- *Corresponding Author:
- Vladimir Biocanin
Faculty of Stomatology in Pancevo, University Business Academy, Pancevo, Zarka Zrenjanina 13000, Serbia
E-mail: vladimirbiocanin@gmail.com
| Date of Received | 24 March 2025 |
| Date of Revision | 26 March 2025 |
| Date of Acceptance | 28 April 2025 |
| Indian J Pharm Sci 2025;87(2):88-93 |
Abstract
Medicinal plants contain various phytochemicals, some of which prevent or can be used in the treatment of many diseases, including cancer. Therefore, they have a significant role not only in the traditional ethno medicine, but also in the healthcare system of a large number of the world’s population. The aim of this study was to investigate and evaluate possible Antiproliferative potential of ethanoic extracts of Origanum vulgare, Centaurium erythraea, Salvia officinalis, Achillea millefolium, Hypericum perforatum and Chelidonium majus on human cervix adenocarcinoma (HeLa) and human myeloid leukemia (K562) cancer cell lines. The stock solutions of investigated extracts were prepared in ethanol at concentration of 1 mg/ml and diluted with complete nutrient medium Roswell Park Memorial Institute 1640. The medium was supplemented with 3 mm L-glutamine, 100 μg/ml streptomycin, 100 IU/ml penicillin, 10 % heat-inactivated fetal bovine serum, and 25 mM Hepes, adjusted to pH 7.2. Cell survival was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide assay 72 h post-treatment. The half-maximal inhibitory concentration values were calculated using a dose-response growth curve. With regard to all investigated extracts, the HeLa cell line was more resistant than K562 cell line. Origanum vulgare and Salvia officinalis/Achillea millefolium extracts exhibited higher cytotoxicity towards K562 cells (half-maximal inhibitory concentration 0.345 μg/ml and half-maximal inhibitory concentration 0.88 μg/ml, respectively) compared to HeLa cells (half-maximal inhibitory concentration 0.795 μg/ml and half-maximal inhibitory concentration 0.975 μg/ml, respectively). The extracts of Centaurium erythraea, Hypericum perforatum and Chelidonium majus expressed no active Antiproliferative (cytotoxic) effects on HeLa and K562 cancer cell lines. The active Antiproliferative effects of Origanum and of Salvia/Achillea extracts suggest that these extracts could be recommended as a good source of biologically active compounds with potential benefit effects.
Keywords
Origanum vulgare, Centaurium erythraea, Salvia officinalis, Achillea millefolium, Hypericum perforatum, Chelidonium majus, Antiproliferative effect, cytotoxicity, Hela cells, K562 cells
Some oncological diseases may significantly impact individuals, especially as they are experienced over life-long time-periods[1]. Chronic Myeloid Leukemia (CML) is a type of a cancer that starts in certain blood-forming cells of the bone marrow. The only risk factors for CML are radiation exposure, age and gender. Targeted therapy drugs are the main treatment for CML. However, there are other options: Interferon therapy, chemotherapy, radiation therapy, surgery or stem cell transplant[2]. Adenocarcinoma of the cervix starts in the glandular cells of the endocervix. It is a less common type of cervical cancer, but it may be more aggressive than other types of cervical cancer. Treatment can include radiation therapy and surgery[3]. In most cases, treatments lead to unpleasant side effects. This has encouraged researchers to focus on medicinal plants as natural products with fewer side effects and with the ability to induce apoptosis in cancer cells, but not in healthy cells[4,5]. Some of the plants that are the most commonly used in Serbian traditional medicine and in which people have great confidence are Achillea millefolium (A. millefolium), Hypericum perforatum (H. perforatum) and Salvia officinalis (S. officinalis). Serbia is classified as one of 158 world centres of biodiversity[6]. Medicinal plants synthesize terpenoids, simple phenols, phenolic acids, flavonoids and alkaloids as secondary metabolites[7]. Because of their antimicrobial, antioxidant, anticancer and antiinflammatory activities, in some parts of the world, more than 60 % of cancer patients use medicinal plants as therapy[8]. Flavonoids and alkaloids achieve antitumor effects by using different mechanisms, such as: Free radicals scavenging, inhibition of enzymes involved in reactive oxygen species formation, prevention of cellular and extracellular compounds oxidation and intranucleosomal Deoxyribonucleic Acid (DNA) fragmentation[9,10]. In this study, we investigated the potential Antiproliferative effects of ethanolic extracts of five different medicinal plants (Origanum vulgare (O. vulgare), Centaurium erythraea (C. erythraea), S. officinalis, A. millefolium, H. perforatum and Chelidonium majus (C. majus)) on cell lines derived from human cervix adenocarcinoma (HeLa) cells and human myeloid leukemia (K562) cells.
The stock solution of each extract was prepared in ethanol at a concentration of 1 mg/ml and then diluted with complete nutrient medium (Roswell Park Memorial Institute 1640 (RPMI-1640)) supplemented with 3 mM L-glutamine, 100 μg/ml streptomycin, 100 IU/ml penicillin, 10 % heatinactivated Fetal Bovine Serum (FBS) and 25 mM Hepes, and adjusted to pH 7.2 by a bicarbonate solution. Human cervix adenocarcinoma HeLa cells were cultured as monolayers in nutrient medium, while human myeloid leukemia K562 cells were maintained as a suspension culture. The cells were grown at 37° in 5 % Carbon Dioxide (CO2) and a humidified air atmosphere.
HeLa cells (2.500 cells per well) were seeded into 96-well microtiter plates and 20 h later, after cell adherence, five different concentrations of the extract were added to the wells. Final concentrations were in the range from 5 to 80 μg/ml. Only nutrient medium was added to the cells in the control wells. The investigated extract was added to a suspension of leukemia K562 cells (5.000 cells per well) 2 h after cell seeding, in the final concentrations from 2.5 to 40 μg/ml. Nutrient medium with the corresponding concentrations of compounds, but devoid of cells was used as a blank.
Cell survival was determined by the 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide Assay (MTT) test 72 h after the investigated extract was added[11,12]. Briefly, 20 μl of MTT solution (5 mg/ml in phosphate-buffered saline) was added to each well. Samples were incubated for a further four hours at 37° under a humidified atmosphere with 5 % CO2. Then, 100 μl of 10 % Sodium Dodecyl Sulfate (SDS) was added to the wells. Absorbance was measured at 570 nm the next day. To achieve cell survival (%), absorbance at 570 nm of a sample was divided by the absorbance of the control sample (the absorbance of cells grown only in nutrient medium), after subtraction of absorption of the blank. Concentrations of the extract which induced a 50 % decrease in malignant and normal cell survival (half-maximal inhibitory concentration (IC50) values) were calculated from a dose-response growth curve using Microsoft® Excel® 2019 software.
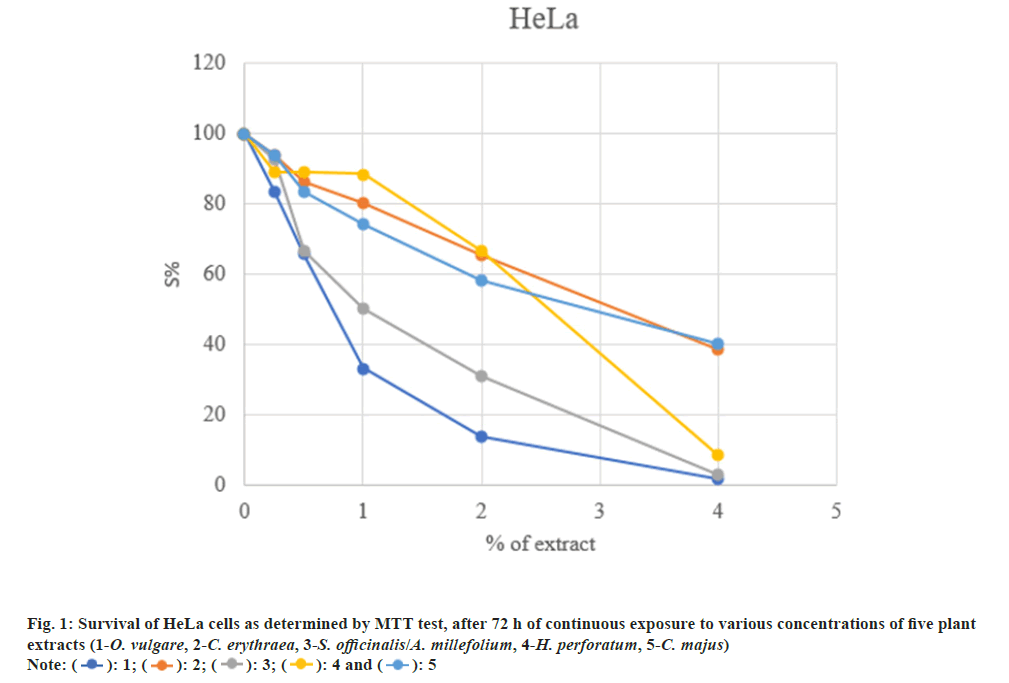
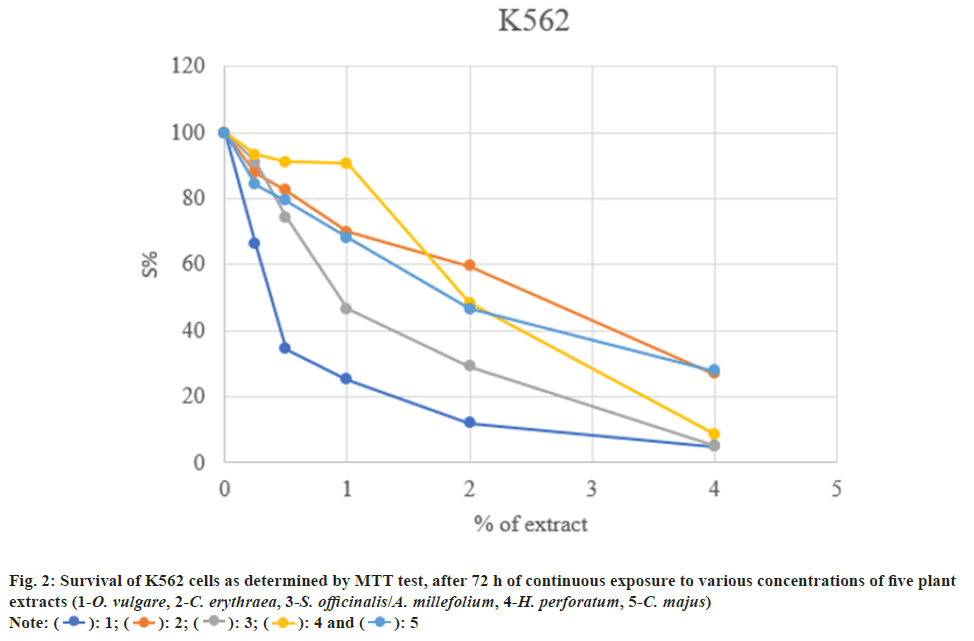
The cytotoxicity of five plant extracts was tested on selected cancer cell lines: Human cervix adenocarcinoma (HeLa) and human myeloid leukemia (K562). Concentrations of the ethanol extracts which induced IC50 are presented in Table 1.
| Tincture | IC50 (µg/ml) Av±SD | |
|---|---|---|
| HeLa | K562 | |
| O. vulgare | 0.795±0.055 | 0.345±0.035 |
| C. erythraea | 3.57±0.43 | 2.71±0.13 |
| S. officinalis/A. millefolium | 0.975±0.035 | 0.88±0.06 |
| H. perforatum | 2.38±0.19 | 1.88±0.08 |
| C. majus | 3.02±0.10 | 1.74±0.10 |
Table 1: Concentrations of the Ethanol Extracts Which Induced A 50 % Decrease (IC50) in Malignant Cells Survival
The examined extracts possess high to moderate cytotoxicity on the investigated tumor cells. The highest cytotoxicity was found in K562 cells (IC50=0.345 μg/ml), and somewhat weaker towards HeLa cells (IC50=3.57 μg/ml) (fig. 1 and fig. 2).
Considering that about 15 % of all new cases of leukemia are CML, some predictions estimate that about one person in 526 will get CML in their life time in the United States[2]. In 2022, an estimated 660 000 women were diagnosed with cervical cancer worldwide and about 350 000 women died from the disease[13]. In a questionnaries, conducted by Markovi? et al.[14] the informants from South-eastern Serbia have reported (4.13 %) to prepare medicinal plants in a form of alcohol extracts. For example, H. perforatum and A. millefolium are frequently used for treating digestive and reproductive group of ailments. C. majus is used to treat cancerous, while C. erythraea is used to treat endocrine diseases. S. officinalis is used to treat infectious group of ailments. Due to the presence of some compounds such as flavonoid and phenolic acid derivatives, Origano species have been related to the ability to inhibit the proliferation of some cancer cells[15]. Among three oregano species, the highest content of total phenols was found in Lippia graveolens (L. graveolens), followed by Luigi palmieri (L. palmieri) and Hedeoma patens which presented the lowest content. L. graveolens significantly inhibited the proliferation of breast cancer cell lines (Michigan Cancer Foundation-7 (MCF-7) and Metastatic Breast Cancer-231 (MDAMB-231)). A remarkable decrease in cell proliferation was observed on the MDA-MB-231 cells at 24 h of treatment with 150 μg/ml of the extract in comparison with MCF-7 cells, where the inhibitory effect was observed up to 72 h of treatment. Quercetin-3-O-hexoside was the only compound identified in this Oregano species, compared to the other two analysed[16]. Similarlly, quercetin and baicalein, found in polyphenol-rich extracts of L. graveolens, inhibited the growth of breast cancer cells[17]. Carvacrol as monoterpenoid phenol was found at high percentage (90.4 %) in O. vulgare essential oil, which water extract displayed outstanding activity against hepatocellular carcinoma (IC50 27.2 μg/ml) and human breast adenocarcinoma (IC50 10.8 μg/ml) cell lines[18]. Results of this study showed that K562 cell line proved to be more sensitive to the effect of O. vulgare extract compared to the HeLa cell line (IC50 0.345 μg/ml vs. IC50 0.795 μg/ml, respectively). This extract expressed active Antiproliferative effect. Phenolic acids (rosmarinic and p-coumaric) were the predominant compounds detected in all samples of different C. erythraea extracts[19]. Although extracts of Centaurium erythraea (C. erythraea) presented a high ferric reductive capacity and high capacities to Scavenage the radical cation, none of them showed Antiproliferative activity towards a variety of cancerous cell lines (IC50>200 μg/ml)[20]. With regard to C. erythraea extract, the HeLa cell line was the most resistant (IC50 3.57 μg/ml) in this study. At a concentration of around 2.71 μg/ml, the extract inhibited K562 cell survival by 50 %. So far, there is no data on the Antiproliferative effect of S. officinalis. Although photometric determination showed higher percent of phenols in S. officinalis than in Echinacea angustifolia (E. angustifolia) (12.5 % vs. 9.07 %), S. officinalis extract manifested a slightly weaker cytotoxic activity (IC50 122.22 μg/ml) on cell lines derived from human cervix adenocarcinoma (HeLa) cells compared to E. angustifolia (IC50 43.52 μg/ml) and Mellisa officinalis (IC50 70.41 μg/ml). That could mean, that some other chemical constituents may be important for Antiproliferative effects[21]. However, some authors noted that variability between phenolic and flavonoid amounts depends on the plant extracts and the used solvents[20]. Spectrophotometrically and chromatographically characterization of different A. millefolium extracts corroborated the presence of various polyphenols, among which phenolic acids (chlorogenic acid, isomers of dicaffeoylquinic acids), flavonoids (luteolin, apigenin) and their corresponding glucosides dominated[22]. Some authors revealed the capacity of A. millefolium aqueous extract to selectively inhibit PC-3 human prostate cancer cells in a time and concentration dependent fashion[23]. Also, hydroalcoholic extract of some other Achillea species, at concentration of 25 μg/ml, expressed cytotoxicity towards the MCF-7 and MDA-Mb-468 human breast adenocarcinoma cell lines[24]. However, Kongul et al.[25] were the only who showed the antitumor potential of A. millefolium aqueous extract (IC50 30.67 mg/ml) on MCF7 human breast cancer cell line. In the present investigation, treatment of HeLa and K562 cell lines with the mixture of S. officinalis and A. millefolium extracts, resulted in a similar sensitivity of both cell lines (IC50 0.975 μg/ml and IC50 0.88 μg/ml, respectively) and in a active Antiproliferative effect. Hypericum contains various polyphenols: Flavonoids (quercetin), phenolic acids (chlorogenic acid), various naphtodianthrones (hypericin, pseudohypericin) and phloroglucinols (hyperforin). Hypericin, pseudohypericin and hyperforin are considered to be biologically active compounds[26,27]. Previous investigations found crude extracts (100 μg/ml) of Hypericum caprifoliatum, Gymnanthemum myrianthum and to a lesser extent Hypocalymma connatum to be the most active on HT-29 human colon carcinoma cells and H-460 nonsmall cell lung carcinoma[28]. Recent investigation demonstrated antiproliferative effect of H. perforatum extract against PC-3 human prostate cancer cells in a concentration and time dependent manner (IC50 0.60 mg/ml)[23]. In contrast, H. perforatum extract did not show Antiproliferative effect against HeLa cells (IC50>200 μg/ml). That could be explained by the use of different Hypericum species and different human cancer lines[29]. Similarly to the mentioned study, this work demonstrated the absence of active antiproliferatve effect of H. perforatum extract on HeLa and K562 cell lines (IC50 2.38 μg/ml and IC50 1.88 μg/ml, respectively). Apart polyphenols, C. majus contains a vast array of alkaloids (chelidonine, homochelidonine, chelerytrine, sanguinarine, berberine, coptizine, protopine) which induce cytotoxic effect in a wide range of cancer cell lines such as: prostate, breast, lung, liver and colon[30-32]. With regard to chelilutine, another alkaloid found in C. majus, human promyelocytic leukemia HL-60 cell line was least resistant (IC50 0.16 μg/ml), while human ovarian carcinoma A-2780 and human cervix adenocarcinoma HeLa cell lines exhibited sensitivities of IC50=0.45 and 0.84 μg/ml, respectively[10]. In this work, compared to K562 cells (IC50 1.74 μg/ml), the IC50 value of C. majus extract on HeLa cells is about two times higher and amounts 3.02 μg/ml. The differences in obtained results might be due to different protocols, cell culture conditions or difference in the composition of alkaloids[30]. In this study, the most sensitive cell line was K562. The moderate antiproliferative effect of most investigated extracts on HeLa and K562 cell lines is perhaps a consequence of using a low concentrations of active compounds, inadequate solvent or because the plants were collected in the autumn.
The active antiproliferative effects of Origanum and of Salvia/Achillea extracts found in this study suggest that these extracts could be recommended as adjunctive treatment of human cervix adenocarcinoma and human myeloid leukemia.
Conflict of interests:
The authors declared no conflict of interests.
References
- Hewison A, Roman E, Smith A, McCaughan D, Sheridan R, Patmore R, et al. Chronic myeloid leukaemia: A qualitative interview study exploring disease impact from patient and practitioner perspectives. Eur J Oncol Nurs 2023;67:102421.
[Crossref] [Google Scholar] [PubMed]
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: A review. JAMA 2025.
[Crossref] [Google Scholar] [PubMed]
- Priya S, Kumar MA. A review on cervical cancer and current preventive measures. Res J Pharm Technol 2019;12(11):5641-5.
- Kandelous HM, Salimi M, Khori V, Rastkari N, Amanzadeh A, Salimi M. Mitochondrial apoptosis induced by Chamaemelum nobile extract in breast cancer cells. Iran J Pharm Res 2016;15(Suppl):197-204.
[Google Scholar] [PubMed]
- Cao XH, Zhao SS, Liu DY, Wang Z, Niu LL, Hou LH, Wang CL. ROS-Ca2+ is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis. Chem Biol Interact 2011;190(1):16-27.
[Crossref] [Google Scholar] [PubMed]
- Radovanovi? K, Gavari? N, A?imovi? M. Anti-inflammatory properties of plants from Serbian traditional medicine. Life 2023;13(4):874.
[Crossref] [Google Scholar] [PubMed]
- Khalid EB, Ayman EM, Rahman H, Abdelkarim G, Najda A. Natural products against cancer angiogenesis. Tumor Biol 2016;37:14513-36.
[Crossref] [Google Scholar] [PubMed]
- Madhuri S, Pandey G. Some anticancer medicinal plants of foreign origin. Current science. 2009;96:779-83.
- Han N, Bakovic M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J Bioanal Biomed S 2015;12(005):1948-59.
- Slunská Z, Gelnarová E, Hammerová J, Táborská E, Slaninová I. Effect of quaternary benzo (c) phenanthridine alkaloids sanguilutine and chelilutine on normal and cancer cells. Toxicol In Vitro 2010;24(3):697-706.
[Crossref] [Google Scholar] [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Method 1983;65(1-2):55-63.
[Crossref] [Google Scholar] [PubMed]
- Ohno M, Abe T. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J Immunol Method 1991;145(1-2):199-203.
[Crossref] [Google Scholar] [PubMed]
- Chidebe RC, Osayi A, Torode JS. The Global Fund, Cervical Cancer, and HPV infections: What can low-and middle-income countries do to accelerate progress by 2030? Eclin Med 2025;81.
- Markovi? MS, Pljevljakuši? DS, Mateji? JS, Nikoli? BM, Zlatkovi? BK, Rakonjac LB, et al. Traditional uses of medicinal plants in Pirot District (southeastern Serbia). Genet Resources Crop Evolu 2024;71(3):1201-20.
- Marrelli M, Cristaldi B, Menichini F, Conforti F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem Toxicol 2015;86:16-24.
[Crossref] [Google Scholar] [PubMed]
- Criollo-Mendoza MS, Ramos-Payán R, Contreras-Angulo LA, Gutiérrez-Grijalva EP, León-Félix J, Villicaña C, et al. Cytotoxic activity of polyphenol extracts from three oregano species: Hedeoma patens, Lippia graveolens and Lippia palmeri, and antiproliferative potential of Lippia graveolens against two types of breast cancer cell lines (MDA-MB-231 and MCF-7). Molecules 2022;27(16):5240.
[Crossref] [Google Scholar] [PubMed]
- Kapinova A, Kubatka P, Golubnitschaja O, Kello M, Zubor P, Solar P, et al. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ Health Prev Med 2018;23:1-8.
[Crossref] [Google Scholar] [PubMed]
- Erenler R, Çarl?k ÜE, Ayd?n A. Antiproliferative activity and cytotoxic effect of essential oil and water extract from Origanum vulgare L. Sigma J Eng Nat Sci 2023;41(1):202-8.
- Mihaylova D, Vrancheva R, Popova A. Phytochemical profile and in vitro antioxidant activity of Centaurium erythraea Rafn. Bulgarian Chem Commun 2019;51(6).
- Bouyahya A, Bakri Y, Et-Touys A, Assemian IC, Abrini J, Dakka N. In vitro antiproliferative activity of selected medicinal plants from the North-West of Morocco on several cancer cell lines. Eur J Integr Med 2018;18:23-9.
- Ceni?-Miloševi? D, Tambur Z, Bokonji? D, Ivan?aji? S, Stanojkovi? T, Grozdani? N, et al. Antiproliferative effects of some medicinal plants on HeLa cells. Arch Biol Sci 2013;65(1):65-70.
- Ivanovi? M, Gruji? D, Cerar J, Islam?evi? Razboršek M, Topali?-Trivunovi? L, Savi? A, et al. Extraction of bioactive metabolites from Achillea millefolium L. with choline chloride based natural deep eutectic solvents: A study of the antioxidant and antimicrobial activity. Antioxidants 2022;11(4):724.
[Crossref] [Google Scholar] [PubMed]
- Hosseini MS, Hosseini F, Ahmadi A, Mozafari M, Amjadi I. Antiproliferative Activity of Hypericum perforatum, Achillea millefolium, and Aloe vera in interaction with the prostatic activity of CD82. Rep Biochem Mol Biol 2019;8(3):260.
- Galavi HR, Saravani R, Shahraki A, Ashtiani M. Anti-proliferative and apoptosis inducing potential of hydroalcoholic Achillea wilhelmsii C. Koch extract on human breast adenocarcinoma cell lines MCF-7 and MDA-Mb-468. Pak J Pharm Sci 2016;29(6):2397-403.
[Google Scholar] [PubMed]
- Köngül E, Ta? Ö, Pa?ayeva L, Karatoprak G?. Analysis of the cytotoxic effects of Achillea millefolium L. extracts on MCF7 cell line. Proceeding 2017;1(10):1077.
- Tatsis EC, Boeren S, Exarchou V, Troganis AN, Vervoort J, Gerothanassis IP. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007;68(3):383-93.
[Crossref] [Google Scholar] [PubMed]
- Colasanti A, Kisslinger A, Liuzzi R, Quarto M, Riccio P, Roberti G, et al. Hypericin photosensitization of tumor and metastatic cell lines of human prostate. J Photochem Photobiol B 2000;54(2-3):103-7.
[Crossref] [Google Scholar] [PubMed]
- Ferraz A, Faria DH, Benneti MN, Da Rocha AB, Schwartsmann G, Henriques A, et al. Screening for antiproliferative activity of six southern Brazilian species of Hypericum. Phytomedicine 2005;12(1-2):112-5.
[Crossref] [Google Scholar] [PubMed]
- Ceni?-Miloševi? D, Tambur Z, Ivancaji? S, Stanojkovi? T, Grozdani? N, Kuliši? Z, et al. Antiproliferative effects of Tanaceti partheni, Hypericum perforatum and propolis on HeLa cells. Arch Biol Sci 2014;66(2):705-12.
- Deljanin M, Nikolic M, Baskic D, Todorovic D, Djurdjevic P, Zaric M, et al. Chelidonium majus crude extract inhibits migration and induces cell cycle arrest and apoptosis in tumor cell lines. J Ethnopharmacol 2016;190:362-71.
[Crossref] [Google Scholar] [PubMed]
- Noureini SK, Esmaili H. Multiple mechanisms of cell death induced by chelidonine in MCF-7 breast cancer cell line. Chem Biol Interact 2014;223:141-9.
[Crossref] [Google Scholar] [PubMed]
- Jakovljevic ZD, Stankovic SM, Topuzovic DM. Seasonal variability of Chelidonium majus L. secondary metabolites content and antioxidant activity. EXCLI J 2013;12:260.
[Google Scholar] [PubMed]