- Corresponding Author:
- T. Phaechamud
Departments of Pharmaceutical Technology, Silpakorn University, Nakorn Pathom 73000, Thailand
E-mail: thawatchaienator@gmail.com
| Date of Submission | 22 January 2013 |
| Date of Revision | 12 March 2013 |
| Date of Acceptance | 10 April 2013 |
| Indian J Pharm Sci 2013;75(4):385-392 |
Abstract
To characterize the thermal behavior and texture analysis of doxycycline hyclate thermosensitive gels developed for periodontitis treatment containing zinc oxide prepared by using poloxamer (Lutrol ® F127) as polymeric material and N-methyl pyrrolidone was used as cosolvent. The thermosensitive gel comprising doxycycline hyclate, Lutrol® F127, and N-methyl pyrrolidone were characterized for the thermal behavior and texture analysis. The topography of the system after the dissolution test was characterized with scanning electron microscope. Differential scanning calorimetric thermogram exhibited the endothermic peaks in the systems containing high amount of N-methyl pyrrolidone in solvent. The sol-gel transition temperature of the systems decreased as the zinc oxide amount was increased. The addition of doxycycline hyclate, zinc oxide, and N-methyl pyrrolidone affected the syringeability of systems. The addition of zinc oxide into the doxycycline hyclate-Lutrol ® F127 systems decreased the diameter of inhibition zone against Staphylococcus aureus, Escherichia coli, and Candida albicans since zinc oxide decreased the diffusion and prolonged release of doxycycline hyclate. From scanning electron microscope analysis, the porous surface of 20% w/w Lutrol® F127 system was notably different from that of gel comprising doxycycline hyclate which had interconnected pores and smooth surfaces. The number of pores was decreased with increasing zinc oxide and the porous structure was smaller and more compact. Therefore, the addition of zinc oxide could increase the syringeability of doxycycline hyclate-Lutrol® F127 system with the temperature dependence. Zinc oxide decreased inhibition zone against test microbes because of prolongation of doxycycline hyclate release and reduced size of continuous cells. Furthermore, zinc oxide also increased the compactness of wall surfaces of Lutrol® F127.
Keywords
Doxycycline hyclate, texture analysis, thermosensitive, topography, zinc oxide
Periodontitis treatment with local antimicrobial administration into the periodontal pocket could be performed with in situ forming gel system. Local drug delivery limits the drug to its target site, with little or no systemic uptake, so a much smaller dose is required for effective therapy and harmful side effects can be minimized or eliminated [1]. Tetracycline-loaded bioadhesive semisolid, polymeric system based upon hydroxyethyl cellulose and polyvinylpyrrolidone [2] and metronidazole-loaded systems based upon Carbopol 974P, hydroxyethylcellulose, and polycarbophil have been reported [3]. Tetracycline and serratiopeptidasecontaining pluronic gel has been designed [4]. Doxycycline hyclate (DH) shows the interference for bacterial protein synthesis and inhibition of tissue collagenase activity. It has an extra antiinflammatory effect by inhibiting the proinflammatory cytokines, IL-1, and TNF-a [5,6]. The Atrigel® is an injectable biodegradable delivery system containing 10% DH based on poly-DL-lactide dissolved in N-methyl-2-pyrrolidone (NMP) [7]. NMP is thermally stable lactam of 4-methylaminobutyric acid, which is colorless with a high boiling point, low viscosity, low toxicity, and good biocompatibility while the solubility parameter is similar to that of ethanol and dimethyl sulfoxide (DMSO) [8]. Zinc oxide (ZnO) is the interesting compound because of its antimicrobial activity [9,10].
Many reports showed antimicrobial properties of ZnO. This material exhibited bactericidal action against S. aureus and P. aeruginosa [11]. Lutrol® F127 (Poloxamer 407) is a synthetic polymer consisting of hydrophilic polyoxyethylene and hydrophobic polyoxypropylene blocks arranged in a triblock structure. Its aqueous solution shows the thermosensitive property which is general liquid at low temperature but changes to gel at higher temperature [12]. Recently, a dynamic mechanical analysis was used to determine the sol-gel transition in a more reproducible manner [13]. Our previous study reported that Lutrol® F127 system showed Newtonian flow at low temperature (4°), which altered to non-Newtonian flow with increasing temperature and viscosity [14]. In addition, there was the decrease of gelation temperature of Lutrol® F127 as the ZnO amount was increased [14]. The power law kinetic implied that the release rate of DH decreased as the amount of ZnO was increased by developing systems. Systems comprising of ZnO could prolong the drug release from Lutrol® F127 systems [14]. This 10 mg/ml developed sample was not toxic to human gingival fibroblasts and macrophage cell lines; however, Lutrol® F127 and DH showed a slight effect as compared to a control group [15]. Typically, it is important to indicate the ability of the product to be delivered from a syringe through a needle, in order to fulfill the requirement for the ease or feasibility of application. Syringeability of various systems was examined to determine the effect of temperature and the addition of excipients on the force required to expel the prepared product. Topography characterization by scanning electron microscope (SEM) could be employed to investigate the structure of polymer. Therefore, it was interesting to use SEM for characterization of gel structure after the test of drug release.
This study was to characterize the thermal behavior of DH thermosensitive gels containing ZnO dispersed in Lutrol® F127 solution. NMP was used as a cosolvent in the systems since it has been recommended as an injectable vehicle with a lactam like structure. Therefore, it might promote the antimicrobial activity of the developed systems. The texture analysis of systems was conducted and the topography of systems after testing dissolution was characterized by scanning electron microscope (SEM).
Materials and Methods
Lutrol® F127 (lot No. WPT×607B) was purchased from Vita Co. Ltd., Bangkok, Thailand. Zinc oxide BP (lot no. 000150, Vidhyasom, Thailand), doxycycline hyclate (Batch No. 20071121, Huashu Pharmaceutical Corporation., Shijiazhuang, China) were supplied by T. Man Pharma Ltd., Part., Bangkok, Thailand. N-methyl-2-pyrrolidone (NMP) (Fluka, New Jersey, USA) was used as received. Sabouraud Dextrose Agar (SDA), Sabouraud Dextrose Broth (SDB), Tryptic Soy Agar (TSA), and Tryptic Soy Broth (TSB) (Difco™, USA) were used as media for antimicrobial test.
Preparation of Lutrol® F127 systems containing zinc oxide and DH
The 20% w/w Lutrol® F127 aqueous solutions were prepared by the cold method as described previously [16]. Briefly, Lutrol® F127 was slowly added to cold water containing different amounts of NMP (4°). Each of dispersions was left in a refrigerator for 24 h at 4° until the solution was clear. To manipulate the various formulation properties, ZnO and DH were added into the systems. The systems containing Lutrol® F127 (20% w/w), DH (5% w/w), and different amount of ZnO were also prepared.
Differential scanning calorimetry
The thermal properties of prepared systems were analyzed by a differential scanning calorimetry (Pyris Sapphrie DSC, Standard 115V, Perkin Elmer instruments, Japan). Samples (5-7 mg) were accurately weighed into aluminum pans and sealed. The heating rates were 5 and 10°/min under nitrogen purge at the temperature of 30-300°.
Texture analysis
Normally, an abrupt change in the storage modulus or viscosity reflects the sol-gel transition. Therefore, the textural analysis of 150 ml prepared systems was done using a TA.XT2i texture analyzer (Charpa Techcenter, Godalming, Stable micro Systems Ltd., UK) equipped with a 5 kg load cell and Texture Expert software. The sol-gel transition temperature of Lutrol® F127 gels containing various ingredients was determined from oscillating measurements at 1 Hz and heated in a temperature controllable water bath from 4 to 45° at a heating rate of 1°/min. The sol-gel transition temperature was calculated by ‘‘time cure test’’ obtained by plotting the G’ as a function of temperature. The gelling temperature was defined as the point where the G’ was halfway between G’ for the solution and G’ for the gel as mentioned previously [17].
Syringeability
The ease with which gel systems containing samples could be expelled after filling into syringes and through their fitted needles was measured using a texture analyzer in compression mode. A sample was filled into a 1 ml syringe and was held up with a clamp at the upper probe of the texture analyzer. It was then moved downward until it was attached to the syringe barrel base. A constant force of 0.1 N was applied to the base and the distance required to expel the contents for a barrel length of 20 mm was measured. All of the experiments were triplicately done (n=3) at temperatures of 4 and 20°. Force displacement profiles were performed, which the force at a distance of 10 mm was selected to indicate the syringeability as the force to expel the sample during drug administration.
Antimicrobial studies
Antimicrobial activities of prepared DH and ZnO-DH gels were determined using agar cup diffusion method. The standard microbes used in this study were S. aureus (ATCC 6538P), E. coli (ATCC 10536), and C. albicans (ATCC 17110). The actively growing broth culture of microbes was prepared and the turbidity was adjusted to obtain 108 cells/ml approximately. Then the swab was spread onto the agar plate in three directions to ensure that the plate area was completely covered and the spread culture was dried at ambient condition. The sterilized cylinder cups were placed carefully on the surface of the swabbed agar. The prepared gels were filled into the cylinder cup and incubated at 37° for 24 h. The antimicrobial activities were measured as the diameter (cm) of clear zone for growth inhibition. The tests were conducted in triplicate and the mean inhibition zone±standard deviation were calculated.
Determination of surface or morphology of systems
The release studies were performed using the dialysis method as described previously [16]. One gram of gel was put in a dialysis tube (MW cutoff, 6000-8000). The dialysis tube was then placed in a glass bottle containing 100 ml phosphate buffer pH 7.2, maintained at 37°, and stirred at 100 rpm. Release medium of each sample (10 ml) was collected periodically and replaced with fresh dissolution medium. The concentration of DH was determined using a UV/ Vis spectrophotometer (Perkin-Elmer, Germany) at 349 nm (n=3). The analysis of drug release from these developed systems was done following a previous report [14]. Samples after dissolution test were freeze dried using the freeze dryer (Triad™ Labconco, Kansan City, MO, USA) for 48 h until the samples were dried to avoid the collapse of porous structures. The dried sample was placed on a double adhesive carbon that adhered to an aluminum foil which was stuck on metal stub before being sputter-coated with gold using the sputter coater (Cressington 108, Cressington Scientific Instruments, Watford, England) prior to testing. Micrographs were taken with SEM (Maxim 200 Camscan, Cambridge, England) at an accelerating voltage of 15 kV. The morphology of samples was observed for the porosity of pore structure, surface structure, and drug crystalline. The samples containing ZnO were also prepared as with the above mentioned method before observing with SEM by both routine secondary electron imaging (SEI) and backscattered electron imaging (BEI) modes.
Results and Discussion
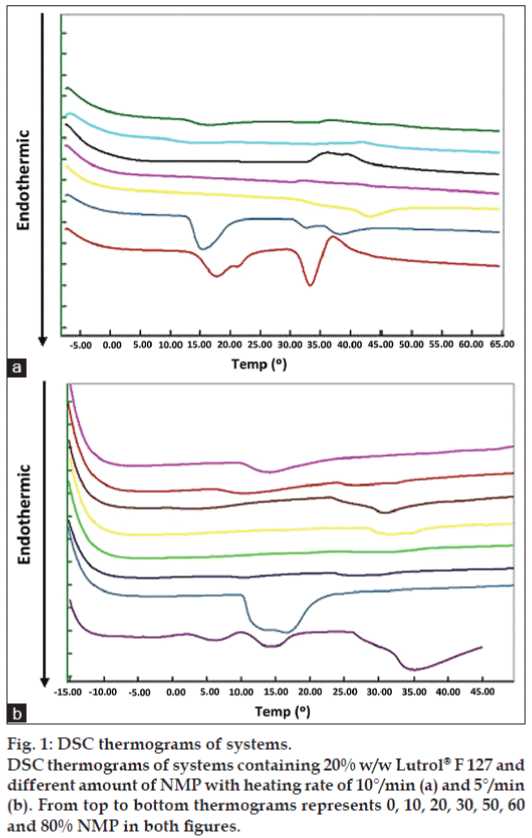
The micelle transition of the formulation was investigated using DSC. The critical micellization temperature (CMT) was taken as the onset of the endothermic peak. This peak has previously been attributed to a hydrophobic entropy gain on micellization [18-20]. DSC investigations of Lutrol® F127 gel containing different NMP concentrations are shown in fig. 1. The CMT did not correlate with NMP amount. The decrease of heating rate to 5°/ min was also conducted. There were endothermic peaks appearing for the systems containing 60 and 80% w/w NMP (fig. 1b). The CMT and gelation temperature of Lutrol® F68 and Lutrol® F127 were found to interconnect and influenced by cosolutes, such as electrolytes and hydrophobic substances [20]. Both CMT and gelation temperature increased upon diluting the system with water, and decreased with an increasing pH of the system due to the solubility reduction of active ingredients. The ratio between two block copolymers presented in the system had an impact on CMT and the gelation temperature, resulting in a decrease of onset temperature of both processes with an increased Lutrol® F127. However, the mentioned characteristics were not obtained from this study.
Practically, the texture analysis could provide the informative data on the dynamic properties, such as the G’ and the viscous modulus (G”). The sol-gel transition temperature for the Lutrol® F127 systems is shown in fig. 2. The G’ was notably low for a solution and increased drastically with temperature as a result of gel formation as previously reported [16,21]. When the sol-gel transition occurred G’ became independent of further increase in temperature. The sol-gel transition temperature of the Lutrol® F127 system containing 5% w/w DH and 20% w/w NMP was about 28° whereas it was decreased with increasing ZnO amount. The results indicated the change of sol-gel transition which was similar to those where a sol-gel transition was determined by the visual method.
Syringeability of various Lutrol® F127 systems was examined to determine the effects of the incorporated excipient and temperature on the force required to expel the product. The systems examined for syringeability are listed in Table 1. The effect of temperature on syringeability of the system was notably evident. The syringeability was tested at the storage temperature (4°) and expelled temperature (20°) [21]. The syringeability of system 1 was 7.11±0.14 N at 4° and 13.68±1.49 N at 20°, which was a two fold increase in resistance to syringing over this temperature range. When the 5% w/w DH was added into Lutrol® F127 system (system 2), the syringeability was increased to 8.65±0.48 N at 4° and 17.31±1.77 N at 20° that were slightly higher than those of system 1. The syringeability of the 20% w/w Lutrol® F127 system containing 5% w/w DH and 20% w/w NMP (system 3) were 17.15±0.54 N and 21.21±1.37 N at 4 and 20°, respectively. The syringeability of the systems containing 1% w/w ZnO were increased to 22.07±4.24 N at 4° and 25.79±2.30 N at 20°. The syringeability correlated well with the rheology results indicating that the added ZnO increased shear stress of the DH-Lutrol® F127 systems. Therefore, the addition of DH, ZnO, and NMP affected the syringeability of systems. These results corresponded to the experiment previously determining the rheology of poloxamer for ophthalmic use [17].
| System | Syringeabilitya (N) | |
|---|---|---|
| at 4° | at 20° | |
| 20% Lutrol® F127 | 7.11 ± 0.14 | 13.68 ± 1.49 |
| 20% Lutrol® F127, 5% DH | 8.65 ± 0.48 | 17.31 ± 1.77 |
| 20% Lutrol® F127, 5% DH | 20% NMP 17.15 ± 0.54 | 21.21 ± 1.37 |
| 20% Lutrol® F127, 5% DH, 20% NMP, 1% ZnO | 22.07 ± 4.24 | 25.79 ± 2.30 |
aSyringeabilty expressed in term of mean ± SD
Table 1: Syringeability of various systems
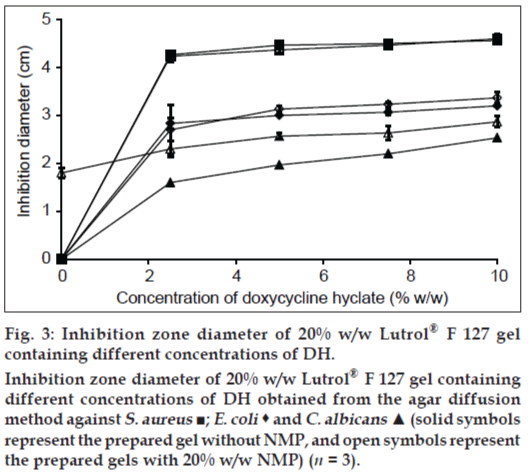
Antimicrobial activities of 20% w/w Lutrol® F127 gels containing DH are shown in fig. 3. The inhibition zone diameter against C. albicans of the gel base containing 20% w/w NMP was 1.8±0.1 cm, whereas the gel base with or without 20% w/w NMP did not exhibit the inhibition zone against S. aureus and E. coli, indicating that the addition of 20% w/w NMP could promote the antifungal activity against C. albicans. The antimicrobial activity of DH gel exhibited a dose response. The addition of NMP into the gel system increased the inhibition zone against C. albicans; therefore, NMP enhanced the antifungal activity of DH. The solubility parameter of NMP is similar to that of ethanol and DMSO [22]; therefore, it could solubilize the lipid in fungal cell membrane and enhance the drug penetration.
Figure 3: Inhibition zone diameter of 20% w/w Lutrol® F 127 gel
containing different concentrations of DH.
Inhibition zone diameter of 20% w/w Lutrol® F 127 gel containing
different concentrations of DH obtained from the agar diffusion
method against S. aureus ■; E. coli  and C. albicans ▲ (solid symbols
represent the prepared gel without NMP, and open symbols represent
the prepared gels with 20% w/w NMP) (n = 3).
and C. albicans ▲ (solid symbols
represent the prepared gel without NMP, and open symbols represent
the prepared gels with 20% w/w NMP) (n = 3).
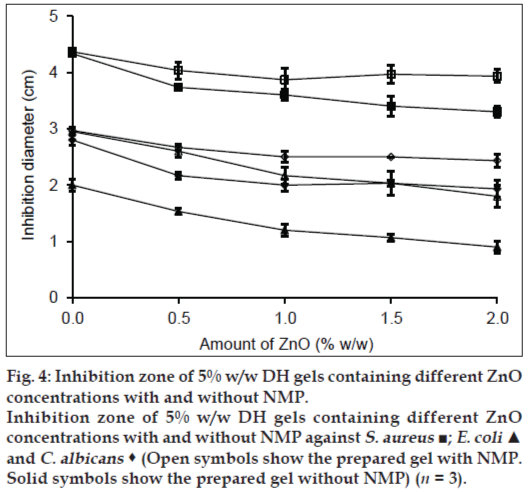
Effect of ZnO on the antimicrobial activity of DH gels is shown in fig. 4. Inhibition zone diameters against S. aureus and E. coli of DH gels with or without NMP were not different whereas 20% w/w NMP could enhance the antifungal activity against C. albicans. The inhibition zone of the prepared gel containing 0.5% w/w ZnO were lower than that without ZnO and indicated that the diffusion of DH from gel was decreased with ZnO. For S. aureus, the inhibition zone of DH-Lutrol® F127 gel decreased as amount of ZnO was increased, whereas the inhibition zone of the prepared gel with 20% NMP did not depend on the ZnO amount. For E. coli and C. albicans, the inhibition zone of the DH-Lutrol® F127 gel with or without 20% w/w NMP decreased when the amount of ZnO were increased. These results showed that the amount of ZnO affected the diffusion of drug from gels. The complex formation between doxycycline and metal ion such as Zn2+ has been reported. This species could attach either to the tricarbonyl methane or the phenol diketone area of this drug molecule [23]. Therefore, the drug release depended on the dissociation of this complex which retarded the drug diffusion into the agar of antimicrobial test and also prolonged drug release in dissolution study as presented in our previous work [14]. The prolongation of drug release with ZnO and better antimicrobial activity were the properties of systems for sustaining DH.
Figure 4: Inhibition zone of 5% w/w DH gels containing different ZnO
concentrations with and without NMP.
Inhibition zone of 5% w/w DH gels containing different ZnO
concentrations with and without NMP against S. aureus ■; E. coli ▲
and C. albicans  (Open symbols show the prepared gel with NMP.
Solid symbols show the prepared gel without NMP) (n = 3).
(Open symbols show the prepared gel with NMP.
Solid symbols show the prepared gel without NMP) (n = 3).
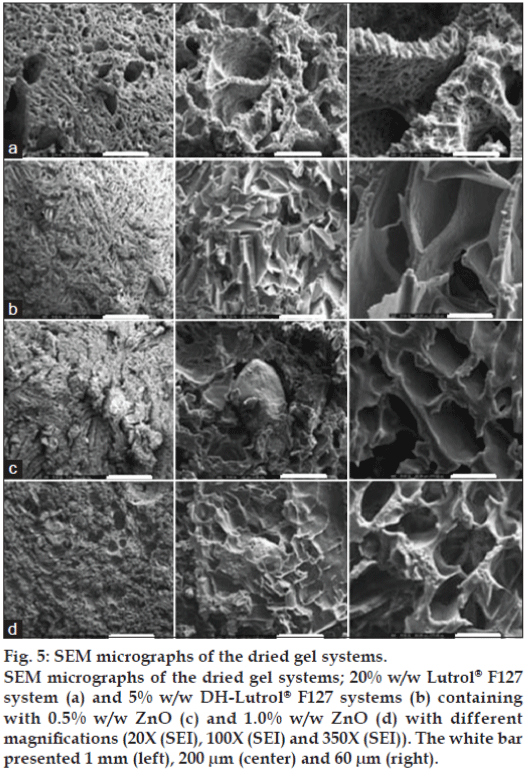
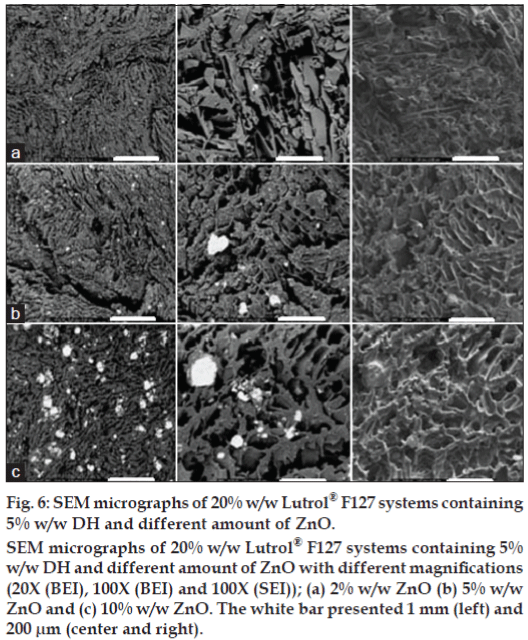
Surface and cross section morphology of the prepared systems after dissolution test were characterized using SEM. Digital images acquired in a SEM could be obtained by SEI mode or BEI mode. The SEI mode is generally used for imaging the surfaces of structures. In the BEI mode, the obtained image contrast depends on the atomic number of the structure under the electron beam. The higher the average atomic weight the brighter the region will appear in the image. Therefore, the bright area in the image indicated the deposited ZnO particles. Freeze-drying technique was utilized for preparing samples to maintain the porous structure without any collapse during water sublimation. The SEM images of Lutrol® F127 dried gel containing 5% w/w DH and different amounts of ZnO at different magnifications are shown in figs. 5-7. The porous surface was evident for 20% w/w Lutrol® F127 system (fig. 5a). The enlarged images of the surface of the wall were found at magnification 100× and 350× which the porous structure was more evident. The structures of 20% w/w Lutrol® F127 systems were notably different from those of systems comprising DH. The structure of the DH-Lutrol® F127 system contained the interconnected pores, whereas the wall surface was smooth (fig. 5b). The pores were decreased with an increased ZnO contents and the porous structure was more compact and smaller. This was explained with entanglement between ZnO and Lutrol® F127 being enhanced by increasing ZnO content. For samples of 0.5, 1.0, 2.0, 5.0, and 10.0% w/w ZnO content, the SEM micrographs were further magnified to clarify their structure as presented in figs. 5c-d and 6a-c. The addition of Aerosil® could transform the structure of poloxamer system from the cubic into the hexagonal phase [24]. Therefore, this study suggested that increasing the ZnO amount could decrease the size of the interconnected pores and increase the compactness of the wall surface. Topography from BEI mode of SEM analysis revealed the distribution of ZnO particles in freeze-dried structure. The denser ZnO particles were evident in the system containing higher amount of this compound.
Figure 5: SEM micrographs of the dried gel systems.
SEM micrographs of the dried gel systems; 20% w/w Lutrol® F127
system (a) and 5% w/w DH-Lutrol® F127 systems (b) containing
with 0.5% w/w ZnO (c) and 1.0% w/w ZnO (d) with different
magnifications (20X (SEI), 100X (SEI) and 350X (SEI)). The white bar
presented 1 mm (left), 200 μm (center) and 60 μm (right).
Figure 6: SEM micrographs of 20% w/w Lutrol® F127 systems containing
5% w/w DH and different amount of ZnO.
SEM micrographs of 20% w/w Lutrol® F127 systems containing 5%
w/w DH and different amount of ZnO with different magnifications
(20X (BEI), 100X (BEI) and 100X (SEI)); (a) 2% w/w ZnO (b) 5% w/w
ZnO and (c) 10% w/w ZnO. The white bar presented 1 mm (left) and
200 μm (center and right).
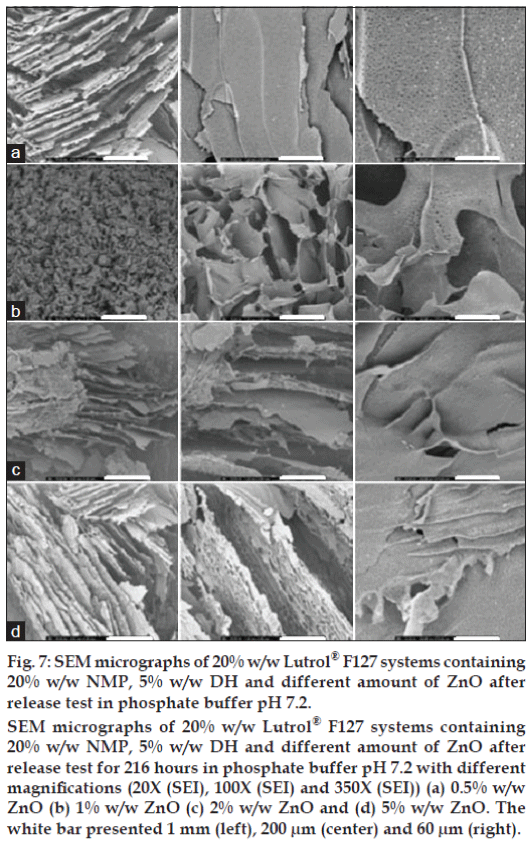
Figure 7: SEM micrographs of 20% w/w Lutrol® F127 systems containing
20% w/w NMP, 5% w/w DH and different amount of ZnO after
release test in phosphate buffer pH 7.2.
SEM micrographs of 20% w/w Lutrol® F127 systems containing
20% w/w NMP, 5% w/w DH and different amount of ZnO after
release test for 216 hours in phosphate buffer pH 7.2 with different
magnifications (20X (SEI), 100X (SEI) and 350X (SEI)) (a) 0.5% w/w
ZnO (b) 1% w/w ZnO (c) 2% w/w ZnO and (d) 5% w/w ZnO. The
white bar presented 1 mm (left), 200 μm (center) and 60 μm (right).
The SEM photographs of cross-sectional morphology of Lutrol® F127 dried gel containing 5% w/w DH, 20% w/w NMP and different amount of ZnO after release test for 216 h in phosphate buffer pH 7.2 [14] at different magnifications are shown in fig. 7. The interconnected pore diameter decreased after the release test. The structure of Lutrol® F127 dried gel seemed fragile and the pore surface was reduced. Lutrol® F127 has a molecular weight of 12 600, which could not diffuse passing the dialysis tube (MW cutoff: 6000-8000). Therefore, this polymeric gel structure without drug was more compact after freeze-drying.
The physical properties of the gel systems depended on the ingredients added into the systems. The sol-gel transition temperature of the Lutrol® F127 system containing 5% w/w DH and 20% w/w NMP was about 28° and decreased as the ZnO amount was increased. The syringeability of DH-Lutrol® F127 system was increased as increasing the amount of zinc oxide. However, their syringeability decreased when the temperature was decreased. The inhibition zones against S. aureus, E. coli, and C. albicans were smaller since ZnO delayed the diffusion and prolonged the DH release. The morphology of freeze-dried Lutrol® F127 system showed the interconnected pores with small porosity on their surfaces, whereas the addition of DH changed the structure of Lutrol® F127 that was the interconnected pores and the surfaces was smooth. The sizes of interconnected pores decreased with increasing ZnO content and the structure was more compact and smaller. However, the structure of the Lutrol® F127 system comprising ZnO after studying DH release was the interconnected pore.
Acknowledgements
This research work was kindly supported by the Research, Development and Engineering (RD and E) Fund through National Nanotechnology Center (NANOTEC), National Science and Technology Development Agency (NSTDA), Thailand (Grant No. NM-B22-NE6-17-50-13) and the Faculty of Pharmacy, Silpakorn University.
References
- Esposito P, Ortesi R, Cervellati F, Menegatti E, Nastruzzi C. Biodegradable microparticles for sustained delivery of tetracycline to the periodontal pocket: Formulatory and drug release studies. J Microencapsul 1997;14:175-87.
- Jones DS, Woolfson AD, Djokic J, Coulter WA. Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm Res 1996;13:1734-8.
- Jones DS, Woolfson AD, Brown AF, O’Neill MJ. Mucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: In vitrorelease kinetics, syringeability, mechanical and mucoadhesive properties. J Control Release 1997;49:71-9.
- Maheshwari M, Miglani G, Mali A, Paradkar A, Yamamura S, Kadam S. Development of tetracycline-serratiopeptidase-containing periodontal gel: Formulation and preliminary clinical study. AAPS PharmSciTech 2006;7:E1-10.
- Alexander MB, Damoulis PD. The role of cytokines in the pathogenesis of periodontal disease. Curr Opin Periodontol 1994;1:39-53.
- Reynolds JJ, Meikle MC. Mechanisms of connective tissue matrix destruction in periodontitis. Periodontol 2000 1997;14:144-57.
- Schwach AK, Vivien CN, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm 2000;50:83-99.
- Sanghri R, Narazaki R, Machatha SG, Yalkowsky SH. Solubility improvement of drug using N-methyl pyrrolidone. AAPS PharmSciTech 2008;9:366-76.
- Sawai J, Shoji S, Igarashi H, Hashimoto A, Kokugan T, Shimizu M, et al. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J Ferment Bioeng 1998;86:521-52.
- Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 2003;54:177-82.
- Coleman NJ, Bishop AH, Booth SE, Nicholson JW. Ag+- and Zn2+-exchange kinetics and antimicrobial properties of 11 angstrom tobermorites. J Eur Ceram Soc 2009;29:1109-17.
- Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res 2006;23:2709-28.
- Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev 2002;54:37-51.
- Mahadlek J, Charoenteeraboon J, Choopun S, Phaechamud T. Role of zinc oxide on rheology of thermosensitive gel developed for periodontitis treatment. Adv Mater Res 2010;93-94:479-84.
- Phaechamud T, Mahadlek J, Aroonrerk N, Choopun S, Charoenteeraboon J. Antimicrobial activities of ZnO-doxycycline hyclate thermosensitive gel. ScienceAsia 2012;38:64-74.
- Schmolka IR. Artiicial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res 1972;6:571-82.
- Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an insitugel for ophthalmic use. Eur J Pharm Sci 1998;6:105-12.
- Alxandridis P, Holtzwarth JF, Hatton TA. Micellization of poly (ethylene oxide)-poly (propyleneoxide) poly (ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules 1994;27:2414-25.
- Wanka G, Hoffmann H, Ulbricht W. Phase diagrams and aggregation behavior of poly (oxyethylene)-poly (oxypropylene)-poly (oxyethylene) triblock copolymers in aqueous solutions. Macromolecules 1994;27:4115-59.
- Scherlund M, Malmsten M, Holmqvist P, Brodin A. Thermosetting microemulsions and mixed micellar solutions as drug delivery systems for periodontal anesthesia. Int J Pharm 2000;194:103-16.
- Kelly HM, Deasy PB, Ziaka E, Claffey N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int J Pharm 2004;274:167-83.
- Hansen CM, Just L. Prediction of environmental stress cracking in plastics with Hansen solubility. Ind Eng Chem Res 2001;40:21-5.
- Novak-Pekli M, Mesbah ME, Petho G. Equilibrium studies on tetracycline-metal ion systems. J Pharm Biomed Anal 1996;14:1025-9.
- Maheshwari M, Paradkar A, Yamamura S, Kadam S. Preparation and characterization of pluronic–colloidal silicon dioxide composite particles as liquid crystal precursor. AAPS Pharm Sci Tech 2006;7:E1-7.
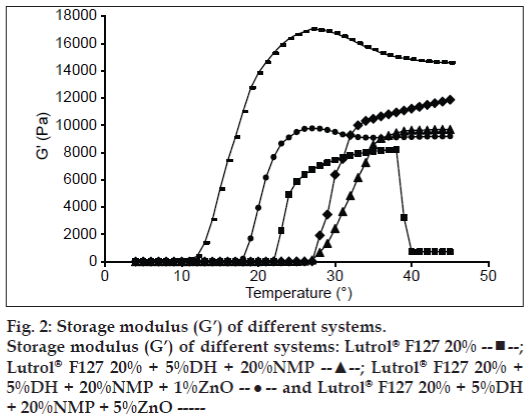
 ;
Lutrol® F127 20% + 5%DH + 20%NMP --▲--; Lutrol® F127 20% +
5%DH + 20%NMP + 1%ZnO --•-- and Lutrol® F127 20% + 5%DH
+ 20%NMP + 5%ZnO -----
;
Lutrol® F127 20% + 5%DH + 20%NMP --▲--; Lutrol® F127 20% +
5%DH + 20%NMP + 1%ZnO --•-- and Lutrol® F127 20% + 5%DH
+ 20%NMP + 5%ZnO -----