at 3308-3360 cm-1 due to NH functional group. 1H NMR spectra of compounds 4a-j showed doublet in the range of δ 1.74-2.10 due to two methyelene proton at C-5 of pyrazoline ring. The proton at C-4 position of pyrazoline ring also showed a triplet in the range of δ 3.74-3.87. All the aromatic protons were observed in the expected regions. Mass spectra of the compounds showed M+1 in agreement with their molecular formula.
All the synthesized compounds were screened for in vitro antioxidant activity by DPPH free radical scavenging method. Result obtained from in vitro antioxidant activity is summarized in Table 1. The investigation of antioxidant screening revealed that some of the tested compounds showed moderate to good antioxidant activity. Particularly, compounds 4b, 4g 4h and 4j have shown moderate antioxidant activity with IC50 value at 13.19, 15.68, 22.41 and 16.14 μg/ml as compared to standard, ascorbic acid.
| Compound code | R | Antioxidant activity (IC50value in µg/ml) |
|---|---|---|
| 4a | 3-bromo-4-nitro | 74.81 |
| 4b | 3,4,5-trimethoxy | 13.19 |
| 4c | 2,4-difluoro | 54.11 |
| 4d | 4-fluoro | 40.93 |
| 4e | 2-hydroxy-4-nitro | 41.91 |
| 4f | 2-chloro-4-fluoro | 66.94 |
| 4g | 2,4-dimethoxy | 15.68 |
| 4h | 2,4,6-trimethoxy | 22.41 |
| 4i | 2-nitro | 34.59 |
| 4j | 3-bromo-4-methoxy | 16.14 |
| Standard | Ascorbic acid | 7.19 |
IC50 - Concentration required to achieving half maximal inhibition
TABLE 1: ANTIOXIDANT ACTIVITY OF STANDARD AND SYNTHESIZED COMPOUNDS
All the synthesized compounds were screened in vivo for their antidiabetic activity by streptozotocin induced diabetic model in albino rat. Blood glucose levels in treatment of diabetic rats with synthesized derivatives were presented in Table 2. Among the synthesized compounds, only the compounds 4b, 4g, 4h, 4i and 4j showed 51.84, 36.07, 38.54, 40.46 and 43.04% blood glucose lowering activity respectively.
| Group | Blood glucose level (mg/dl) | % antidiabetic activity | ||||
|---|---|---|---|---|---|---|
| 0 day | 7 day | 14 day | 21 day | 28 day | ||
| Normal control | 91.38±2.41 | 90.69±3.08 | 91.72±2.17 | 90.76±2.02 | 92.87±1.27 | - |
| Diabetic control | 267.40±4.23 | 270.65±4.33 | 276.44±3.88 | 284.20±3.35 | 287.45±4.80 | - |
| 4a | 277.66±3.98 | 275.17±3.59 | 269.77±3.67 | 270.25±3.08 | 273.77±4.88 | 1.4 |
| 4b | 268.99±2.55 | 254.02±4.86* | 239.85±2.98* | 165.12±3.70* | 129.54±1.67* | 51.84 |
| 4c | 275.28±6.24 | 274.85±4.63 | 273.78±4.01 | 269.65±3.96 | 272.68±5.48 | 0.94 |
| 4d | 277.33±4.69 | 272.03±6.27 | 265.15±5.30 | 269.51±4.59 | 273.28±3.91 | 1.28 |
| 4e | 280.16±3.96 | 276.01±6.97 | 274.43±3.91 | 270.60±6.25 | 274.09±4.68 | 2.17 |
| 4f | 274.81±4.41 | 267.05±4.17 | 265.23±3.72 | 270.45±5.35 | 273.44±4.92 | 0.49 |
| 4g | 272.91±3.13 | 251.35±4.12* | 235.44±5.13* | 210.95±3.21* | 174.49±4.70* | 36.07 |
| 4h | 270.42±2.94 | 249.43±4.01* | 238.31±3.97* | 215.54±4.38* | 166.18±2.52* | 38.54 |
| 4i | 271.56±2.49 | 249.36±2.60* | 217.06±4.92* | 168.86±3.33* | 161.67±3.27* | 40.46 |
| 4j | 281.24±2.77 | 250.11±2.38* | 230.30±4.01* | 180.91±2.78* | 160.17±3.84* | 43.04 |
| Rosiglitazone | 268.81±4.07 | 183.88±3.69* | 153.65±3.50* | 119.53±3.35* | 110.81±3.53* | 58.78 |
Values are expressed as mean±SEM (n=6). *P?0.01, compared to diabetic control.
TABLE 2: ANTIDIABETIC EFFECT OF SYNTHESIZED TEST COMPOUNDS
In conclusion, a few number of new pyrazole containing tetrazole derivatives were synthesized by rapid and high yield synthetic route and evaluated for their in vitro antioxidant and in vivo antidiabetic activities. Presence of electron donating groups either at o-, m- or p-position, and electron withdrawing groups at o-, m-position of the phenyl ring attached to pyrazole nucleus as substituent elicits a moderate antioxidant and antidiabetic activity.
Acknowledgments:
The authors thank Director, Department of Pharmaceutical Technology, Meerut Institute of Engineering & Technology, Meerut, for providing laboratory facilities. Authors are also grateful to IIT New Delhi for providing spectral data.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- Ugochukwu NH, Babady NE, Cobourne M, Gasset SR. The effect of Gongronemalatifoliumextracts on serum lipid profile and oxidative stress in hepatocytes of diabetic rats.J Biosci2003;28:1-5.
- Scoppola A, Montecchi FR, Mezinger G, Lala A. Urinary mevalonate excretion rate in type 2 diabetes: role of metabolic control. Atherosclerosis2001;156:357-61.
- Owu DU, Antai AB, Udo?a KH, Obembe AO, Obasi KO, Eteng MU. Vitamin C improves basal metabolic rate and lipid profile in alloxan-induced diabetes mellitus in rats. J Biosci2006;31:575-9.
- Kesavulu MM, Giri R, Kameswara RB, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab2000;26:387-92.
- Nayeemunnisa A. Alloxan diabetes-induced oxidative stress and impairment of oxidative defense system in rat brain: neuroprotective effects of Cichoriumintybus. Int J Diabetes Metab 2009;17:105-9.
- Ceriello A. Oxidative stress and glycemic regulation. Metabolism 2000;49:27-29.
- Gumieniczek A. Effects of pioglitazone on hyperglycemia-induced alterations in antioxidative system in tissues of alloxan-treated diabetic animals. ExpToxicolPathol 2005;56,321-6.
- Chaudhry J, Ghosh NN, Roy K, Chandra R. Antihyperglycemic effect of a thiazolidinedione analogue and its role in ameliorating oxidative stress in alloxan-induced diabetic rats. Life Sci 2007;80:1135-42.
- Arteel GE,SiesH. The biochemistry of selenium and the glutathione system. Environ ToxicolPharmacol 2001;10:153-8.
- Schumacher RF, Rosario AR, Souza ACG, Acker CI, Nogueira CW, Zeni G. The potential antioxidant activity of 2,3-dihydroselenophene, a prototype drug of 4-aryl-2,3-dihydroselenophenes. Bioorg Med Chem 2011;19:1418-25.
- Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food SciNutr 2004;44:575-86.
- Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today 2004;9:557-66.
- Young IS, Torney JJ, Trimble ER.The effect of ascorbate supplementation on oxidative stress in the streptozotocin diabetic rat.Free RadicBiol Med 1992;13:41-6.
- Schocken MJ, Creekmore RW, Theodoridis G, Nystrom GJ, Robinson RA. Microbial transformation of the tetrazolinone herbicide f5231.Appl Environ Micobiol 1989;55:1220-2.
- Benson FR. The chemistry of the tetrazoles. Chem Rev 1974;41:1-61.
- Burger A. Isosterism and bioisosterism in drug design. Prog Drug Res 1991;37:287-371.
- Gowd MR, Pasha MA. A versatile and efficient synthesis of 5-substituted-1-H tetrazoles. J ChemSci 2011;123:75-9.
- Malik MA, Al-Thabaiti SA, Malik MA. Synthesis, structure optimization and antifungal screening of novel tetrazole ring bearing acyl-hydrazones. Int J MolSci 2012;13:10880-98.
- Muralikrishna S, Raveendrareddy P, Ravindranath LK, Harikrishna S, Jagadeeswara PR.Synthesis characterization and antitumor activity of thiazole derivativescontaining indole moiety bearing-tetrazole. Der Pharm Chem 2013;6:87-93.
- Bacher SC, Lahiri SC, Synthesis of chloro and bromo substituted 5-(indan-1'-yl)tetrazoles and 5-(indan-1'-yl)methyltetrazoles as possible analgesic agents. Pharmazie 2004;59:435-8.
- Bepary S, Das BK, Bachar SC, Kundu JK, Rouf ASS, Datta BK. Anti-inflammatory activity of indanyltetrazole derivatives. Pak J Pharm Sci 2008;3:295-8.
- Momose Y, Maekawa T, Odaka H, Ikeda H, Sodha T. Novel 5-substituted-1H-tetrazole derivatives as potent glucose and lipid lowering agents. Chem Pharm Bull 2002;50:100-11.
- Mohite PB, Bhaskar VH. Potential pharmacological activities of tetrazoles in the new millennium. Inter J Pharm Tech Res 2011;3:1557-66.
- Admac J, Waisser K, Kunes J, Kaustova J. A note on the antitubercular activities of 1-aryl-5-benzylsulfanyltetrazoles. Arch Pharm 2005;338:385-9.
- Katritzky AR, Jain R, Petrukhin R, Denisenko S, Schelenz T. QSAR correlations of the algistatic activity of 5-amino-1-aryl-1H-tetrazoles. SAR QSAR Environ Res 2001;12:259-66.
- Sridhar R, Perumal PJ, Etti S, Shanmugam G, Ponnuswamy MN, Prabavathy VR,et al. Design, synthesis and antimicrobial activity of 1H-pyrazole carboxylates. Bioorg Med ChemLett 2004;14:6035-40.
- Bondock S, Fadaly W, Metwally MA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety.Eur J Med Chem 2010;45:3692-701.
- Barsoum FF, Girgis AS. Facile synthesis of bis(4,5-dihydro-1H-pyrazole-1-carboxamides) and their thio-analogues of potential PGE(2) inhibitory properties. Eur J Med Chem 2009;44:2172-7.
- Castagnolo D, Logu AD, Radi M, Bechi B, Manetti F, Magnani M,et al. Synthesis, biological evaluation and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis. Bioorg Med ChemLett 2008;16:8587-91.
- Aziz MA, Rahma GA, Hassan AA. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem 2009;44:3480-7.
- Burguete A, Pontiki E, Litina DH, Villar R, Vicente E, Solano B,et al. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues.Bioorg Med ChemLett 2007;17:6439-43.
- Cheng LV, Zhu HL, Huan Q, Sun J, Zhou Y. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agent. Bioorg Med ChemLett2010;18:4606-14.
- Sabbagh OI, Baraka MM, Ibrahim SM, Pannecouque P, Andrei G, Snoeck R,et al. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem2009;44:3746-53.
- Cheng XW, Ming B, Gong GH. Tetrazolium compounds: synthesis and application in medicine. Molecules 2015;20:5528-53.
- Kaushik N, Kumar N, Kumar A. Synthesis of substituted 5-phenyl-1-(5-phenyl)-isoxazol-3-yl)-1H-tetrazole as antioxidant agents. J AdvSci Res2015;6:14-19.
- Kaushik N, Kumar N, Kumar A. Synthesis of triazolothiadiazine derivatives as antioxodant agents. Int J Pharm PharmSci2015;7:120-23.
- Malladi SR, Anisetti R, Rao PR. A facile synthesis, in vitro anti-inflammatory and antioxidant activity of novel benzimidazolylpyrano[2,3-d][1,3]thiazolocarbonitriles. Indian J Pharm Sci 2014;76:510-8.
- Litch?eld JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J PharmacolExpTher1949;96:99-113.
- Raghavan PV. Expert Consultant, CPCSEA, OECD, guideline no 420,2000.
- Jarret RJ, Keen H, Hardwick C. Instant blood sugar measurement using Dextrostix and a reflectance meter. Diabetes1970;19:724-6.
- Mariappan G, Saha BP, Datta S, Kumar D, Haldar PK.Design, synthesis and antidiabetic evaluation of oxazolones derivatives. J ChemSci 2011;123:335-41.
- Bhaskar VH, Mohite PB. Synthesis, characterization and evaluation of anticancer activity of some tetrazole derivatives.J Optoelect Biomed Mat 2010;2:249-59.
- Bhaskar VH, Mohite PB. A facile synthesis, characterisation and in vitroanti-inflammatory activity of novel N-substituted tetrazoles. J Optoelect Biomed Mat 2011;3:87-93.