- *Corresponding Author:
- L. GAO
Department of Neurosurgery, Shanghai Tenth People's Hospital, Tongji University, Shanghai 200070, China
E-mail: lianggaoh@126.com
| Date of Received | 19 April 2022 |
| Date of Revision | 24 March 2023 |
| Date of Acceptance | 04 October 2023 |
| Indian J Pharm Sci 2023;85(6):1761-1770 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the impact and potential mechanisms of erianin on the differentiation of neural stem cells into neurons in vitro cell model of traumatic brain injury. The brain injury tissue extraction liquid was extracted from traumatic brain injury rats. Neural stem cells were divided into different groups and cultured. The control, extract, vector, CCT020312 (p-extracellular signal regulated kinase activator), erianin and erianin+CCT020312 groups. The oxidative stress, inflammatory response, mitochondrial deoxyribonucleic acid damage level, neuronal differentiation, and signal pathway activation status of the cell model were evaluated. When brain injury tissue extract was added to the cell culture medium, the oxidative stress, inflammatory reaction, mitochondrial deoxyribonucleic acid damage level, and activation status of the extracellular signal regulated kinase 1/c-Jun signaling pathway significantly increased, leading to a significant decrease in the number of differentiated neurons. Furthermore, when CCT020312 was also added, it further exacerbates the changes in the above-mentioned indicators. However, when erianin was added simultaneously with the brain injury tissue extract to the cell culture medium, it significantly reduced the oxidative stress, inflammatory reaction, mitochondrial deoxyribonucleic acid damage level, and activation status of the extracellular signal regulated kinase 1/c-Jun signaling pathway, promoted cell differentiation into neurons. However, CCT020312 was able to reverse the aforementioned effects of erianin. The results indicate erianin in vitro cell models exert neuroprotective effects through antioxidative and anti-inflammatory actions, promoting the differentiation of neural stem cells into neurons, which may be associated with its inhibition of extracellular signal regulated kinase 1/c-Jun signaling pathway activation.
Keywords
Traumatic brain injury, erianin, neural stem cells, neuronal differentiation, oxidative stress, inflammation, extracellular signal regulated kinase 1/c-Jun signaling pathway
Studies have observed the presence of neuroblast cells expressing immature neuronal markers in the surrounding areas of focal ischemia[1], Traumatic Brain Injury (TBI)[2,3], and subarachnoid hemorrhage[4]. However, the differentiation into mature neurons is rarely observed. Despite the enhanced response of endogenous Neural Stem Cells (NSCs) to TBI, their self-repair capacity is limited, especially in the hostile environment characterized by extensive cell death and inflammation following TBI, resulting in low survival rates of these newly generated cells. Secondary injuries, including excitotoxicity caused by the neurotransmitter glutamate, oxidative stress, and neuroinflammatory responses[5-7], persist for hours to months after the primary injury and impair the endogenous neuronal regeneration. These secondary injuries are critical determinants for the differentiation of proliferating NSCs into mature neurons[8].
Oxidative stress, characterized by the imbalance between Reactive Oxygen Species (ROS) and antioxidants, plays a critical role in the secondary injury that follows TBI[9]. Neuroinflammation is involved in a wide range of processes and plays a vital role in various aspects of the Central Nervous System (CNS) physiology and pathology. Neuroinflammation is a complex physiological response that holds significant value in eliminating pathogens and promoting the regeneration of damaged brain tissue. However, excessive or uncontrolled inflammation can lead to autoimmune disorders and tissue damage[10]. The resolution of these micro environmental pressures experienced by neural regeneration, and the promotion of the differentiation of endogenous NSCs into mature neurons, has garnered increasing attention.
Erianin is a small molecule biphenyl compound belonging to the family of Orchidaceae plants. It is extracted from plants of the Eulophia genus. Erianin exhibits a wide range of pharmacological effects and has been found to be involved in various biological processes such as programmed cell death, angiogenesis, anti-inflammatory, and antioxidant activities. Studies have shown its specific functions in the treatment of diseases such as tumors, inflammation, diabetic nephropathy, retinopathy, and ulcerative colitis, making it a promising drug for the treatment of multiple ailments[11-16]. This study aimed to investigate the impact and potential mechanisms of erianin on the differentiation of NSCs into neurons in vitro.
Materials and Methods
Cell line:
OriCell® Sprague-Dawley (SD) rat NSCs were purchased from Saiye (Guangzhou) Biotechnology Co., Ltd. After cell recovery, they were seeded into culture dishes at a density of 3×105 viable cells/ ml and supplemented with an adequate amount of NSCs culture medium (Dulbecco's Modified Eagle Medium (DMEM)/F12 basal medium 100 ml+basic Fibroblast Growth Factor (bFGF) 20 ng/ml+Epidermal Growth Factor (EGF) 20 ng/ ml+B27 2 ml), followed by gentle mixing. The cells were placed into a culture incubator at 37°, 5 % Carbon dioxide (CO2), and saturated humidity. Fresh culture medium was replaced or sub cultured based on the cell growth status.
Brain injury tissue extracts preparation:
The TBI rat model was based on the description by Yi et al.[3]. 3 d after the TBI, the rats were deeply anesthetized, and the head was disinfected with iodine and alcohol. The skull was then opened. Tissue from the injured area surrounding the cortex by 3 mm was taken and weighed, then placed in a sterile grinder. 1 ml of basal culture medium was added to the grinder according to the weight of 100 g of brain tissue. The tissue was thoroughly ground for 5 min, left to settle for 5 min, and then placed in a high-speed centrifuge at a speed of 12 000 rpm for 15 min. The supernatant was collected and stored in a -80° freezer for later use.
NSCs differentiation culture and grouping:
After coating the glass slide with poly-L-lysine, it is placed in a 24-well culture plate, and NSCs (density of 1×105 cells/ml) are seeded. They are then divided into control group, extract group, vector group, CCT020312 group, erianin group and erianin+CCT020312 group. For the control group, DMEM/F12 complete medium was added. For the extract group, DMEM/F12 complete medium containing 20 μl/ml of TBI tissue extract was added. For the CCT020312 group, DMEM/ F12 complete medium containing 20 μl/ml of TBI tissue extract and 5 μM CCT020312 (a selective p-Extracellular Signal Regulated Kinase (ERK) activator, MedChemExpress) was added. For the erianin group, DMEM/F12 complete medium containing 20 μl/ml of TBI tissue extract and 10 ng/ ml erianin (Weikeqi-Biotech) was added. For the erianin+CCT020312 group, DMEM/F12 complete medium containing 20 μl/ml of TBI tissue extract, 10 ng/ml erianin, and 5 μM CCT020312 was added. For the vector group, DMEM/F12 complete medium containing 20 μl/ml of TBI tissue extract and an equal amount of DMSO was added. The culture plate was then placed in a 37°, 5 % CO2 humidified incubator and cultured for 7 d, with medium change once in between.
Detection of oxidative stress and inflammatory factor indicators:
After 7 d of cell culture, discarded the culture supernatant, washed once with Phosphate Buffer Saline (PBS), digested the cells with trypsin, and collected the cells. Malondialdehyde (MDA) and Superoxide Dismutase (SOD) reagent kits (Beyotime) were used to evaluate the levels of MDA and SOD. Levels of Neurofilament Medium (NEFM), Tumor Necrosis Factor-Alpha (TNF-α), and Interleukin-10 (IL-10) were measured using Enzyme-Linked Immunosorbent Assay (ELISA) kits (Abcam). The above operations strictly followed the instructions of the assay kits.
Mitochondrial Deoxyribonucleic Acid (mtDNA) was evaluated by quantitative Polymerase Chain Reaction (qPCR):
After each group of cells was cultured for 7 d, they were washed once with PBS, digested with trypsin, centrifuged at 1500 g for 5 min, discarded the supernatant, collected the cells, and added the sample lysis solution. Used DNA extraction kit (Beyotime) to extract genomic DNA. qPCR amplification was conducted according to the instructions of the BeyoRT™ SYBR Green qPCR Mix kit (Beyotime). The primers used were as follows; mitochondrial Nicotinamide Adenine Dinucleotide Hydrogen (NADH) dehydrogenase 1 (ND1): sense 5'-TGAATCCGAGCATCCTACC-3', anti-sense 5'-ATTCCTGCTAGGAAAATTGG-3'; Apolipoprotein B (APOB): sense 5'-CGTGGGCTCCAGCATTCTA-3', antisense 5'-TCACCAGTCATTTCT GCCTTTG-3'. The parameter setting was as follows; pre-denaturation at 95° for 2 min, followed by 95° for 15 s, 60° for 15 s, for a total of 40 cycles. Using APOB as the reference gene, the relative copy number of mtDNA was calculated using the 2-ΔΔCt method.
Western blot:
After 7 d of cell culture in each group, the cells were washed once with PBS and then treated with lysis buffer. Following complete decomposition, the supernatant was collected through centrifugation at 10 000-14 000 g for 3-5 min and stored at -20°. The protein concentration in the samples was determined using the Bicinchoninic Acid (BCA) reagent (Beyotime). Subsequently, Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) electrophoresis and membrane transfer were performed. The Polyvinylidene Fluoride (PVDF) membrane was thoroughly washed with Tris-Buffered Saline with 0.1 % Tween® 20 Detergent (TBST) and then placed in a blocking solution at room temperature for 1 h. After blocking, the membrane was incubated overnight at 4° with the primary antibody, followed by incubation with the secondary antibody at room temperature for 1.5 h. The primary antibodies used included rabbit anti-Doublecortin (DCX) (1:500, Abcam), rabbit anti-ERK1 (1:600, Abcam), rabbit anti-p-ERK1 (1:600, Abcam), rabbit anti After 7 d of cell culture in each group, the cells were washed once with PBS and then treated with lysis buffer. Following complete decomposition, the supernatant was collected through centrifugation at 10 000-14 000 g for 3-5 min and stored at -20°. The protein concentration in the samples was determined using the Bicinchoninic Acid (BCA) reagent (Beyotime). Subsequently, Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) electrophoresis and membrane transfer were performed. The Polyvinylidene Fluoride (PVDF) membrane was thoroughly washed with Tris-Buffered Saline with 0.1 % Tween® 20 Detergent (TBST) and then placed in a blocking solution at room temperature for 1 h. After blocking, the membrane was incubated overnight at 4° with the primary antibody, followed by incubation with the secondary antibody at room temperature for 1.5 h. The primary antibodies used included rabbit anti-Doublecortin (DCX) (1:500, Abcam), rabbit anti-ERK1 (1:600, Abcam), rabbit anti-p-ERK1 (1:600, Abcam), rabbit antic- Jun (1:800, Abcam), rabbit anti-p-c-Jun (1:800, Abcam), and mouse anti-β-actin (1:1000, Abcam). The secondary antibodies used were IRDye800- conjugated goat anti-rabbit Immunoglobulin G (IgG) and IRDye700-conjugated goat anti-mouse IgG. The resulting bands were collected and analyzed for optical density using the Odyssey 3.0 analysis system.
Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) assay:
After culturing the cells in each group for 7 d, discarded the culture medium, added 4 % formaldehyde solution to each well and fixed it at room temperature for 30 min. Washed three times with PBS, added 50 μl PBS containing 0.5 % Triton X-100, and incubated at room temperature for 5 min. Used the TUNEL kit for apoptosis cell detection according to the instruction. Finally, each well was added with 50 μl Hoechst 33258 (1:3000, Beyotime) and incubated at 37°, avoiding light, for 0.5 h. The slides were washed with PBS three times. After sealing the slides with anti-fluorescence quenching mounting medium, positive cells were counted using a fluorescence microscope.
Immunofluorescence:
Each group of cells was cultured for 3 d (DCX staining) and 7 d (Mitogen-Activated Protein (MAP) 2 staining). After discarding the culture medium, 4 % paraformaldehyde solution was added to each well at room temperature for fixation for 30 min. PBS was used to wash the wells three times, followed by the addition of 50 μl of PBS containing 0.5 % Triton X-100 at room temperature for incubation for 5 min. The primary antibody incubation solution was then added and incubated at 4°, avoiding light, overnight. This was followed by the addition of the secondary antibody incubation solution and incubation at 4°, avoiding light, for 6 h. PBS was used to wash the wells three times. Hoechst 33258 (1:3000, Beyotime) was added and incubated at 37°, avoiding light, for 0.5 h. PBS was used to wash the wells three times. The slides were sealed with an anti-fluorescence quenching sealing solution, and the positive cells were quantified using a fluorescence microscope. The primary antibodies used were rabbit anti-DCX (1:400, Abcam) and rabbit anti-MAP2 (1:500, Abcam). The secondary antibody used was Alexa Fluor® 488 goat anti-rabbit (1:1000, Abcam).
Statistical analysis:
The data obtained in this study followed a normal distribution and are presented as mean±Standard Deviation (SD). One-way Analysis of Variance (ANOVA) was used for pairwise comparisons among the three groups, and independent t-tests were used for comparisons between two groups. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 22.0 software. Relevant statistical plots were generated using GraphPad Prism 7.0 software. Statistical significance was set at p<0.05.
Results and Discussion
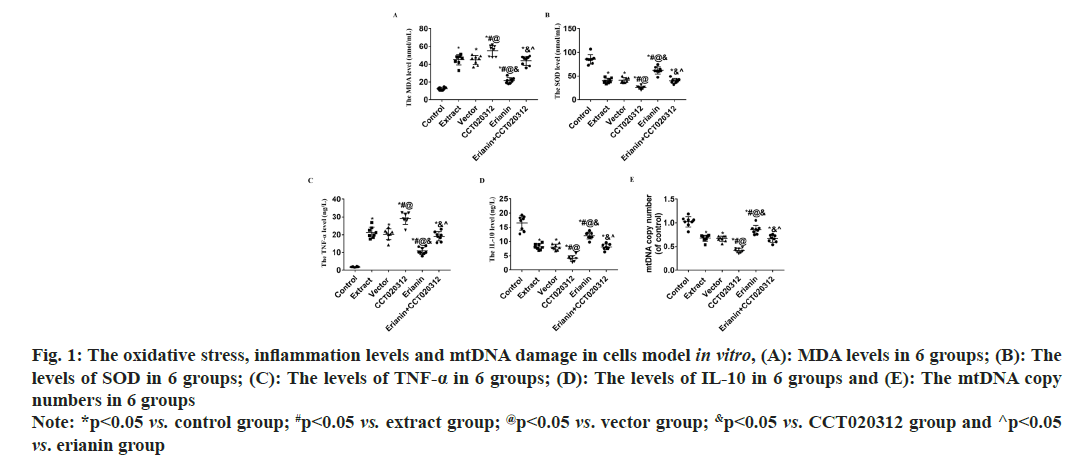
Compared to the control group, the addition of TBI tissue extraction liquid led to a significant increase in MDA levels in the extract, vector and CCT020312 groups (p<0.05), with the CCT020312 group exhibiting a more pronounced increase (p<0.05). Following erianin treatment, the MDA level in the erianin group decreased significantly but remained higher than that of the control group (p<0.05). In the presence of both erianin and CCT020312, the MDA level in the erianin+CCT020312 group was comparable to that of the extract and vector groups (p>0.05). Compared to the control group, the addition of TBI tissue extraction liquid resulted in a significant decrease in SOD levels in the extract, vector and CCT020312 groups, with the CCT020312 group showing a more significant decrease (p<0.05). After erianin treatment, the SOD level in the erianin group increased significantly but remained lower than that of the control group (p<0.05). The SOD levels in the erianin+CCT020312 group, when treated with both erianin and CCT020312, were similar to those of the extract and vector groups (p>0.05) as shown in fig. 1A and fig. 1B.
Fig. 1: The oxidative stress, inflammation levels and mtDNA damage in cells model in vitro, (A): MDA levels in 6 groups; (B): The levels of SOD in 6 groups; (C): The levels of TNF-α in 6 groups; (D): The levels of IL-10 in 6 groups and (E): The mtDNA copy numbers in 6 groups Note: *p<0.05 vs. control group; #p<0.05 vs. extract group; @p<0.05 vs. vector group; &p<0.05 vs. CCT020312 group and ^p<0.05 vs. erianin group
Compared to the control group, the addition of TBI tissue extraction liquid resulted in a significant increase in TNF-α levels in the extract, vector and CCT020312 groups, with the CCT020312 group showing a more pronounced increase (p<0.05). After the addition of TBI tissue extraction liquid and treatment with erianin, the TNF-α level in the erianin group decreased significantly but remained higher than that of the control group (p<0.05). When treated with both erianin and CCT020312 simultaneously, the TNF-α level in the erianin+CCT020312 group was similar to that of the extract and vector groups (p>0.05) as shown in fig. 1C.
Compared to the control group, the addition of TBI tissue extraction liquid led to a significant decrease in IL-10 levels in the extract, vector and CCT020312 groups, with the CCT020312 group demonstrating a more significant decrease (p<0.05). After the addition of TBI tissue extraction liquid and treatment with erianin, the IL-10 level in the erianin group increased significantly but remained lower than that of the control group (p<0.05). When treated with both erianin and CCT020312 simultaneously, the IL-10 level in the erianin+CCT020312 group was similar to that of the extract and vector groups (p>0.05) as shown in fig. 1D.
Comparatively, the addition of TBI tissue extraction liquid significantly reduced the copy number of mtDNA in the extract, vector and CCT020312 groups compared to the control group, with the CCT020312 group showing a more significant reduction (p<0.05). After the application of erianin with the addition of TBI tissue extraction liquid, the erianin group showed a significant increase in mtDNA copy number, but still lower than the control group (p<0.05). When erianin and CCT020312 were used in combination, the erianin+CCT020312 group showed a similar mtDNA copy number to that of the extract and vector groups (p>0.05).
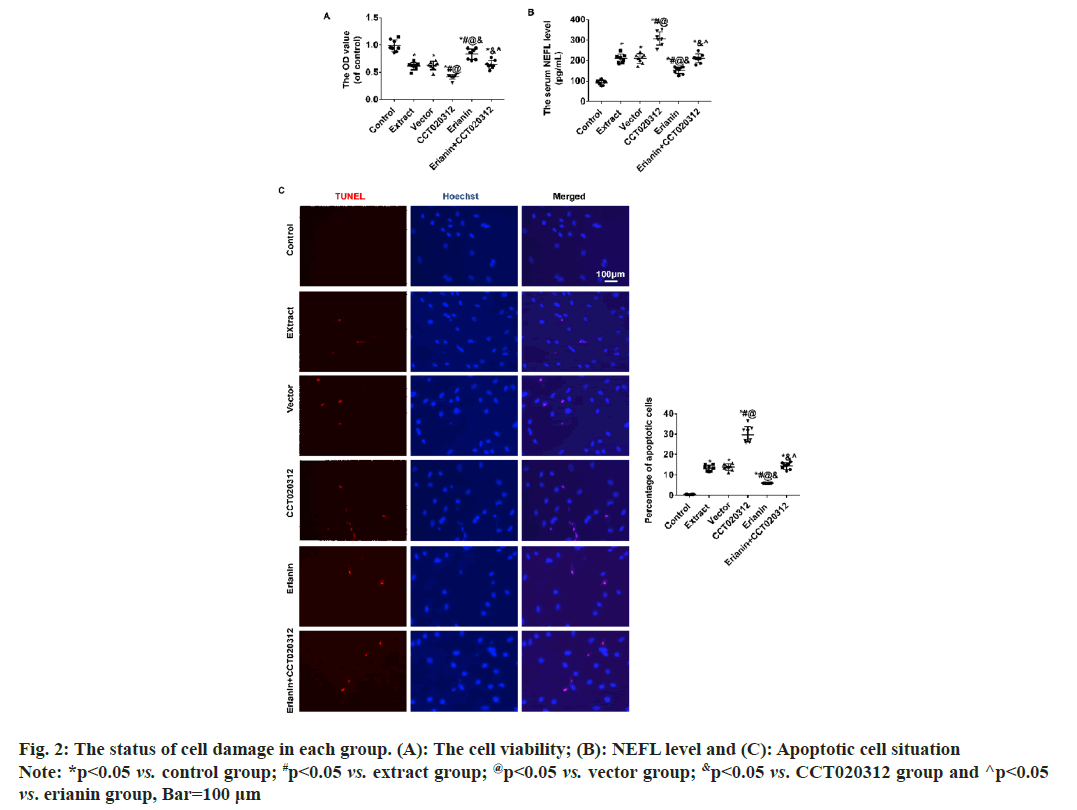
Compared to the control group, the addition of TBI tissue extraction liquid resulted in a significant decrease in cellular activity in the extract, vector and CCT020312 groups. The decrease in the CCT020312 group was particularly noteworthy (p<0.05). Following treatment with erianin, the cellular activity in the erianin group increased significantly but remained lower than that of the control group (p<0.05). When treated with both erianin and CCT020312 simultaneously, the cellular activity in the erianin+CCT020312 group was similar to that of the extract and vector groups (p>0.05) as shown in fig. 2A.
Compared to the control group, the level of Neurofilament Light chain (NEFL) and the percentage of apoptotic cells increased significantly in the extract, vector, and CCT020312 groups when TBI tissue extraction liquid was added. The increase in the CCT020312 group was particularly pronounced (p<0.05). After treatment with erianin, the level of NEFL and the percentage of apoptotic cells in the erianin group decreased significantly but remained higher than those in the control group (p<0.05). When treated with both erianin and CCT020312 simultaneously, the level of NEFL and the percentage of apoptotic cells in the erianin+CCT020312 group were similar to those in the extract and vector groups (p>0.05) as shown in fig. 2B and fig. 2C.
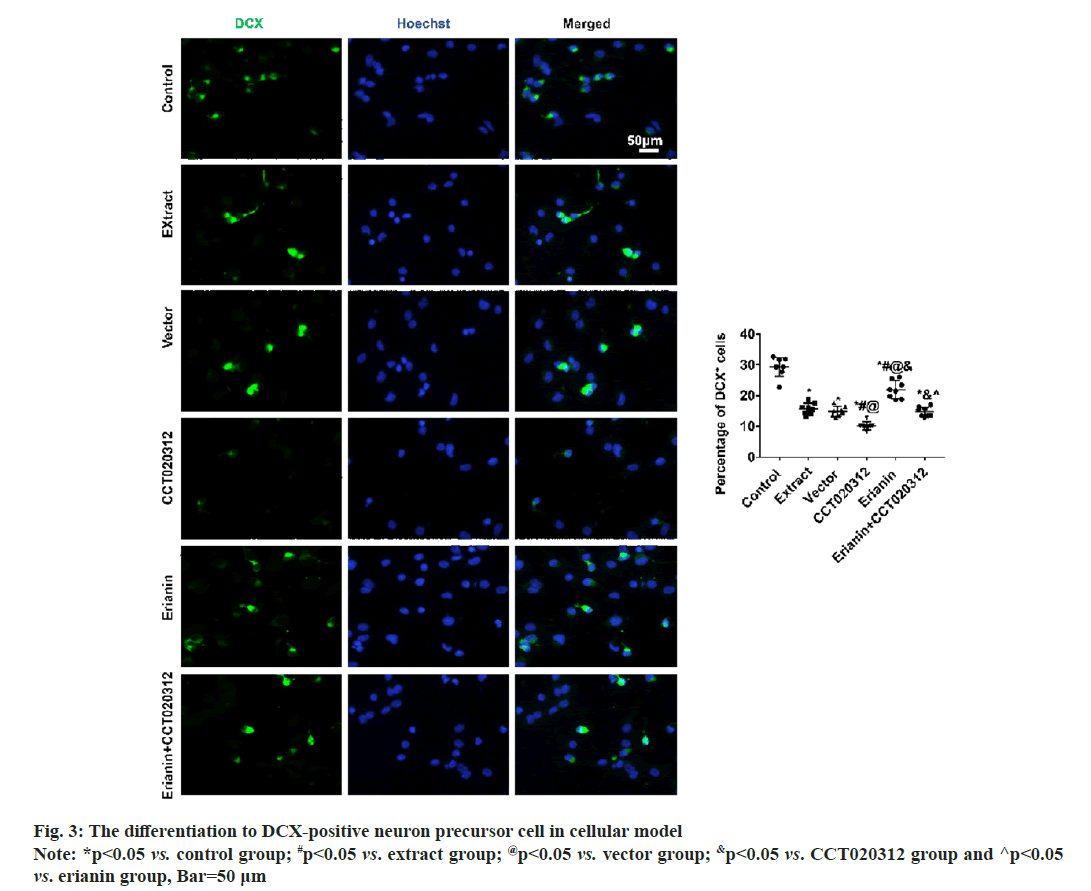
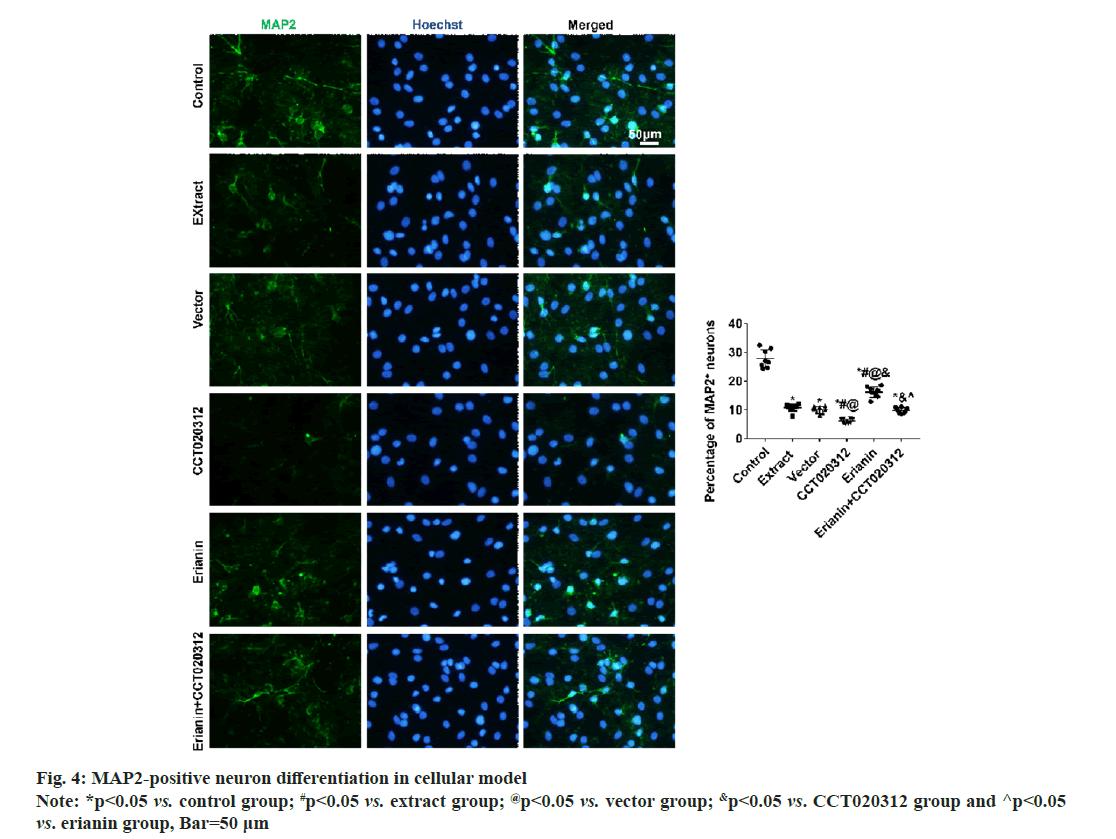
After 3 d and 7 d of cultivation in vitro, immunofluorescence staining for DCX and MAP2 revealed that the percentages of DCX-positive neuronal precursor cells and MAP2-positive neurons in the extract, vector and CCT020312 groups treated with the TBI tissue extraction liquid were significantly reduced compared to the control group, with the CCT020312 group showing a more significant decrease (p<0.05). After the addition of the TBI tissue extraction liquid and treatment with erianin, the percentages of DCX-positive neuronal precursor cells and MAP2-positive neurons in the erianin group significantly increased, but remained lower than those in the control group (p<0.05). Furthermore, when erianin and CCT020312 were applied together, the percentages of DCX-positive neuronal precursor cells and MAP2-positive neurons in the erianin+CCT020312 group were similar to those in the extract group and vector group (p>0.05) as shown in fig. 3 and fig. 4.
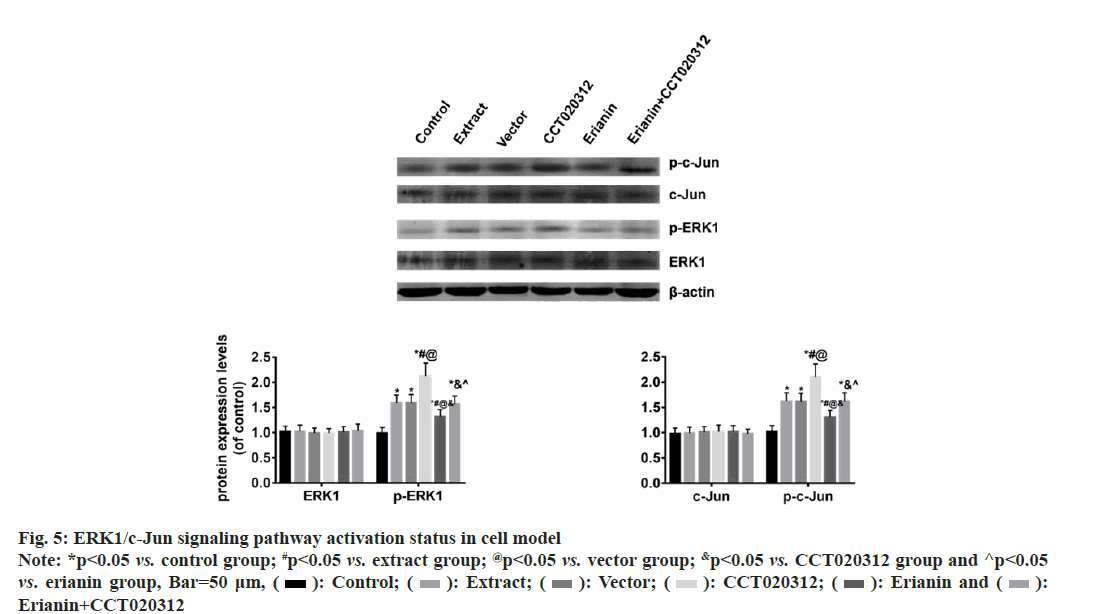
The Western blot results indicated that there were no significant changes in the total levels of ERK1 and c-Jun proteins in each cell group. However, the levels of p-ERK1 and p-c-Jun proteins were significantly increased in the groups treated with TBI tissue extraction liquid, namely the extract, vector and CCT020312 groups, compared to the control group. Notably, the CCT020312 group exhibited a more pronounced increase (p<0.05). Conversely, when erianin was added along with the TBI tissue extraction liquid, the levels of p-ERK1 and p-c-Jun proteins in the erianin group demonstrated a significant reduction; nevertheless, they were still higher than those in the control group (p<0.05). Furthermore, simultaneous treatment with erianin and CCT020312 resulted in levels of p-ERK1 and p-c-Jun proteins in the erianin+CCT020312 group similar to those in the extract and vector groups (p>0.05) as shown in fig. 5.
Fig. 5: ERK1/c-Jun signaling pathway activation status in cell model Note: *p<0.05 vs. control group; #p<0.05 vs. extract group; @p<0.05 vs. vector group; &p<0.05 vs. CCT020312 group and ^p<0.05 vs. erianin group, Bar=50 μm, ( ): Control; (
): Control; ( ): Extract; (
): Extract; ( ): Vector; (
): Vector; ( ): CCT020312; (
): CCT020312; ( ): Erianin and (
): Erianin and ( ):
Erianin+CCT020312
):
Erianin+CCT020312
Following a TBI, there is a significant increase in the level of ROS within the brain, which may persist for several days. It is well-established that ROS, including hydrogen peroxide, superoxide anion, hydroxyl radicals and peroxyl radicals, have the potential to cause irreversible oxidation of macromolecules and inflict cellular damage[17]. The brain tissue, due to its elevated oxidative metabolic activity, comparatively low antioxidant capacity, and inadequate repair mechanisms, is particularly vulnerable to oxidative harm. In the context of TBI, ROS production can occur through mitochondrial leakage, the arachidonic acid cascade, and catecholamine oxidation[18]. Oxidative damage primarily manifests as lipid peroxidation affecting neuronal, glial and vascular cell membranes, as well as myelin phospholipids. Therefore, the level of MDA serves as a reliable indicator of the extent of oxidative stress.
Within moments of experiencing a TBI, a potent and sterile immune response is triggered. This immune response is characterized by the release of danger signals from neurons and glial cells, activation of resident innate immune cells, recruitment and infiltration of peripheral immune cells, and the secretion of various inflammatory molecules[19]. Different cell factors secreted within the CNS have pro-inflammatory effects following TBI. Notably, the levels of IL-1β and TNF-α, well-known pro-inflammatory cytokines, are elevated in both humans and animals after TBI[20]. ROS play a role in activating transcription factors involved in redox regulation, such as Mitogen- Activated Protein Kinases (MAPK), Nuclear Factor Kappa B (NF-κB), and Activator Protein 1 (AP-1), thereby contributing to the inflammatory process[21]. Additionally, damaged neurons, meninges, and the Blood-Brain Barrier (BBB) release Damage-Associated Molecular Patterns (DAMPs) that selectively bind to corresponding receptors. This binding event subsequently triggers a series of cascades involving NF-κB in activated B cells, MAPK, and ERK. Consequently, various chemokines and cytokines are released, leading to the amplification of the inflammatory response[22,23].
Therefore, addressing the aforementioned micro environmental pressures faced by neuro regeneration has attracted increasing attention in promoting the differentiation of endogenous NSCs into mature neurons. Extensive efforts have been made in the search for effective drugs to promote adult neuro regeneration, representing potential targets for treating neurological disorders. Modern medicine is predominantly driven by phase I, II, III and even IV clinical trials, for centuries, Traditional Chinese Medicine (TCM) has been utilized not only in China but also in other Asian countries like Korea and Japan, with herbal remedies tested on humans for thousands of years, rather than on animals, and their specific effects have been well-documented. Of all the drugs approved by the Food and drug Administration (FDA), 25 % are from Chinese Herbal Medicine (CHM)[24]. Erianin is a type of CHM, in which protective effects have been observed in multiple diseases. However, whether erianin can promote neuronal differentiation after TBI remains unclear. Therefore, we first extracted the injured brain tissue extract and added it to the NSCs during the process of neuronal differentiation to simulate the in vivo microenvironment and establish an in vitro cell injury model. Based on this, we applied erianin for intervention treatment. Firstly, we observed the levels of oxidative stress and inflammation in the in vitro cell model. To avoid interference from the injury brain tissue extract on the detection results, we discarded the culture medium and directly tested the cells. The results showed that the addition of the injury brain tissue extract significantly increased the levels of MDA and TNF-α in the cells, while decreasing the levels of SOD and IL-10. If erianin was simultaneously added, the levels of MDA and TNF-α in the cells decreased significantly, while the levels of SOD and IL-10 increased significantly, although they did not reach the levels of the control group. These inflammatory factors may mainly originate from the differentiated glial cells in the culture. Astrocytes are widely distributed in the CNS and perform various functions. After brain injury, the phenotype of astrocytes undergoes changes, and reactive astrocytes exhibit an inflammatory phenotype in CNS injuries and diseases[25-27]. Whether erianin has an effect on reactive astrocytes remains to be further investigated in our subsequent research. We also observed mitochondrial damage in cell models during in vitro experiments. The results indicated that the addition of injured brain tissue extract significantly decreased the level of mtDNA copies in cells, while the co-addition of erianin partially restored the level of mtDNA copies. The degree of tissue damage and mitochondrial dysfunction following TBI injury may be linked to the severity of ROS-related damage[28]. Mitochondrial damage and genetic changes occur when heightened ROS levels surpass the capability of the mitochondrial redox homeostasis system to neutralize them[29]. The resulting mitochondrial shape damage after TBI ultimately causes a collapse in cellular bioenergy and cell death[30]. We also observed neuronal damage in cell models during our study. The results showed that the addition of injured brain tissue extract increased the level of NEFL in cells and significantly elevated the percentage of apoptotic cells. However, the co-addition of erianin decreased the level of NEFL in cells and significantly reduced the percentage of apoptotic cells, although it did not fully restore them to the level of the control group. In our in vitro experiments, we used immunofluorescence staining for DCX and MAP2 to detect neuronal differentiation in cell models. The results indicated that the addition of injured brain tissue extract significantly decreased the percentages of DCX-positive neuronal progenitor cells and MAP2-positive neurons. However, the co-addition of erianin significantly restored the percentages of DCX-positive neuronal progenitor cells and MAP2-positive neurons. These findings suggest that erianin promotes the differentiation of NSCs into mature neurons. To investigate whether erianin exerts its effects by inhibiting the ERK/c- Jun signaling pathway, we conducted in vitro cell experiments. In the cell injury model, we added erianin intervention along with the activation agent of the ERK signaling pathway, CCT020312. The results showed that when erianin was added alone in the cell injury model, the protein levels of p-ERK1 and p-c-Jun were significantly reduced. However, when CCT020312 was added simultaneously, the protein levels of p-ERK1 and p-c-Jun in the cells were significantly increased. Moreover, the previously observed antioxidant, anti-inflammatory, neuroprotective and promotion of NSCs differentiation into neurons effects of erianin were partially reversed. Furthermore, adding CCT020312 together with the injured brain tissue extract worsened the extent of cell damage. These findings further suggest that erianin may exert its aforementioned effects by inhibiting the ERK/c-Jun signaling pathway.
In summary, erianin can inhibit oxidative stress, inflammatory response, and mtDNA damage in an in vitro cell injury model. It can also partially promote the recovery from oxidative stress, high inflammation levels, and mtDNA damage, protecting cells from apoptosis. Erianin exerts its neuroprotective effects through antioxidative and anti-inflammatory mechanisms, promoting the differentiation of NSCs into neurons. CCT020312, the activator of the ERK signaling pathway, can block this effect, suggesting that the neuroprotective properties of erianin could be linked to its suppression of the ERK1/c-Jun signaling pathway.
Ethical approval:
This study was approved by the Research Ethics Committee of People’s Hospital of Hai’an.
Authors' contributions:
The experimental design was conducted by Liang Gao, while Qingquan Li, Xiaokui Gan, Ming Zhang and Guangmin Zhang performed the experiments. The data analysis phase was carried out by Yingbin Li, and the paper was authored by Qingquan Li. All the authors have thoroughly reviewed and approved the final draft of the manuscript to ensure its accuracy.
Conflict of interests:
The authors declared no conflict of interests.
References
- Taylor SR, Smith C, Harris BT, Costine BA, Duhaime AC. Maturation-dependent response of neurogenesis after traumatic brain injury in children. J Neurosurg Pediatr 2013;12(6):545-54.
[Crossref] [Google Scholar] [PubMed]
- Zheng W, ZhuGe Q, Zhong M, Chen G, Shao B, Wang H, et al. Neurogenesis in adult human brain after traumatic brain injury. J Neurotrauma 2013;30(22):1872-880.
- Yi X, Jin G, Zhang X, Mao W, Li H, Qin J, et al. Cortical endogenic neural regeneration of adult rat after traumatic brain injury. PloS One 2013;8(7):e70306.
[Crossref] [Google Scholar] [PubMed]
- Sgubin D, Aztiria E, Perin A, Longatti P, Leanza G. Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J Neurosci Res 2007;85(8):1647-55.
[Crossref] [Google Scholar] [PubMed]
- Dorsett CR, McGuire JL, dePasquale EA, Gardner AE, Floyd CL, McCullumsmith RE. Glutamate neurotransmission in rodent models of traumatic brain injury. J Neurotrauma 2017;34(2):263-72.
[Crossref] [Google Scholar] [PubMed]
- Wu AG, Yong YY, Pan YR, Zhang L, Wu JM, Zhang Y, et al. Targeting Nrf2-mediated oxidative stress response in traumatic brain injury: Therapeutic perspectives of phytochemicals. Oxid Med Cell Long 2022;2022:e34009.
[Crossref] [Google Scholar] [PubMed]
- Zhao Q, Li H, Li H, Xie F, Zhang J. Research progress of neuroinflammation-related cells in traumatic brain injury: A review. Medicine 2023;102(25):e34009.
[Crossref] [Google Scholar] [PubMed]
- Denniss RJ, Barker LA. Brain trauma and the secondary cascade in humans: Review of the potential role of vitamins in reparative processes and functional outcome. Behavioral Sci 2023;13(5):388.
[Crossref] [Google Scholar] [PubMed]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J 2012;5(1):9-19.
[Crossref] [Google Scholar] [PubMed]
- Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell 2010;140(6):771-6.
[Crossref] [Google Scholar] [PubMed]
- Chen MF, Liou SS, Kao ST, Liu IM. Erianin protects against high glucose-induced oxidative injury in renal tubular epithelial cells. Food Chem Toxicol 2019;126:97-105.
[Crossref] [Google Scholar] [PubMed]
- Dou B, Hu W, Song M, Lee RJ, Zhang X, Wang D. Anti-inflammation of Erianin in dextran sulphate sodium-induced ulcerative colitis mice model via collaborative regulation of TLR4 and STAT3. Chem Biol Interact 2020;324:109089.
[Crossref] [Google Scholar] [PubMed]
- Zhang T, Ouyang H, Mei X, Lu B, Yu Z, Chen K, et al. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2–NF-κB signaling pathway. FASEB J 2019;33(11):11776-90.
[Google Scholar] [PubMed]
- Lv J, Wang Z, Liu H. Erianin suppressed lung cancer stemness and chemotherapeutic sensitivity via triggering ferroptosis. Environ Toxicol 2023.
[Crossref] [Google Scholar] [PubMed]
- Yang Z, Liu R, Qiu M, Mei H, Hao J, Song T, et al. The roles of ERIANIN in tumor and innate immunity and its’ perspectives in immunotherapy. Front Immunol 2023;14:1170754.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhang Q, Wei F, Liu N. Progressive study of effects of erianin on anticancer activity. Onco Targets Ther 2019;12:5457.
[Crossref] [Google Scholar] [PubMed]
- Hakiminia B, Alikiaii B, Khorvash F, Mousavi S. Oxidative stress and mitochondrial dysfunction following traumatic brain injury: From mechanistic view to targeted therapeutic opportunities. Fundam Clin Pharmacol 2022;36(4):612-62.
[Crossref] [Google Scholar] [PubMed]
- Ismail H, Shakkour Z, Tabet M, Abdelhady S, Kobaisi A, Abedi R, et al. Traumatic brain injury: Oxidative stress and novel anti-oxidants such as mitoquinone and edaravone. Antioxidants 2020;9(10):943.
[Crossref] [Google Scholar] [PubMed]
- Lee H, Lee S, Cho IH, Joong Lee S. Toll-like receptors: Sensor molecules for detecting damage to the nervous system. Curr Protein Peptide Sci 2013;14(1):33-42.
[Crossref] [Google Scholar] [PubMed]
- Kumar RG, DiSanto D, Awan N, Vaughan LE, Levochkina MS, Weppner JL, et al. Temporal acute serum estradiol and tumor necrosis factor-α associations and risk of death after severe traumatic brain injury. J Neurotrauma 2020;37(20):2198-210.
[Crossref] [Google Scholar] [PubMed]
- Parsons AL, Bucknor EM, Castroflorio E, Soares TR, Oliver PL, Rial D. The interconnected mechanisms of oxidative stress and neuroinflammation in epilepsy. Antioxidants 2022;11(1):157.
[Crossref] [Google Scholar] [PubMed]
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem 2010;114(1):13-27.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Jin H, Tan H, Cai X, Sun Y. Erythrocyte-derived extracellular vesicles aggravate inflammation by promoting the proinflammatory macrophage phenotype through TLR4–MyD88–NF-κB–MAPK pathway. J Leukocyte Biol 2022;112(4):693-706.
[Crossref] [Google Scholar] [PubMed]
- Najmi A, Javed SA, Al Bratty M, Alhazmi HA. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 2022;27(2):349.
[Crossref] [Google Scholar] [PubMed]
- Hasel P, Rose IV, Sadick JS, Kim RD, Liddelow SA. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci 2021;24(10):1475-87.
- Kihara Y, Jonnalagadda D, Zhu Y, Ray M, Ngo T, Palmer C, et al. Ponesimod inhibits astrocyte-mediated neuroinflammation and protects against cingulum demyelination via S1P1-selective modulation. FASEB J 2022;36(2):e22132.
[Crossref] [Google Scholar] [PubMed]
- Borggrewe M, Grit C, Vainchtein ID, Brouwer N, Wesseling EM, Laman JD, et al. Regionally diverse astrocyte subtypes and their heterogeneous response to EAE. Glia 2021;69(5):1140-54.
[Crossref] [Google Scholar] [PubMed]
- Li J, Wang X, Qin S. Molecular mechanisms and signaling pathways of reactive astrocytes responding to traumatic brain injury. Histol Histopathol 2021;36(9):921-9.
[Crossref] [Google Scholar] [PubMed]
- Gupta R, Saha P, Sen T, Sen N. An augmentation in histone dimethylation at lysine nine residues elicits vision impairment following traumatic brain injury. Free Radical Biol Med 2019;134:630-43.
[Crossref] [Google Scholar] [PubMed]
- Tan HP, Guo Q, Hua G, Chen JX, Liang JC. Inhibition of endoplasmic reticulum stress alleviates secondary injury after traumatic brain injury. Neural Regen Res 2018;13(5):827-36.
[Crossref] [Google Scholar] [PubMed]