- Corresponding Author:
- M. A. Neelakantan
Chemistry Research Centre, National Engineering College, K. R. Nagar, Kovilpatti-628 503
E-mail: maneels@rediffmail.com
| Date of Submission | 4 August 2009 |
| Date of Revision | 1 December 2009 |
| Date of Acceptance | 20 February 2010 |
| Indian J Pharm Sci, 2010, 72 (2): 216-222 |
Abstract
Some new mixed ligand complexes (1-5) of type ML'B (M(II)=Mn(II), Co(II), Ni(II), Cu(II) and Zn(II); HL'= o-vanillidene-2-aminobenzothiazole; B= 1,10-phenanthroline) and Schiff base metal complexes of types (ML 2 ") (6-10) and (M 2 L") (11-15) (HL"= o-vanillidene-2-amino-N-(2-pyridyl)-benzene sulfonamide) were synthesized and characterized by elemental analysis and spectral (IR, 1 H NMR and 13 C NMR) studies. The free ligands and their metal complexes have been screened for their in vitro biological activities against bacteria, fungi and yeast. The metal complexes show more potent activities compared with Schiff base ligands.
Keywords
Antibacterial activities, antifungal activities, mixed ligand complexes, o-vanillin, schiff base, spectral studies
Metal complexes of N and S chelating ligands have attracted considerable attention because of their interesting physicochemical properties and pronounced biological activities. The N and S atoms play a key role in the coordination of metals at the active sites of numerous metallobimolecules. o-vanillin is a natural aldehyde found in Andropogen nardus. It is used to treat bellyaches and also used in spicery[1]. Schiff bases containing o-vanillin possesses antifungal, antibacterial properties[2] and it acts as a weak inhibitor of tyrosinase, display both antimutagenic and comutagenic properties in Escherichia coli[3]. Heterocycles containing thiazole ring is present in a number of pharmacologically and biologically active compounds. Compounds containing benzothiazole and sulphonamide derivatives were used as antifungal[4,5], antiinfl ammatory[6], antiHIV[7], anticancer[8], anticarbonic anhydrase[9], diuretic, hypoglycaemic[10], antithyroid[11], antimalarial and in therapeutic fields. In view of the pronounced biological activities of these compounds, we report herein the synthesis and characterization of (i) mixed ligand complexes of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) derived from 1,10–phenanthroline and o-vanillidene-2-aminobenzothiazole (HL') and (ii) Schiff base metal complexes of o-vanillidene-2-amino-N-(2-pyridyl)-benzene sulfonamide (HL"). The biological screening of free ligands and their complexes against different bacteria, fungi and yeast are reported.
Materials and Methods
All the chemicals and solvents used were of AR grade (Merck, Mumbai, India) except o-vanillin (Fluka, Switzerland) and 2-amino-N-(2-pyridyl)- benzene sulfonamide (Sigma, USA). Melting points of all the compounds were determined in open glass capillaries and are uncorrected. The purity of the Schiff bases was ascertained by TLC on Silica gel–G plates and spots were visualized by using iodine vapours. The elemental analysis was performed using a Thermo Finnigan Flash EA 1112 CHNS analyzer at Central Electro Chemical Research Institute (CECRI), Karaikudi, India. The conductometric measurements of the complexes were carried out in DMSO solution using Systronics 611 conductivity bridge. Vibrational spectra were recorded using KBr pellets on FT-IR Shimadzu 8400S spectrophotometer, in the region 4000 – 400 cm-1 range. The 1H NMR and 13C NMR of Schiff base ligands and their diamagnetic zinc complexes in DMSO-d6 were recorded on Perkin Elmer R-32 spectrometer using Tetramethylsilane as internal standard at IIT, Chennai, India.
Biological evaluation
The newly synthesized ligands and their metal complexes were screened in vitro for their antibacterial activity against bacteria: Escherichia coli, Pseudomonas aeruginosa, Salmonella Typhi and Vibrio parahaemolyticus by well diffusion method[12] using agar nutrient. The antifungal activities were tested against fungus: Aspergillus Niger, Penicillium, Trichoderma virida and yeast: Saccharomyces cerevisiae by well diffusion method using potato dextrose agar as the medium. Ampicillin and nystatin are used as control for bacteria and fungi, respectively. The suspension of each microorganism was added to a sterile agar medium, then poured into sterile Petri plates and left to solidifi cation. The well was dug in the agar media using sterile metallic borer in each plate. The test solution (3×10-3 M) was prepared by dissolving the compounds in DMSO and the well was fi lled with the test solution using micropipette. The plates were incubated for 24 h in the case of bacteria and 72 h for fungi at 35°. The extracts were subjected to further assay with a series on time basis (24, 48 and 72 h). During this period, the test solution was diffused and affected the growth of the inoculated microorganisms. Activity was determined by measuring the diameter of the zone showing complete inhibition (mm). Growth of inhibition was compared with the control. The zone of inhibition is given as the average of three independent determinations.
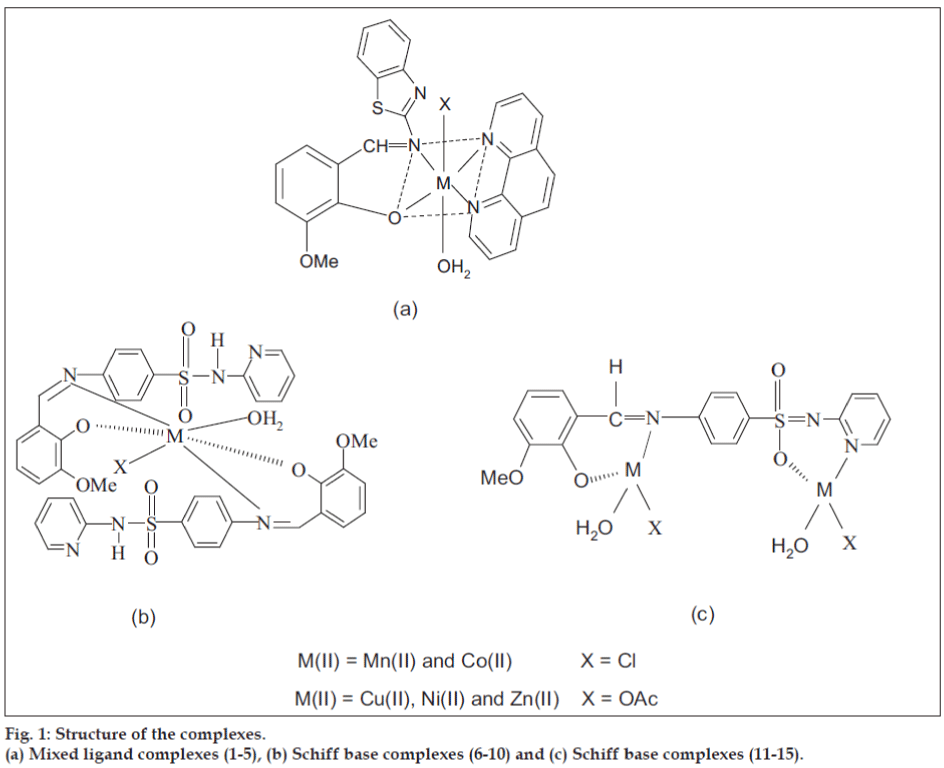
Synthesis of mixed ligand complexes (1-5)
The Schiff base (HL') was synthesized[13] by the condensation of 20 ml of o-vanillin (0.030 g/10 mmol) with 2-amino benzothiazole (0.030 g/10 mmol) in ethanol (1:1 molar ratio). Ten millilitres of Schiff base (0.028 g/10 mmol) and 1,10-phenanthroline (0.020 g/10 mmol) in ethanol was added drop wise to 10 ml of 10 mmol metal salts in hot ethanol (0.020g of MnCl2.4H2O, 0.024g of CoCl2.6H2O, 0.025 g of Ni(CH3COO)2.4H2O, 0.020 g of Cu(CH3COO)2.H2O and 0.022 g of Zn(CH3COO)2. 2H2O). The reaction mixture was refluxed for 3 h on a water bath and the volume of the solution was reduced to half of its original volume. The solid compound (fig. 1) obtained was fi ltered off, washed with water, diethyl ether and dried in vacuum over CaCl2. The color, elemental analysis data, molar conductivity and melting point of Schiff base (HL') and its mixed ligand complexes (1-5) are given in Table 1.
| Compound | Color | Mol. formulaa | Mol. Wt. | Yield (%) | Λm (Ω-1 cm2 mol-1) | Mp (0) |
|---|---|---|---|---|---|---|
| HL' | Reddish yellow | C15H12N2O2S | 284.00 | 65 | 6.78 | 180 |
| HL" | Orange | C19H17N3O4S | 383.42 | 94 | 4.23 | 198 |
| 1 | Pale yellow | MnC27H21O3N4SCl | 571.74 | 67 | 11.38 | 205 |
| 2 | Green | CoC27H21O3N4SCl | 575.73 | 62 | 17.39 | 145 |
| 3 | Light brown | NiC29H24O5N4S | 599.04 | 69 | 14.22 | 169 |
| 4 | Dark brown | CuC29H24O5N4S | 603.90 | 67 | 9.42 | 202 |
| 5 | Yellow | ZnC29H24O5N4S | 605.76 | 65 | 6.78 | 130 |
| 6 | Brown | MnC38H34O9N6S2Cl | 896.43 | 54 | 5.50 | 210 |
| 7 | Lignt Brown | CoC38H34O9N6S2Cl | 899.88 | 64 | 8.10 | >300 |
| 8 | Lignt Green | NiC40H37O11N6S2 | 900.22 | 59 | 3.00 | 285 |
| 9 | Black | CuC40H37O11N6S2 | 904.93 | 63 | 5.13 | 290 |
| 10 | Dark yellow | ZnC40H37O11N6S2 | 906.86 | 57 | 3.40 | 220 |
| 11 | Light brown | Mn2C19H19O6N3SCl2 | 718.27 | 58 | 9.80 | 225 |
| 12 | Brown | Co2C19H19O6N3SCl2 | 725.17 | 51 | 13.90 | >300 |
| 13 | Dark Green | Ni2C23H25O10N3S | 725.85 | 68 | 12.90 | 293 |
| 14 | Black | Cu2C23H25O10N3S | 663.28 | 83 | 10.80 | 320 |
| 15 | Light yellow | Zn2C23H25O10N3S | 739.13 | 56 | 12.40 | 254 |
aC, H and N are within the limit of ± 0.3% and S±0.4%; HL'- o-vanillidene-2-aminobenzothiazole; HL"- o-vanillidene-2-amino-N-(2-pyridyl)-benzene sulfonamide
Table 1: Physio-chemical properties of schiff base and metal complexes
Compound 1, yield: 67%, mp: 205°, IR (KBr) cm-1: 1639 (C=N, azomethine), 1557 (C=N, thiazole ring), 1217 (C–O, phenolic), 746 (C–S–C, thiazole ring), 569 (Mn–N), 424 (Mn–O), 3396 and 856 (H2O molecule). Compound 2, yield: 62%, mp: 145°, IR (KBr) cm-1: 1618 (C=N, azomethine), 1558 (C=N, thiazole ring), 1215 (C–O, phenolic), 747 (C–S–C, thiazole ring), 572 (Co–N), 430 (Co–O), 3387, 848 (H2O molecule). Compound 3, yield: 69%, mp: 169°, IR (KBr) cm-1: 1624 (C=N, azomethine), 1558 (C=N, thiazole ring), 1207 (C–O, phenolic), 747 (C–S–C, thiazole ring), 567 (Ni–N), 420 (Ni–O), 3387, 850 (H2O molecule). Compound 4, yield: 67%, mp: 199o, IR (KBr) cm-1: 1606 (C=N, azomethine), 1560 (C=N, thiazole ring), 1217 (C–O, phenolic), 748 (C–S–C, thiazole ring), 540 (Cu–N), 422 (Cu–O), 3380 and 852 (H2O molecule). Compound 5, yield: 65%, mp: 130°, IR (KBr) cm-1: 1641 (C=N, azomethine), 1561 (C=N, thiazole ring), 1213 (C–O, phenolic), 748 (C–S–C, thiazole ring), 563 (Zn–N), 437 (Zn– O), 3321, 852 (H2O molecule), 1H NMR (TMS, DMSO-d6) d ppm: 7.67 (s, 1H, HC=N, azomethine), 6.80–7.40 (d, 4H, Ar–H), 6.90 (s, 1H, HC=N, thiazole ring), 3.70 (s, 3H, OCH3), 1.80 (s, 3H, OOCCH3).
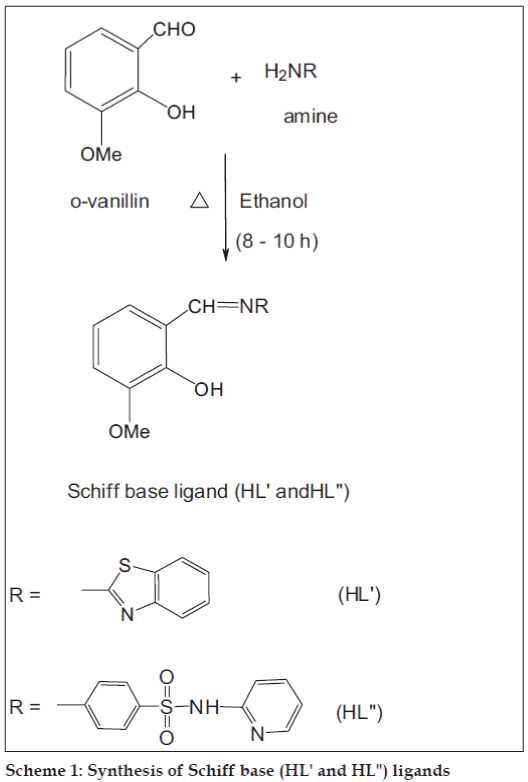
Synthesis of Schiff base
The Schiff base (HL") was prepared by refl uxing a mixture of equimolar quantities of o-vanillin (10 ml, 0.300 g/10 mmol) and 2-amino-N-(2-pyridyl)-benzene sulfonamide (10 ml, 0.490 g/10 mmol, Scheme 1) in hot ethanol. After 8 h of refluxing, the reaction mixture was kept at room temperature overnight and the orange colored product was fi ltered, washed with distilled water, diethyl ether and recrystallized from the same solvent. Yield: 85%, mp: 198°, IR (KBr) cm-1: 1631 (C=N, azomethine), 1269 (C–O, phenolic), 1361, 1138 (SO2), 3246 (N–H, sulfonamide group), 1025 (–HC=N, pyridine ring nitrogen), 1H NMR (TMS, DMSO-d6) d ppm: 9.92 (s, 1H, HC=N, azomethine), 12.82 (s, –OH), 7.60–8.00 (d, 4H, Ar – H), 8.40–8.60 (s, pyridine –HC=N), 11.04 (s, NH), 3.48 (s, 3H, OCH3), 2.30 (s, 3H, CH3), 5.72 (s, 2H, SO2NH group), 13C NMR (TMS, DMSO-d6) d ppm: 164.66 (HC=N, azomethine), 155.29 (–C–OH, phenolic), 151.44 (HC=N, pyridine ring), 142.04–140.36 (C=C, pyridine ring), 148.51 (C–O–C), 121.69–117.96 (Ar, C=C), 56.24 (OCH3).
Synthesis of metal complexes (6-15)
Ten millilitres of metal salts (10 mmol, 0.02 g of MnCl2.4H2O, 0.024 g of CoCl2.6H2O, 0.025 g of Ni(CH3COO)2.4H2O, 0.02 g of Cu(CH3COO)2. H2O and 0.022 g of Zn(CH3COO)2. 2H2O) in hot ethanol was added to 10 ml of the Schiff base ligand (0.038 g/10 mmol) in ethanol (1:1). 10 ml of metal salts (20 mmol, 0.04 g of MnCl2.4H2O, 0.048 g of CoCl2.6H2O, 0.05 g of Ni(CH3COO)2.4H2O, 0.04 g of Cu(CH3COO)2. H2O and 0.044 g of Zn(CH3COO)2. 2H2O) in hot ethanol was added to 10 ml of the Schiff base ligand (0.038 g/10 mmol) in ethanol (2:1). The mixture was refl uxed for 8 h on water bath. The volume of the solution was reduced to one-third of its original volume and left overnight. The precipitate was fi ltered off, washed with 50% (v/v) ethanol-water mixture, diethyl ether and dried over fused CaCl2 in a vacuum desiccator. The elemental analysis, molar conductivity value, color and melting point of Schiff base (HL") and its metal(II) complexes (6-15) are given in Table 1.
Compound 6, yield: 54%, mp: 210°, IR (KBr) cm-1: 1599 (C=N, azomethine), 1293 (C–O, phenolic), 1362, 1130 (SO2), 3248 (N–H, sulfonamide group), 1026 (–HC=N, pyridine ring nitrogen), 594 (Mn–N), 521 (Mn–O), 3398, 976, 774 (–OH, H2O molecule). Compound 7, yield: 64%, mp: >300°, IR (KBr) cm-1: 1602 (C=N, azomethine), 1272 (C–O, phenolic), 1364, 1132 (SO2), 3244 (N–H, sulfonamide group), 1030 (C=N, pyridine ring nitrogen), 604 (Co–N), 520 (Co–O), 3405, 976, 759 (–OH, H2O molecule). Compound 8, yield: 59%, mp: 285°, IR (KBr) cm-1: 1610 (C=N, azomethine), 1286 (C–O, phenolic), 1364, 1135 (SO2), 3246 (N–H, sulfonamide group), 1029 (C=N, pyridine ring nitrogen), 588 (Ni–N), 503 (Ni–O), 3398, 968, 767 (–OH, H2O molecule), 1616,1402 (–COO, acetate group). Compound 9, yield: 63%, mp: 290°, IR (KBr) cm-1: 1616 (C=N, azomethine), 1291 (C–O, phenolic), 1362, 1138 (SO2), 3248 (N–H, sulfonamide group), 1027 (C=N, pyridine ring nitrogen), 569 (Cu–N), 513 (Cu–O), 3415, 980, 775 (–OH, H2O molecule), 1626, 1412 (–COO, acetate group). Compound 10, yield: 57%, mp: 220°, IR (KBr) cm-1: 1598 (C=N, azomethine), 1278 (C–O, phenolic), 1363, 1136 (SO2), 3247 (N–H, sulfonamide group), 1026 (C=N, pyridine ring nitrogen), 596 (Zn–N), 507 (Zn–O), 3382, 962, 769 (–OH, H2O molecule), 1631,1408 (–COO, acetate group). 1H NMR (TMS, DMSO-d6) δ ppm: 10.12 (s, 1H, HC=N, azomethine), 7.65–7.97 (d, 4H, Ar–H), 8.38–8.61 (s, pyridine, –HC=N), 11.00 (s, NH), 3.46 (s, 3H, OCH3), 2.31 (s, 3H, CH3), 5.70 (s, 2H, SO2NH group), 4.73 (s, 2H, H2O molecule), 13C NMR (TMS, DMSO-d6) δ ppm: 169.42 (HC=N, azomethine), 150.94 (HC=N, pyridine ring), 141.98–140.52 (C=C pyridine ring C), 147.91 (C–O–C), 122.76–118.72 (Ar, C=C), 55.82 (OCH3).
Compound 11, yield: 58%, mp: 225°, IR (KBr) cm-1: 1615 (C=N, azomethine), 1279 (C–O, phenolic), 1006 (C=N, pyridine ring nitrogen), 589 (Mn–N), 518 (Mn–O), 3403, 987, 779 (–OH, H2O molecule). Compound 12, yield: 51%, mp: >300°, IR (KBr) cm-1: 1599 (C=N, azomethine), 1286 (C–O, phenolic), 1013 (C=N, pyridine ring nitrogen), 586 (Co–N), 516 (Co–O), 3394, 997, 765 (–OH, H2O molecule). Compound 13, yield: 68%, mp: 293°, IR (KBr) cm-1: 1605 (C=N, azomethine), 1298 (C–O, phenolic), 1350, 1009, 669 (C=N, pyridine ring nitrogen), 605 (Ni–N), 513 (Ni–O), 3379, 986, 776 (–OH, H2O molecule), 1631, 1405 (–COO, acetate group). Compound 14, yield: 83%, mp: 320°, IR (KBr) cm-1: 1620 (C=N, azomethine), 1287 (C–O, phenolic), 1012 (C=N, pyridine ring nitrogen), 605 (Cu–N), 517 (Cu–O), 3415, 980, 775 (–OH, H2O molecule), 1632, 1408 (– COO, acetate group). Compound 15, yield: 56%, mp: 254°, IR (KBr) cm-1: 1612 (C=N, azomethine), 1304 (C–O, phenolic), 1006 (C=N, pyridine ring nitrogen), 579 (Zn–N), 510 (Zn–O), 3410, 989, 787 (–OH, H2O molecule), 1623, 1408 (–COO, acetate group). 1H NMR (TMS, DMSO-d6) d ppm: 10.03 (s, 1H, HC=N, azomethine), 7.64–7.99 (d, 4H, Ar–H), 8.23–8.54 (s, pyridine, –HC=N), 3.40 (s, 3H, OCH3), 2.28 (s, 3H, CH3), 11.86 (s, 2H, SO2NH group), 4.67 (s, 2H, H2O molecule), 13C NMR (TMS, DMSO-d6) d ppm: 172.26 (–HC=N, azomethine), 164.49 (HC=N, pyridine ring), 140.19 – 138.25 (C=C pyridine ring C), 148.17 (C–O–C), 124.27 – 119.58 (Ar, C= C), 56.15 (OCH3).
Results and Discussion
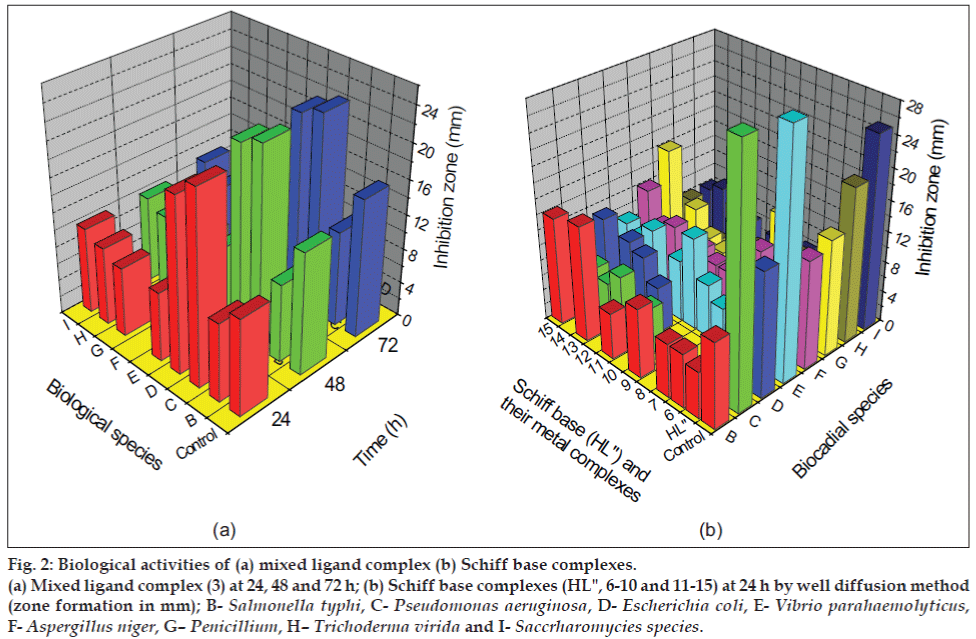
The zone of inhibition of the complexes against the growth of bacteria and fungi were given in Table 2 and Table 3, respectively. A representative graph is given in fig 2. From the table it is evident that the complexes (1-5) and (11-15) have higher antibacterial activity than the complexes (6-10). The complexes (1-5) and (6-10) shows higher antifungal activity than the complexes (11-15). In general, the synthesized metal complexes have higher biological activities compared to the free ligands. The increased inhibition activity of the metal complexes can be explained on the basis of Tweedy’s chelation theory[14]. In metal complexes, on chelation the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of p- electrons over the whole chelate ring. The large ring size of 1,10-phenanthroline moiety makes the complexes more lipophillic[15]. This increased lipophillicity enhances the penetration of the metal complexes into lipid membranes and block the metal binding sites in the enzymes[16]. Metal complexes also disturb the respiration process of the cell and thus block the synthesis of proteins, which restricts further growth of the organisms. This enhancement in inhibiting the growth of bacteria and fungi can also be explained on the basis of their structure. The azomethine linkage and hetero aromatic moiety in the synthesized complexes exhibit extensive biological activities[17,18] due to increased liposolubility of the molecules in crossing cell membrane of the microorganism. The presence of electron donor group (-OCH3) in the complexes also plays a role in enhancing the inhibition activity. The antibacterial activity is found to be in the order; Control>(1-5)>(11-15)>(6- 10)>HL'>HL". The antifungal activity is found to be in the order; Control>(6-10)>(1-5)>(11-15)>HL'>HL".
Figure 2: Biological activities of (a) mixed ligand complex (b) Schiff base complexes.
(a) Mixed ligand complex (3) at 24, 48 and 72 h; (b) Schiff base complexes (HL", 6-10 and 11-15) at 24 h by well diffusion method
(zone formation in mm); B-Salmonella typhi, C-Pseudomonas aeruginosa, D-Escherichia coli, E-Vibrio parahaemolyticus,
F-Aspergillus niger, G–Penicillium, H–Trichoderma virida and I-Saccrharomycies species.
| Compound | Diameter of inhibition zone in mm for different microbial species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella typhi | Pseudomonas aeruginosa | Escherichia coli | Vibrio parahaemolyticus | ||||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| HL' | 7 | 8 | 8 | 8 | 9 | 9 | 10 | 11 | 12 | 8 | 9 | 9 | |
| HL" | 6 | 7 | 7 | 7 | 8 | 8 | - | - | - | 7 | 8 | 8 | |
| 1 | 9 | 10 | 10 | 9 | 11 | 12 | 9 | 11 | 11 | 9 | 10 | 13 | |
| 2 | - | - | - | 14 | 15 | 16 | - | - | - | 8 | 8 | 11 | |
| 3 | 9 | 9 | 11 | 22 | 23 | 23 | 20 | 22 | 22 | 8 | 9 | 9 | |
| 4 | - | 9 | 10 | 8 | 10 | 10 | - | - | - | 10 | 10 | 10 | |
| 5 | 9 | 10 | 10 | - | - | - | 12 | 12 | 14 | 10 | 11 | 12 | |
| 6 | 7 | 7 | 8 | - | - | - | - | - | - | 6 | 7 | 9 | |
| 7 | 7 | 7 | 8 | - | - | - | - | - | - | 6 | 7 | 9 | |
| 8 | - | - | - | - | - | - | - | - | - | 5 | 10 | 10 | |
| 9 | 9 | 8 | 7 | - | - | - | - | - | - | 7 | 8 | 9 | |
| 10 | - | - | - | 6 | 7 | 7 | - | - | - | 12 | 13 | 14 | |
| 11 | 6 | 8 | 8 | - | - | - | 6 | 7 | 8 | 8 | 9 | 9 | |
| 12 | - | - | - | 8 | 9 | 9 | 9 | 9 | 10 | - | - | - | |
| 13 | 15 | 15 | 16 | 6 | 7 | 7 | 10 | 12 | 13 | 10 | 10 | 11 | |
| 14 | - | - | - | 7 | 8 | 9 | - | - | - | 8 | 8 | 9 | |
| 15 | 14 | 15 | 17 | 11 | 12 | 12 | 11 | 14 | 14 | 9 | 11 | 12 | |
| Control | 11 | 14 | 16 | 32 | 34 | 34 | 16 | 17 | 18 | 31 | 33 | 34 | |
Each experiment was done in triplicate; [-, less active]; well diffusion method
Table 2: Bacterial activities of the schiff base and metal complexes.
| Compound | Diameter of inhibition zone in mm for different microbial species | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspergillusniger | Penicillium | Trichodermavirida | Saccharomyces cerevisiae | ||||||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||
| HL' | 5 | 6 | 6 | 5 | 6 | 6 | 8 | 8 | 9 | 8 | 9 | 9 | |||
| HL" | 6 | 6 | 6 | - | 6 | 8 | 6 | 7 | 7 | 4 | 5 | 6 | |||
| 1 | - | - | - | 9 | 9 | 10 | 13 | 14 | 14 | 12 | 12 | 13 | |||
| 2 | 9 | 9 | 10 | 9 | 10 | 11 | - | - | - | 8 | 8 | 9 | |||
| 3 | - | 9 | 9 | 8 | 8 | 9 | 9 | 9 | 9 | 10 | 10 | 11 | |||
| 4 | 10 | 11 | 11 | 9 | 10 | 10 | 12 | 14 | 14 | 9 | 10 | 10 | |||
| 5 | - | 10 | 10 | - | 9 | 10 | 12 | 12 | 13 | 10 | 11 | 12 | |||
| 6 | 10 | 12 | 13 | 10 | 11 | 12 | 8 | 9 | 9 | 8 | 11 | 12 | |||
| 7 | 12 | 14 | 16 | 15 | 16 | 16 | 8 | 8 | 8 | 6 | 7 | 8 | |||
| 8 | 10 | 12 | 12 | - | - | - | 4 | 4 | 8 | 6 | 8 | 10 | |||
| 9 | 7 | 9 | 13 | - | - | - | 4 | 6 | 6 | - | - | - | |||
| 10 | 7 | 8 | 16 | 13 | 15 | 16 | 6 | 10 | 10 | 6 | 8 | 8 | |||
| 11 | 8 | 8 | 10 | 6 | 8 | 8 | 4 | 4 | 4 | - | - | - | |||
| 12 | - | - | - | 7 | 8 | 8 | - | - | - | 6 | 6 | 6 | |||
| 13 | 9 | 12 | 12 | 10 | 10 | 11 | 4 | 4 | 4 | - | - | - | |||
| 14 | 8 | 8 | 9 | - | - | - | - | - | - | 9 | 9 | 10 | |||
| 15 | 12 | 13 | 14 | 16 | 18 | 19 | 8 | 12 | 12 | 8 | 8 | 8 | |||
| Control | 14 | 15 | 16 | 15 | 18 | 18 | 20 | 22 | 23 | 25 | 28 | 28 | |||
Each experiment was done in triplicate; [-, less active]; well diffusion method
Table 3: Fungal activities of the schiff base and metal complexes
Acknowledgements
The authors wish to thank the Management, Director and Principal of National Engineering College, Kovilpatti for their keen interest and constant encouragement throughout this investigation.
References
- Zhu RH, Xue QC. Handbook of synthesis practical spicery. Beijing: Light Industry press; 1986. p.167.
- Temel H, Sekerci M. Novel complexes of Manganese(II), Cobalt(II), Copper(II) and Zinc(II) with Schiff base derived from 1,2-bis-(p-amino-phenoxy)ethane and salicylaldehyde. Synth React Inorg Met Org Chem 2001;31:849-57.
- Watanabe K, Ohta T, Shirasu Y. Enhancement and inhibition of mutation by o-vanillin in Escherichia coli. Mutat Res 1989;218:105-9.
- Chavan AA, Pai NR. Synthesis and biological activity of N-substituted-3-chloro-2-azetidinones. Molecules 2007;12:2467-77.
- Chohan ZH, Ul-Hassan M, Khan KM, Supuran CT. In vitro antibacterial, antifungal and cytotoxic properties of sulfonamide - derived Schiff's bases and their metal complexes. J EnzInhib Med Chem 2005;20:183-8.
- Sushilkumar SB, Devanand BS. Synthesis and Anti-inflammatory Activity of [2-(Benzothiazol-2-ylimino)-4-oxo-3-phenylthiazolidin-5-yl]-acetic acid derivatives. J Korean Chem Soc 2003;47:237-40.
- Racane L, Kulenovic VT, Jakic LF, Boykin DW, Zamola GK. Synthesis of bis-substituted amidinobenzothiazoles as potential anti-HIV agents. Heterocycles 2001;55:2085-98.
- Caleta I, Grdisa M, Sermek DM, Cetina M, Kulenovic VT, Pavelic K, et al. Synthesis, crystal structure and antiproliferative evaluation of some new substituted benzothiazoles and styrylbenzothiazoles. Farmaco 2004;59:297-305.
- Supuran CT, Scozzafava A. Carbonic anhydrase and their therapeutic potentials. Exp Opin Ther Pat 2000;10:575-600.
- Ogden RC, Flexner CW. Protease inhibitors in AIDS therapy. Marcel Dekker, New York: 2001.
- Supuran CT, Scozzafava A, Mastrolorenzo A. Bacterial proteases: Current therapeutic use and future prospects for the development of new antibiotics. Exp Opin Ther Pat 2000;111:221-59.
- Pelczar MJ, Chan ECS, Kreig NR. Antibiotics and other chemotherapeutic agents. In: Edwards DD, Pelczar MF, editors. Microbiology: Concepts and applications. 6th ed. New York: McGraw-Hill Inc; 1993. p. 556-88.
- Neelakantan MA, Marriappan SS, Dharmaraja J, Jeyakumar T, Muthukumaran K. Spectral, XRD, SEM and biological activities of transition metal complexes of polydentate ligands containing thiazole moiety. SpectrochimActa A Mol Biomol Spectrosc 2008;71:628-35.
- Tweedy BG. Possible mechanism for reduction of elemental sulfur by moniliniafructicola. Phytopathology 1964;55:910-4.
- Mazumder UK, Gupta M, Bera A, Bhattacharya S, Karki S, Manikandan L, et al. Synthesis, antitumor and antibacterial activity of some Ru(bpy)2+2/4-substituted thiosemi-carbazide complexes. Indian J Chem 2003;42A:313-7.
- Vaghasia Y, Nair R, Soni M, Baluja S, Chanda S. Synthesis, structural determination and antibacterial activity of compounds derived from vanilline and 4-aminoantipyrene. J Serb Chem Soc 2004;69:991-8.
- Neelakantan MA, Rusalraj F, Dharmaraja J, Johnsonraja S, Jeyakumar T, SankaranarayanaPillai M. Spectral characterization, cyclic voltammetry, morphology, biological activities and DNA cleaving studies of amino acid Schiff base metal(II) complexes. SpectrochimActa A Mol Biomol Spectrosc 2008;71:1599-609.
- Chohan ZH. Antibacterial and antifungal ferrocene incorporated dithiothione and dithioketone compounds. Appl Organomet Chem 2006;20:112-6.