- Corresponding Author:
- Priyanka l. Gaikwad
Department of Pharmaceutical Chemistry, Bharati Vidyapeeth’s College of Pharmacy, Belapur, Navi Mumbai-400 614, India
E-mail: priyankalg@yahoo.co.in
| Date of Submission | 05 December 2012 |
| Date of Revision | 20 June 2013 |
| Date of Acceptance | 24 June 2013 |
| Indian J Pharm Sci 2013;75(4):496-500 |
Abstract
Antimicrobial screening of several novel pyrazolothiazol-4(5H)-one derivatives (3a-3j) has been performed. Reaction of aromatic aldehydes with aromatic ketones yielded starting chalcones (1a-1j) which have been subsequently reacted with thiosemicarbazide for obtaining N-thiocarbamoylpyrazole derivatives (2a-2j). These were further cyclized to pyrazolothiazol-4(5H)-one derivatives (3a-3j) in the presence of ethylbromoacetate. The structures of newly synthesized compounds were confirmed by FTIR and 1 H NMR and/or MS. The in vitro antimicrobial activity of these compounds was evaluated against Gram positive bacteria, Gram negative bacteria and fungi. Their minimum inhibitory concentration was determined by tube dilution method. The results showed that most of the compounds have promising antimicrobial activity as compared to standard drugs. Among the test compounds, 2-[5(4-chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-thiazol-4(5H)-one (3e) was found to show the most potent antimicrobial activity.
Keywords
Antimicrobial activity, chalcone, minimum inhibitory concentration, pyrazolothiazol-4(5H)-one, thiosemicarbazide
Antimicrobials are one of the most important weapons in fighting bacterial infections. However, over the past few decades, development of resistance to existing drugs is a constant growing phenomenon that has concerned researchers throughout the world. So there is still need for the development of new class of active antimicrobial agents [1-4].
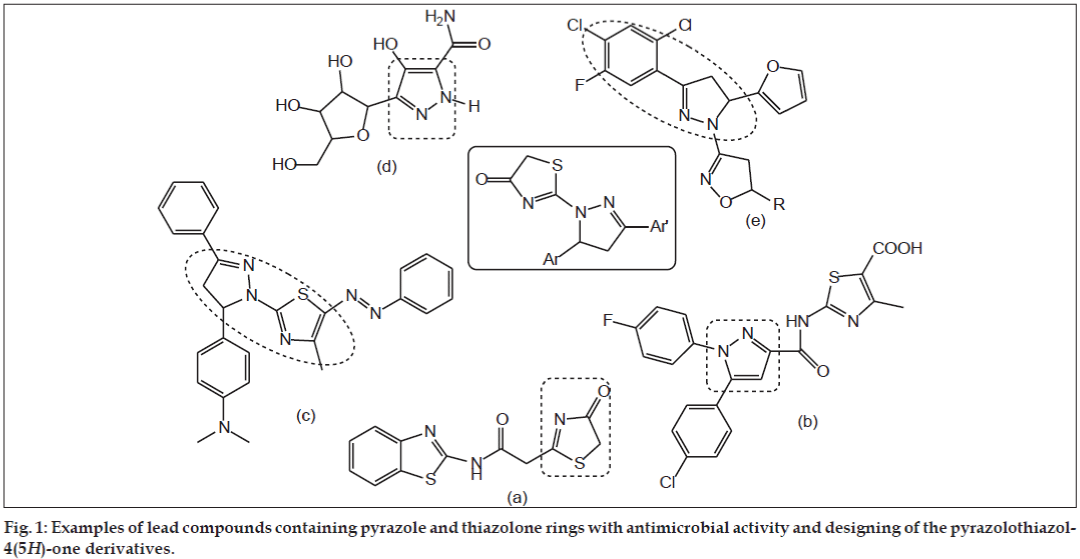
An extensive literature survey revealed that, thiazolone ring has broad spectrum of biological activities such as antimicrobial, antiproliferative, antiviral, antiinflammatory, and anticonvulsant [5,6]. Bondock et al. reported synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety (fig. 1a) [7]. Abdelhamid reported a facile synthesis of 5-arylthiazoles and triazolino [4,3-a] pyrimidines as antimicrobial agents (fig. 1b) [8].
On the other hand, pyrazole ring belongs to the privileged scaffolds in modern medicinal chemistry particularly in discovering new antimicrobial agents. The pyrazole motifs were found to possess an extensive spectrum of pharmacological activities like anticancer, antibacterial, antiinflammatory, analgesic, antidepressant and antioxidant [9]. Ragavan et al. reported synthesis and antimicrobial activities of novel 1,5-diaryl pyrazoles (fig. 1c) [10]. Bekhit and his coworkers reported synthesis and biological evaluation of some thiazolylpyrazole derivatives as dual anti-inflammatory antimicrobial agents (fig. 1d) [11]. Shah et al. reported synthesis and antibacterial studies of some novel isoxazoline derivatives (fig. 1e) [12].
All of these encouraged us to integrate these two heterocycles and screen new pyrazolothiazol-4(5H)- one derivatives, which might exhibit synergistic antimicrobial effect. Herein, we illustrate in the present work the designing, synthesis and biological evaluation of pyrazolothiazol-4(5H)-one derivatives as potential antimicrobial agents.
All reagents and solvents used were purchased from S. D. Fine-Chem. Ltd., Mumbai, India. Melting points were determined by open capillary method on a VMP-D apparatus (Veego Instruments Corporation, Mumbai, India). The completion of the reaction was checked by thin layer chromatography (TLC) on silica gel coated aluminum sheets (silica gel 60) obtained from Merck Ltd., Mumbai, India and visualized using UV lamp. The FTIR spectra were recorded in the range of 4000-400 cm-1 by KBr disk method using FTIR 8400S Shimadzu spectrometer. Spectral analyses were recorded at Sophisticated Analytical Instrumental Facility (SAIF), IIT, Mumbai, India. 1H NMR spectra were recorded on Varian Mercury Plus (300 MHz) spectrometer in DMSO-d6 or CDCl3 as solvent using tetramethylsilane (TMS) as an internal reference standard and values are expressed in δ ppm. Mass spectra were recorded on Varian Inc. mass spectrometer.
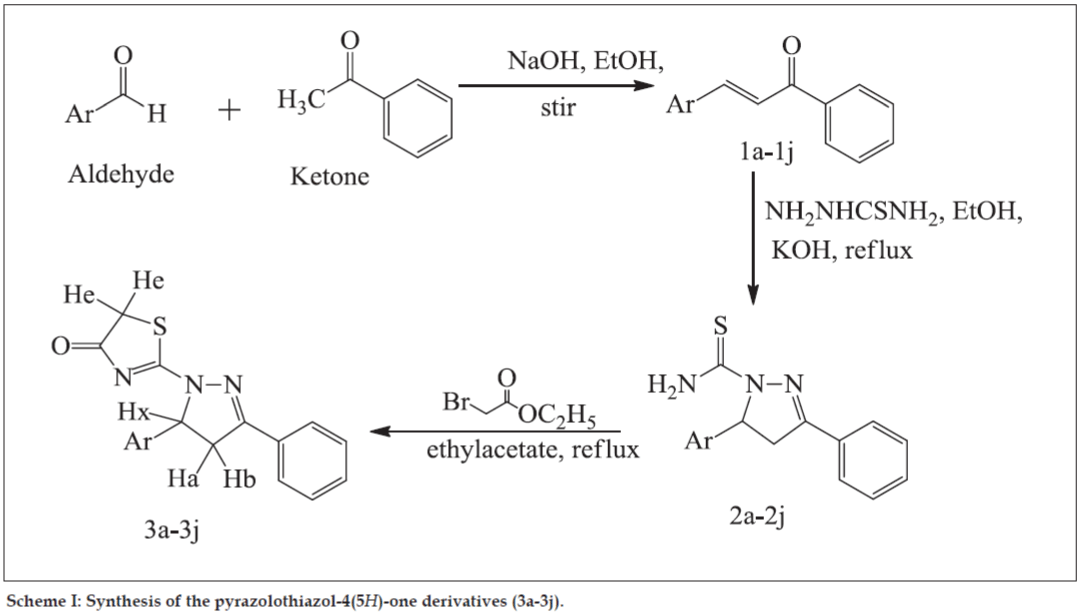
General pathway for synthesis of compounds (3a-3j) was outlined in Scheme 1 and the physicochemical characterization is given in Table 1. For synthesis of chalcones (1a-1j) a suspension of aromatic ketone (40 mmol) and aromatic aldehyde (40 mmol) in ethanolic NaOH was stirred for appropriate period of time. The reaction mixture was poured into cold water to produce solid compound which was recrystallized with suitable solvent in order to obtain pure chalcone.
| Compound | Ar | Molecular formula | Molecular weight | Mp (°) | % yield | Rf value* |
|---|---|---|---|---|---|---|
| 3a | C6H5 | C18H15N3OS | 321.40 | 222 | 72 | 0.32 |
| 3b | C4H4O | C16H13N3O2S | 311.36 | 230 | 68 | 0.35 |
| 3c | C4H4S | C16H13N3OS2 | 327.42 | 233 | 80 | 0.77 |
| 3d | 2-ClC6H4 | C18H14ClN3OS | 355.84 | 272 | 74 | 0.82 |
| 3e | 4-ClC6H4 | C18H14ClN3OS | 355.84 | 249 | 77 | 0.77 |
| 3f | 2-Cl, 4-ClC6H3 | C18H13Cl2N3OS | 390.29 | 287 | 65 | 0.59 |
| 3g | 2-Cl, 6- FC6H3 |
C18H13ClFN3OS | 373.83 | 251 | 81 | 0.85 |
| 3h | 4-FC6H4 | C18H14FN3OS | 339.39 | 210 | 73 | 0.74 |
| 3i | 3-NO2C6H4 | C18H14N4O3S | 366.39 | 299 | 66 | 0.31 |
| 3j | 4-OHC6H4 | C18H15N3O2S | 337.40 | 300 | 62 | 0.35 |
The properties like substituents present, molecular formula, molecular weight, Mp=Melting point, percentage yield and Rf value. *Mobile phase for TLC (hexane:ethyl acetate, 1:1)
Table 1: Physicochemical characterization of newly synthesized compounds (3a-3j)
General method for synthesis of N-thiocarbamoylpyrazole derivatives (2a-2j) described as mixture of chalcone (10 mmol) and thiosemicarbazide (20 mmol) was refluxed in ethanolic KOH. Precipitate formed was filtered under vacuum. Recrystallization was carried out with acetone or ethanol.
For synthesis of pyrazolothiazol-4(5H)-one derivatives (3a-3j), N-thiocarbamoylpyrazole derivative (10 mmol) was refluxed with ethylbromoacetate (33 mmol) for appropriate time. After completion of reaction, residue obtained was recrystallized with suitable solvent.
2-(3,5-Diphenyl-4,5-dihydropyrazol-1-yl)-thiazol- 4(5H)-one (3a). Yield: 72%; mp 222–223°; FTIR (ν, cm-1)=2970 (C-H, aromatic), 1697 (C=O), 1543 (C=N), 1226 (C-N), 700 (C-S); 1H NMR (300 MHz, CDCl3) δ ppm=3.34–3.42 (dd, 1H, Ha), 3.85 (s, 2H, He), 3.90-4.00 (dd, 1H, Hb), 5.76−5.81 (dd, 1H, Hx), 7.21-7.36 (m, 5H, Ar-H), 7.43–7.54 (m, 3H, Ar-H), 7.78-7.85 (dd, 2H, Ar-H). MS: m/z =322 (M+ +1, 100%), 323 (M+ +2, 18.56%).
2-(5-Furan-2-yl-3-phenyl-4,5-dihydropyrazol-1-yl)- thiazol-4(5H)-one (3b). Yield: 68%; mp 230-232°; FTIR (ν, cm-1)=2970 (C-H, aromatic), 1695 (C=O), 1543 (C=N), 1222 (C-N), 690 (C-S); 1H NMR (300 MHz, CDCl3) δ ppm=3.63-3.71 (dd, 1H, Ha), 3.74-3.84 (dd, 1H, Hb), 3.86 (s, 2H, He), 5.84−5.90 (dd, 1H, Hx), 6.31-6.33 (dd, 1H, Ar-H), 6.54-6.55 (d, 1H, Ar-H), 7.30-7.31 (d, 1H, Ar-H), 7.44-7.54 (m, 3H, Ar-H), 7.80-7.84 (dd, 2H, Ar-H).
2-(3-Phenyl-5-thiophen-2-yl-4,5-dihydropyrazol-1-yl)- thiazol-4(5H)-one (3c). Yield: 80%; mp 233−235°; FTIR (ν, cm-1)=2966 (C-H, aromatic), 1701 (C=O), 1543 (C=N), 1211 (C-N), 692 (C-S); 1H NMR (300 MHz, CDCl3) δ ppm=3.53-3.60 (dd, 1H, Ha), 3.87 (s, 2H, He), 3.88-3.97 (dd, 1H, Hb), 6.08-6.13 (dd, 1H, Hx), 6.91-6.94 (dd, 1H, Ar-H), 7.15-7.20 (d, 1H, Ar-H), 7.21-7.23 (dd, 1H, Ar-H), 7.44-7.55 (m, 3H, Ar-H), 7.80-7.84 (dd, 2H, Ar-H).
2- [5-(2-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1- yl]-thiazol-4(5H)-one (3d). Yield: 74%; mp 272-274°; FTIR (ν, cm−1)=2924 (C-H, aromatic), 1697 (C=O), 1541 (C=N), 1222 (C-N), 756 (Aromatic C-Cl), 688 (C-S); MS: m/z =356.1(M+, 100%), 358 (M+ +2, 38.51%), 357 (M+ +1, 24.68%).
2- [5-(4-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-thiazol-4(5H)-one (3e). Yield: 77%; mp 249-251°; FTIR (ν, cm−1)=2929 (C-H, aromatic), 1703 (C=O), 1533 (C=N), 1226 (C-N), 827 (Aromatic C-Cl), 688 (C-S); MS: m/z=356 (M+, 100%), 358 (M+ +2, 39.55%), 357(M+ +1, 19.54%), 255 (M+ −100, 11.82%).
2- [5-(2,4-Dichlorophenyl)-3-phenyl-4,5-dihydropyrazol- 1-yl]-thiazol-4(5H)-one (3f). Yield: 65%; mp 287-289°; FTIR (ν, cm−1) =3074 (C-H, aromatic), 1689 (C=O), 1533 (C=N), 1226 (C-N), 763 (Aromatic C-Cl), 692 (C-S); 1H NMR (300 MHz, CDCl3) δ ppm=3.22-3.29 (dd, 1H, Ha), 3.92 (s, 2H, He), 3.99-4.09 (dd, 1H, Hb), 6.01-6.06 (dd, 1H, Hx), 6.94-6.97 (d, 1H, Ar-H), 7.18-7.19 (dd, 1H, Ar-H), 7.42-7.53 (m, 4H, Ar-H), 7.76-7.80 (dd, 2H, Ar-H).
2- [5-(2-Chloro-6-fluorophenyl)-3-phenyl-4,5- dihydropyrazol-1-yl]-thiazol-4(5H)-one (3g). Yield: 81%; mp 251−253°; FTIR (ν, cm−1)=2966 (C-H, aromatic), 1701 (C=O), 1537 (C=N), 1224 (C-N), 1116 (Aromatic C-F), 790 (Aromatic C-Cl), 694 (C-S); MS: m/z=374 (M+ +1, 100%), 340 (M+ −34, 32.16%), 273 (M+ - 100, 45.84%), 239 (M+ - 135, 31.23%).
2- [5-(4-Fluorophenyl)-3-phenyl-4,5-dihydropyrazol-1- yl]-thiazol-4(5H)-one (3h). Yield: 73%; mp 210-212°; FTIR (ν, cm−1)=2926 (C-H, aromatic), 1708 (C=O), 1568 (C=N), 1161 (C-N), 1124 (Aromatic C-F), 688 (C-S); MS: m/z=340 (M+ +1, 100%), 341(M+ +2, 20.39%).
2- [5-(3-Nitrophenyl)-3-phenyl-4,5-dihydropyrazol- 1-yl]-thiazol-4(5H)-one (3i). Yield: 66%; mp 298-299°C; FTIR (ν, cm−1)=3061 (CH, aromatic), 1697 (C=O), 1539 (C=N), 1222 (C-N), 692 (C-S); MS: m/z=367 (M+ +1, 100%), 368 (M+ +2, 20.54%), 232 (M+ −135, 7.36%).
2- [5-(4-Hydroxyphenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-thiazol-4(5H)-one (3j). Yield: 62%; mp 298-300°; FTIR (ν, cm−1)=3460 (C-OH), 2970 (C-H, aromatic), 1688 (C=O), 1579 (C=N), 1230 (C-N), 693 (C-S); 1H NMR (300 MHz, DMSO-d6) δ ppm=3.35-3.42 (dd, 1H, Ha), 3.91 (s, 2H, He), 4.01-4.11 (dd, 1H, Hb), 5.65-5.70 (dd, 1H, Hx), 6.70-6.73 (d, 2H, Ar-H), 7.01-7.04 (d, 2H, Ar-H), 7.49-7.58 (m, 3H, ArH), 7.83-7.86 (dd, 2H, ArH), 9.48 (s, 1H, OH).
The in vitro antimicrobial activity of newly synthesized compounds (3a-3j) was determined by tube dilution method. In this work, two Gram positive organisms, i.e. Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538P and two Gram negative organisms, i.e. Escherichia coli ATCC 9002(NCTC) and Pseudomonas aeruginosa ATCC 25619 were used to investigate the antibacterial activity. They were also evaluated for their in vitro antifungal activity against Aspergillus niger ATCC 9029 and Candida albicans ATCC 2091. The bacterial and the fungal strains were procured from National Chemical Laboratory, Pune, India. Streptomycin and fluconazole (Sigma Aldrich, Bangalore, India) were used as standard for antibacterial and antifungal studies, respectively. Two-fold dilutions of the compounds and standard drugs were prepared in dimethylsulfoxide (DMSO) ranging from 0.8 to 400 μg/ml. Seeded broth was prepared in nutrient broth from 24 h old bacterial cultures on nutrient agar at 37±1° while fungal spores from 24 h old Sabourauds agar slant cultures were suspended in Sabourauds dextrose broth. The colony forming units of the seeded broth were adjusted in the range of Mcfarland standard number one. Testing was performed at pH 7.4±0.2. A set of assay tubes containing only inoculated broth was kept as control and likewise solvent controls were also run simultaneously. The tubes were incubated in incubators at 37° for bacteria and at 28° for fungi. The minimum inhibitory concentrations were recorded by visual observations after 24 h (bacteria) and 72-96 h (fungi) of incubation. Results of antimicrobial studies have been presented in Table 2.
| Compound | Minimum inhibitory concentration (μg/ml) | |||||
|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | Fungi | ||||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | A. niger | C. albicans | |
| 3a | 200 | 100 | 1.55 | 50 | 12.5 | 25 |
| 3b | 400 | 50 | 400 | 200 | 400 | 400 |
| 3c | 25 | 6.25 | 100 | 400 | 100 | 100 |
| 3d | 3.12 | 12.5 | 6.25 | 25 | 6.25 | 50 |
| 3e | 1.55 | 12.5 | 1.55 | 3.12 | 6.25 | 12.5 |
| 3f | 3.12 | 25 | 12.5 | 6.25 | 12.5 | 6.25 |
| 3g | 6.25 | 3.12 | 6.25 | 12.5 | 3.12 | 12.5 |
| 3h | 6.25 | 3.12 | 25 | 50 | 25 | 200 |
| 3i | 1.55 | 100 | 12.5 | 25 | 50 | 100 |
| 3j | 25 | 200 | 200 | 100 | 50 | 400 |
| Std a | 3.12 | 6.25 | 6.25 | 6.25 | - | - |
| Std b | - | -- | - | - | 8 | 16 |
Std=standard, a=streptomycin and Std, b=fluconazole are standards for antibacterial and antifungal activity, respectively
Table 2: In vitro antimicrobial activity of compounds (3a-3j)
The synthesis of compounds (3a-3j) was followed by the general pathway outlined in Scheme 1. In the first step, chalcones (1a-1j) were obtained by direct condensation of the aromatic aldehydes and the aromatic ketones, using sodium hydroxide as catalyst in ethanol. In the second step, cyclization of different chalcones with thiosemicarbazide under basic condition leads to the formation of N-thiocarbamoylpyrazole derivatives (2a-2j). In the last step, pyrazolothiazol- 4(5H)-one derivatives (3a-3j) were obtained by refluxing compounds (2a-2j) with ethylbromoacetate in ethylacetate. All of the synthesized compounds gave satisfactory analytical and spectroscopic data, which were in full accordance with their depicted structures. The physicochemical characteristics of newly synthesized compounds are given in Table 1.
These synthesized pyrazolothiazol-4(5H)-one derivatives were evaluated for their in vitro antimicrobial activity (Table 2). The study revealed that some of the tested compounds showed moderate to excellent antimicrobial activity against pathogenic strains. Interestingly, among the synthesized compounds, 3e showed excellent antibacterial activity against B. subtilis, E. coli, and P. aeruginosa as compared to standard drug streptomycin. Also 3a, 3e, 3g, 3h and 3i exhibited moderate antibacterial activity. 3c, 3d, 3f and 3g showed similar antibacterial activity as that of standard. Similarly, 3e and 3g showed excellent activity against both A. niger and C. albicans as compared to standard drug fluconazole. Also 3d and 3f showed good activity against A. niger and C. albicans, respectively.
From the above results it was observed that, compounds bearing electronegative substitutions such as Cl or F showed pronounced activity against most of the bacterial and fungal strains. While the compounds bearing aromatic heterocyclic rings without substitutions like furan or thiaphene were found to be inactive. Therefore, in future pyrazolothiazol-4(5H)-one derivatives would represent a novel lead for the development of a new class of antimicrobial agents. These compounds are recommended for further in vivo screening.
Acknowledgements
The authors thank Mr. Sandeep Nikam for his guidance during Antimicrobial activity.
References
- Sarkar A, Kumar KA, DuttaNK, Chakraborty P, DastidarSG.Evaluation of in vitro and in vivo antibacterial activity of dobutaminehydrochloride. Indian J Med Microbiol 2003;21:172-8.
- Gold HS, Moellering RC. Antimicrobial-drug resistance. N Engl J Med1996;335:1445-53.
- Bossche HV, Marichal P, Odds FC. Molecular mechanisms of drug resistance in fungi. Trends Microbiol 1994;2:393-400.
- Cohen ML. Epidemiology of drug resistance implications for a post antimicrobial era. Science 1992;257:1050-5.
- Murphy GI, Holder JC. PPAR-gamma agonists: Therapeuticrole in diabetes, inflammation and cancer. Trends PharmacolSci2000;21:469-74.
- Subtelna I, Atamanyuk D, Szymanska E, Kiec-KononowiczK,Zimenkovsky B, Vasylenko O, et al. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg Med Chem 2010;18:5090-102.
- Bondock S, Fadaly W, MetwallyMA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazolederivatives containing benzothiazole moiety.Eur J Med Chem2010;45:3692-701.
- Abdelhamid AO, Sayed AR, Zaki YH. Reaction of HydrazonoylHalides 511: A Facile Synthesis of 5-Arylthiazoles and Triazolino [4,3-a] pyrimidines as Antimicrobial Agents. Phosphorus Sulfur Silicon Relat Elem 2007;182:1447-57.
- Rahman MD, Siddiqui AA. Pyrazoline derivatives: A worthy insight into the recent advances and potential pharmacological activities. Int J Pharm Sci Drug Res 2010;2:165-75.
- VenkatRagavan R, Vijayakumar V, SuchethaKumari N. Synthesis and antimicrobial activities of novel 1,5-diaryl pyrazoles. Eur J Med Chem2010;45:1173-80.
- Bekhit AA, Fahmy HT, Rostoma SA, Bekhit AA. Synthesis and biological evaluation of some thiazolylpyrazole derivatives as dual anti-inflammatory antimicrobial agents.Eur J Med Chem2010;45:6024-38.
- Shah T, Desai V. Synthesis and antibacterial studies of some novel Schiff isoxazoline derivatives. J Serb ChemSoc 2007;72:443-9.