- *Corresponding Author:
- R. A. Gokarn
Department of Rasashastra and Bhaishajya Kalpana
Mahatma Gandhi Ayurved College Hospital and RC, Salod (H), Wardha-442 001
E-mail: rohit_gn@yahoo.com
| Date of Submission | 16 February 2016 |
| Date of Revision | 01 February 2017 |
| Date of Acceptance | 02 June 2017 |
| Indian J Pharm Sci 2017;79(4):633-640 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Rasasindura is a unique, Ayurvedic mercurial preparation widely used by practitioners. Thi investigation is an attempt to perform acute and chronic oral toxicity evaluation of Rasasindura along with an adjuvant Guduchi Ghana (solidified aqueous extract of Tinospora cordifolia Will.) in rats. Oral acute toxicity study of test drug was carried at the limit dose of 2000 mg/kg orally in rats. For chronic toxicity, Rasasindura with adjuvant was administered at therapeutic equivalent dose (45 mg/kg, orally), therapeutic equivalent dose×5 (225 mg/kg, orally), therapeutic equivalent dose×10 (450 mg/kg, orally) for 90 days and an additional recovery group of therapeutic equivalent dose×10 for 30-day observation after the treatment period. Acute toxicity result showed that drug did not produce any signs and symptoms of toxicity or mortality up to an oral dose of 2000 mg/kg in rats. Chronic toxicity results showed that Rasasindura, even at a level as high as therapeutic equivalent dose×10 level, had no significant effect whatsoever on the ponderal and hematological parameters. Although the drug produced mild to moderate adverse changes (in kidney, liver, intestine, and stomach) at therapeutic equivalent dose×10 dose level, equivalent of which are unlikely to be ever employed in a clinical trial. The observed changes were not seen at the lower dose levels as well as in the recovery study. Hence, it is suggested that the Rasasindura, along with the adjuvant prepared as per the customary method, is safe for consumption at the therapeutic dose level.
Keywords
Rasasindura, Guduchi ghana, Tinospora cordifolia, bhasma, toxicity
Ayurveda, the traditional system of Indian medicine, has enriched the historical background and is one of the great living traditions. Use of processed metals, minerals, and mercurial-processed herbs as a medicament has been an integral part of Ayurvedic practice. These metallic preparations have unique process of preparation, involving Shodhana (purification and/or detoxification) and Marana (incineration and/or calcination). Practitioners developed these methods to detoxify the raw material by chemical transformations and thus modify the properties of materials to enhance therapeutic potential [1,2]. Their extensive use of these medicaments since more than a millennium without any reports of any untoward events can be considered as a testimony to their safety; but no objective-verifiable data exists to support such claims. Pre-clinical studies of Ayurvedic drugs provide scientific basis for their traditional use and to prove that they are safe and efficacious [3].
Rasasindura is one among such mercurial preparations widely used by Ayurveda practitioners. Pharmaceutical processing of Rasasindura involves treating purified mercury with purified sulphur and juices of Aloe vera (Linn.) Burm. f. to form black sulphide of mercury, which is further treated with gradual, intermittent heat to transform into more stable form, i.e. cinnabar by Kupipakwa method [4]. Kupipakwa is a specialized heating system, i.e. gradual, intermittent heat by vertical electrical-muffle furnace. Rasasindura is said to be mercury sulphide, associated with several organic macro-molecules derived from processing with plant extracts [5]. Toxic effects of purified mercury were said to be neutralized in the presence of purified sulphur [6]. As per the classics, Rasasindura is administered with the adjuvant such as Guduchi ghana (solidified aqueous extract of Tinospora cordifolia Will.) [7]. Rasasindura has unique properties to pacify diseases such as diabetes, fistula, fever, lack of appetite, anaemia, oedema etc.; and it is considered equivalent to the elixir, which is known to overcome death [4,7]. Since reports of toxicity evaluation of this classical preparation (along with the adjuvant) was not available during extensive literature review, it was thought worthwhile to undertake the detailed toxicity assessment in albino rats.
Materials and Methods
Aloe vera and T. cordifolia were collected from the botanical garden of Gujarat Ayurved University, Jamnagar. The plant materials were authenticated and voucher specimens of each submitted to Pharmacognosy laboratory of Institute. Rasasindura and Guduchi ghana (solidified aqueous extract of T. cordifolia) were prepared in Department of Rasashastra and Bhaishajya Kalpana, Gujarat Ayurved University, Jamnagar; and SOPs were prepared and documented [4,8]. All chemicals used in the study were of analytical grade.
Experimental animals
Wistar albino rats of either sex weighing 200±20 g body weight were used for the study. The animals were maintained under ideal husbandry conditions in terms of standard conditions of temperature (23±2°), relative humidity (50 to 60%), and exposed to 12 h light-and-dark cycles. All animals were exposed to the same environmental conditions and were maintained on standard diet and drinking water ad libitum. The experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC/10/2012/08, Ph.D.) as per the guideline of Committee for the Purpose of Control and Supervision on Experiments on Animals, India.
Dose selection
As per the classical guideline, the therapeutic dose of Rasasindura is 125 mg/d [9] and should be administered with the adjuvant as solidified aqueous extract of T. cordifolia (375 mg/d). Hence, total dose of drug with adjuvant is 500 mg/d. The suitable dose for rats was calculated by referring to table of Paget and Barnes [10] and was found to be 45 mg/kg rat (considered as therapeutic equivalent dose, TED). The test drug along with adjuvant was administered orally with the help of cannula, in the form of suspension in honey and distilled water solution.
Acute toxicity study
Young, healthy, nulliparous, and non-pregnant Wistar-strain albino, female rats were selected and acclimatized for seven days before the experiment. The Rasasindura along with adjuvant was orally administered at limit dose of 2000 mg/kg to overnight fasted female rats in sequential manner as per the OECD 425 guideline [11]. The rats were observed closely for behavioural changes, signs and symptoms of toxicity, and mortality continuously for the first six hours; and thereafter, periodically up to 14 d. The body weight of each rat was noted on the last day and the rats were sacrificed. The abdomen was opened through mid-line incision to record the autopsy changes, followed by dissecting the important organs for histopathological changes.
Chronic toxicity study
The chronic toxicity study was carried out followed by standard guideline with modification as per experimental need [12,13]. Rats were randomized into six groups of six rats in each with three males and three females. Group (I) was kept as control group, received vehicle as honey solution in distilled water (5 ml/kg, orally). Group (II) to (IV) were administered with test drug Rasasindura along with adjuvant at TED (45 mg/kg, orally), TED×5 (225 mg/kg, orally), and TED×10 (450 mg/kg, orally), respectively. The suspensions of test drugs were administered orally once-a-day for 90 consecutive days in main study. Additional six animals were kept in satellite control group (V) and in the recovery TED×10 treated group (VI) for observation after the treatment period, for reversibility or persistence of any toxic effects. The duration of post-treatment period was fixed as 30 d (total of 120 d, including 90 d treatment period and 30 d recovery period). All the animals were dosed with constant dose volume of 5 ml/kg, orally.
The rats were observed daily, carefully for any overt and apparent signs and symptoms of toxicity. The bodyweight change of an individual rat was noted initially and thereafter weekly during the study period. At the end of experimental periods, blood was withdrawn by the retro-orbital puncture under light-ether anaesthesia using capillary tube for estimation of serum biochemical and haematological parameters. The body weight of each rat was noted on last day and rats were sacrificed. The abdomen was opened through mid-line incision to record the autopsy changes followed by dissecting out the important organs.
Haematological analysis was performed using an automatic haematological analyser (Swelab, Sweden). The parameters were total red blood cell (RBC), haemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), white blood cell (WBC), neutrophils percentage (%N), lymphocyte percentage (%L), eosinophil’s percentage, monocyte percentage, and platelet count (PC).
Serum bio-chemical parameters were carried out using fully automated biochemical random access analyser (BS-200, Lilac Medicare Pvt. Ltd., Mumbai). The parameters were blood glucose [14], urea [15], creatinine [16], total cholesterol [17], HDL-cholesterol [18], triglyceride [19], VLDL-cholesterol, LDL-cholesterol, total protein [20], albumin, globulin [21], alkaline phosphatase [22], SGOT [23], SGPT [24], uric acid [25], direct bilirubin [24], total bilirubin [26], and serum calcium [27].
Bone-marrow smear from the femur bone was prepared using standard procedure. All the important internal organs were carefully dissected namely brain, pituitary, liver, heart, thymus, spleen, kidney, lung, stomach, intestine, testis, prostate, seminal vesicle, uterus, ovary, adrenal gland, trachea, aorta, lymph node, and skin. After noting for any signs of gross lesion and ponderal changes of major organs, all were transferred to 10% phosphate buffered formalin solution for fixation and later on subjected to dehydrating, wax embedding, sectioning, and staining with haematoxylin and eosin (H and E) for histological evaluation by light microscopy. The slides were viewed under trinocular research Carl-Zeiss’s microscope at various magnifications to note down the changes in the microscopic features of the tissues.
Statistical analysis
The data is expressed as mean±standard error of mean for six rats per experimental group. One-way analysis of variance (ANOVA) was used to compare the mean values of quantitative variables among the groups, followed by Dunnet’s multiple t-test for unpaired data to determine significant difference between groups at P<0.05.
Results and Discussion
Acute toxicity study of test drug was carried out to record immediate adverse signs and symptoms of drug in female rats at dose levels that are several folds higher than the therapeutic equivalent dose. Administration of Rasasindura along with adjuvant did not affect any behavioural changes and other parameters observed during the acute toxicity test in female rats. No signs and symptoms of toxicity and mortality were observed up to oral dose of 2000 mg/kg of test drug in rats. Further, drug did not affect the cytoarchitecture of major organs like heart, kidney, liver, uterus, and ovary which suggest that LD50 value may be higher than 2000 mg/kg by oral route. As per UN classification, any substance, which has oral LD50 of more than 2000 mg/kg is considered as low hazard potential and categorized as UN 6.1 PG III [28]. Thus as per the above criterion Rasasindura along with adjuvant can be categorized as substances with low health hazard potential (Class 4 of GHS and UN 6.1 PG III).
There were no behavioural changes observed in Rasasindura treated groups during the course of chronic toxicity study. No symptoms of toxicity and mortality was observed in treated groups at TED×10, TED×5, and TED dose levels in the main study and TED×10 in the recovery study. Normal body weight gain was observed in control rats during main study (90 d) as well as recovery study (120 d). An increase in body weight was found in Rasasindura treated groups at all dose levels. Changes in body weight are an important factor to monitor the health of an animal. Loss of body weight is usually the first sign indicating the onset of an adverse effect. The dose, at which body weight loss is by 10% or more, is considered to be a toxic dose, irrespective of whether or not it is accompanied by any other changes [29]. The percentage change in body weight pattern in test drug treated groups did not differ significantly from the changes observed in the control groups, which suggest the absence of serious toxic effect of Rasasindura during chronic administration in rats.
Out of the nine organs for which relative weights were recorded (Table 1), Rasasindura treated group showed significant decrease in relative weight of liver in TED treated group in comparison to the normal control, whereas in other dose levels the changes were non-significant. A significant increase was observed in relative weight of testis at TED, uterus weight at TED×5, and kidney and testis at TED×10 dose levels; however, the changes were almost reversed in the drug treated recovery group at TED×10 dose level. Normally, decrease in the weight of an organ is indicative of loss of tissue mass in that organ, exception being the secretory organs in which the decrease in weight sometimes is seen along with the increased activity. In this case, the increase in the weight of reproductive organs may be indicative of stimulation of hormone secretion. In the present study, there were no remarkable changes observed in the relative weight of the organs at higher doses of test drugs. Hence, it may be, understood that the drugs do not tend to produce any serious toxic effect on the relative weight of the important internal organs in chronic toxicity studies.
| Relative weight | Control group | Drug treated groups | R Control group | Recovery TED x 10 | ||
|---|---|---|---|---|---|---|
| TED | TED×5 | TED×10 | ||||
| Liver (g/100 g) | 3.57±0.17 | 3.12±0.10* | 3.27±0.06 | 3.26±0.09 | 2.72±0.07 | 2.99±0.11 |
| Heart (mg/100 g) | 280.41±8.88 | 266.04±5.36 | 289.12±9.69 | 281.65±6.78 | 240.44±8.42 | 269.80±12.81 |
| Kidney (mg/100 g) | 720.10±26.28 | 675.17±19.26 | 766.08±30.88 | 804.74±25.63* | 691.42±18.42 | 714.44±10.76 |
| Spleen (mg/100 g) | 194.50±9.32 | 173.56±8.14 | 206.28±18.26 | 189.69±10.14 | 158.85±7.44 | 179.45±8.87 |
| Thymus (mg/100 g) | 172.54±6.73 | 165.65±5.21 | 167.91±7.71 | 158.91±7.60 | 145.56±10.91 | 146.15±12.89 |
| Testis (g/100 g) | 648.26±28.37 | 818.50±41.74* | 684.25±123.10 | 868.99±45.83 | 792.01±61.68 | 661.95±39.86 |
| Prostate (mg/100 g) | 146.97±21.19 | 128.77±11.70 | 129.53±9.04 | 180.36±7.43 | 145.07±24.68 | 167.09±16.38 |
| S. vesicle (mg/100 g) | 504.08±95.14 | 504.55±55.60 | 481.70±32.76 | 527.32±47.38 | 421.09±62.60 | 477.01±58.91 |
| Uterus (mg/100 g) | 230.03±17.72 | 215.88±28.98 | 323.70±26.45* | 222.81±21.52 | 197.89±8.69 | 178.48±9.04 |
The results are expressed as mean±SEM, where n=6. SEM: Standard error of mean. *P<0.05, compared with control group
Table 1: Effect of test drugs on relative weight of organs of rats recorded during chronic toxicity study.
Analysis of the effects of Rasasindura on haematological parameters (Table 2) revealed Non-significant increase in WBC count at each of the dose level studied in the main study; however, contrary effect was observed in the recovery phase in the drug treated group in comparison to the control group. However all the values were within the normal range [30]. The test drug at all dose level did not affect the RBC related parameters. This clearly indicates that the test drug did not affect the cellular and non-cellular elements of the blood to significant extent. Further, with the discontinuation of the drug, most of the values were similar to that observed in the recovery control group, which suggests that the drug is devoid of any serious haematological toxicity, even at higher dose on repeated administration.
| Hematological Parameters | Control group | Drug treated groups | R control group | Recovery TED×10 | ||
|---|---|---|---|---|---|---|
| TED | TED×5 | TED×10 | ||||
| RBC (106/ml) | 7.55±0.08 | 7.69±0.17 | 7.41±0.10 | 7.86±0.22 | 8.67±0.26 | 8.74±0.26 |
| Hemoglobin (g/dl) | 13.85±0.34 | 13.95±0.21 | 13.60±0.16 | 14.00±0.21 | 15.38±0.24 | 15.38±0.33 |
| PCV% | 42.18±0.58 | 42.48±0.95 | 41.40±0.43 | 44.41±1.01 | 48.76±1.22 | 49.60±1.30 |
| MCV (fl) | 55.86±0.41 | 55.25±0.47 | 55.84±0.48 | 56.54±0.75 | 56.32±1.18 | 56.75±0.80 |
| MCH (pg/red cell) | 18.35±0.32 | 18.15±0.24 | 18.35±0.27 | 17.84±0.37 | 17.38±0.53 | 17.63±0.38 |
| MCHC (g/dl) | 32.81±0.49 | 32.87±0.25 | 32.84±0.24 | 31.58±0.33 | 30.84±0.42 | 31.03±0.34 |
| WBC (103/ml) | 6816.66±630.03 | 8114.28±1217.61 | 8114.28±1068.91 | 7585.71±570.47 | 7020.00±575.67 | 6233.33±276.48 |
| Neutrophil% | 24.50±4.32 | 28.42±5.47 | 19.57±2.32 | 17.14±1.79 | 23.00±4.93 | 23.66±2.10 |
| Lymphocyte % | 71.66±4.27 | 66.57±5.81 | 76.85±2.13 | 79.42±2.04 | 75.80±5.06 | 72.00±2.51 |
| Eosinophil% | 2.33±0.21 | 2.71±0.28 | 2.00±0.21 | 2.00±0.21 | 2.60±0.24 | 2.33±0.21 |
| Monocyte% | 1.50±0.22 | 2.28±0.28 | 1.57±0.20 | 1.42±0.20 | 2.60±0.24 | 2.00±0.36 |
| PLT (103/ml) | 1152.83±54.10 | 1267.57±47.57 | 1154.00±62.82 | 1267.85±51.82 | 1115.80±45.11 | 1057.50±83.48 |
The results are expressed as mean±SEM, where n=6. SEM: Standard error of mean
Table 2: Effect of test drugs on hematological parameters in rats recorded during chronic toxicity study.
The effects of Rasasindura on serum bio-chemical parameters are presented in Table 3. Out of eighteen parameters studied, significant decrease was observed in blood glucose level at TED and TED×5 treated groups, while significant increase in creatinine level at TED dose level and triglyceride level at TED×5 dose level in comparison to the control group in main study. However, similar significant changes were not observed in higher dose of Rasasindura. After discontinuation of test drug in the recovery group, the observed changes in glucose level, creatinine, and triglyceride were almost same as seen in the recovery control group. Cholesterol level and HDL-cholesterol level in rats were unaffected by the test drug at all dose level in comparison to the control group; hence, it can be inferred that the observed changes do not cause any serious toxic effect (Table 4).
| Biochemical Parameters | Control group | Drug treated groups | R control group | Recovery TED×10 | ||
|---|---|---|---|---|---|---|
| TED | TED×5 | TED×10 | ||||
| Glucose (mg/dl) | 92.83±8.63 | 68.57±2.61* | 70.85±4.11* | 107.28±3.63 | 117.50±4.86 | 111.66±3.34 |
| Urea (mg/dl) | 69.83±4.88 | 81.00±2.43 | 64.00±4.35 | 69.14±8.74 | 88.60±5.00 | 65.16±3.15 |
| Creatinine (mg/dl) | 0.58±0.03 | 0.70±0.03* | 0.62±0.03 | 0.55±0.02 | 0.56±0.02 | 0.60±0.02 |
| Total protein (g/dl) | 7.08±0.17 | 6.94±0.19 | 6.95±0.24 | 7.12±0.10 | 6.90±0.24 | 7.05±0.10 |
| Albumin (g/dl) | 3.58±0.16 | 3.61±0.15 | 3.67±0.23 | 3.72±0.08 | 3.36±0.14 | 3.35±0.08 |
| Globulin (g/dl) | 3.50±0.17 | 3.12±0.10 | 3.28±0.17 | 3.40±0.09 | 3.33±0.15 | 3.70±0.17 |
| ALP (IU/L) | 233.25±45.02 | 149.00±11.82 | 143.33±23.78 | 263.42±37.67 | 261.40±37.64 | 281.33±51.96 |
| SGOT (IU/L) | 138.16±7.85 | 153.28±9.11 | 142.85±8.42 | 148.85±9.83 | 124.50±8.65 | 143.83±5.17 |
| SGPT (IU/L) | 67.00±7.82 | 53.28±1.37 | 58.28±5.16 | 62.00±6.92 | 57.00±2.70 | 57.00±4.20 |
| Uric acid (mg/dl) | 0.70±0.13 | 0.81±0.09 | 0.84±0.09 | 0.72±0.06 | 0.95±0.16 | 1.05±0.09 |
| D. Bilirubin (mg/dl) | 0.13±0.03 | 0.11±0.01 | 0.10±0.00 | 0.12±0.02 | 0.13±0.03 | 0.13±0.02 |
| T. Bilirubin (mg/dl) | 0.45±0.05 | 0.48±0.07 | 0.41±0.04 | 0.48±0.06 | 0.45±0.07 | 0.48±0.08 |
| Calcium (mg/dl) | 9.58±0.30 | 8.98±0.17 | 9.81±0.25 | 9.22±0.22 | 9.35±0.22 | 9.48±0.09 |
The results are expressed as mean±SEM, where n=6. SEM: Standard error of mean. *P<0.05 compared with control group; @P<0.05 compared with recovery control group
Table 3: Effect of test drugs on biochemical parameters in rats recorded during chronic toxicity study.
| Serum Lipid profile | Control group | Drug treated groups | R control group | Recovery TED x 10 | ||
|---|---|---|---|---|---|---|
| TED | TED x 5 | TED x 10 | ||||
| Total cholesterol (mg/dl) | 34.33±3.45 | 38.42±1.81 | 37.28±3.92 | 30.71±2.01 | 37.50±3.59 | 28.16±1.86@ |
| HDL-cholesterol (mg/dl) | 33.16±3.15 | 37.14±4.50 | 37.85±5.70 | 27.85±2.08 | 31.66±3.41 | 25.16±1.60 |
| Triglyceride (mg/dl) | 61.33±3.67 | 76.14±6.55 | 78.42±6.07* | 70.28±6.80 | 70.16±5.29 | 73.83±8.56 |
| VLDL-cholesterol (mg/dl) | 12.26±0.73 | 15.22±1.31 | 15.68±1.21 | 14.05±1.36 | 14.03±1.05 | 14.76±1.71 |
| LDL-cholesterol (mg/dl) | 11.10±1.68 | 13.32±3.86 | 14.40±3.30 | 11.20±0.80 | 7.43±1.90 | 11.76±2.26 |
The results are expressed as mean ± SEM, where n=6. SEM: Standard error of mean. *P<0.05 compared with control group; @P<0.05 compared with recovery control group
Table 4: Effect of test drugs on serum lipid profile in rats recorded during chronic toxicity study.
Significant decrease in blood urea was observed in recovery TED×10 treated rats in comparison to the recovery control group however same was not observed in main study. Test drugs did not influence the level of serum transaminases, urea, and creatinine to a significant extent in main study; which suggests that the test drug may not affected the liver and kidney function in the treated rats. The observed values of above bio-chemical parameters are within the normal range [30].
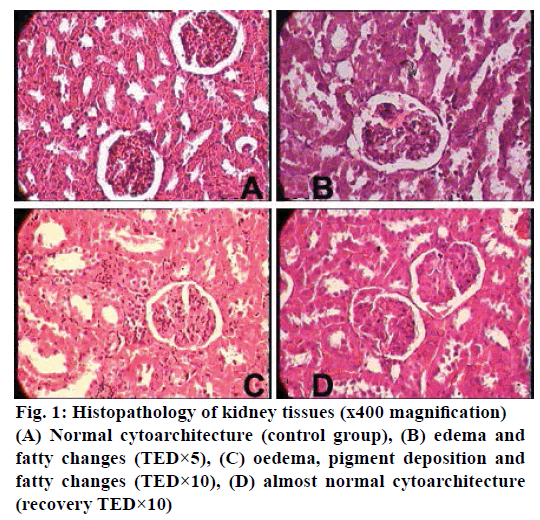
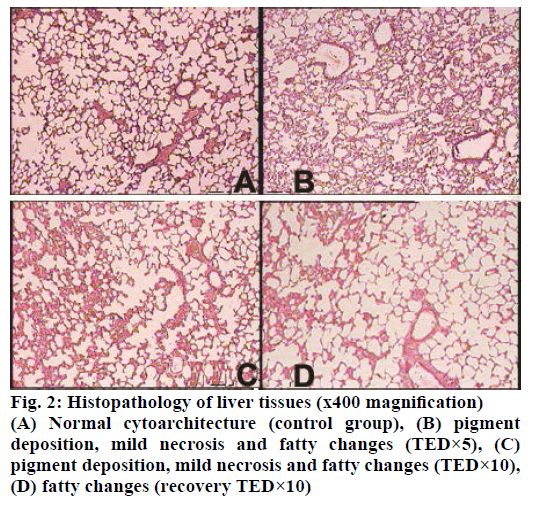
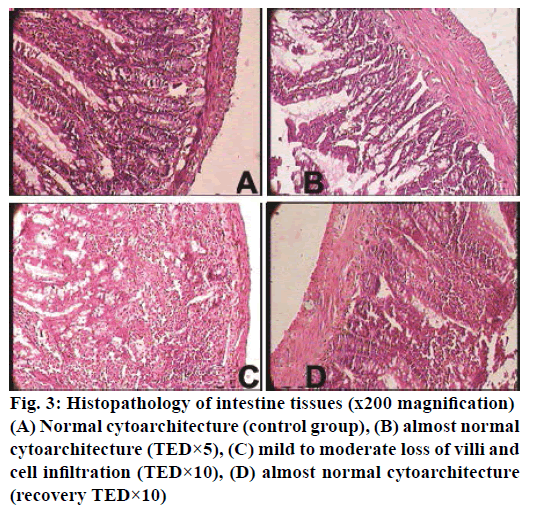
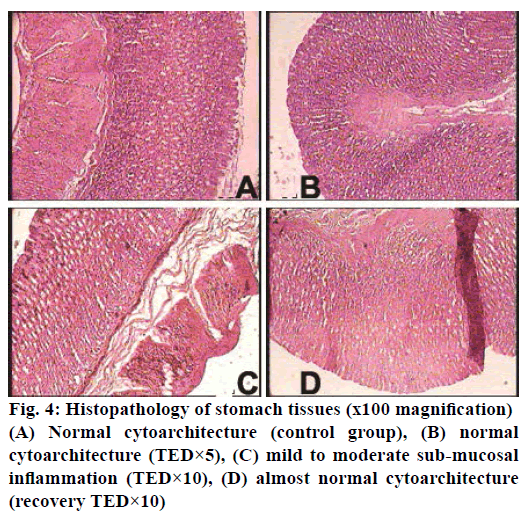
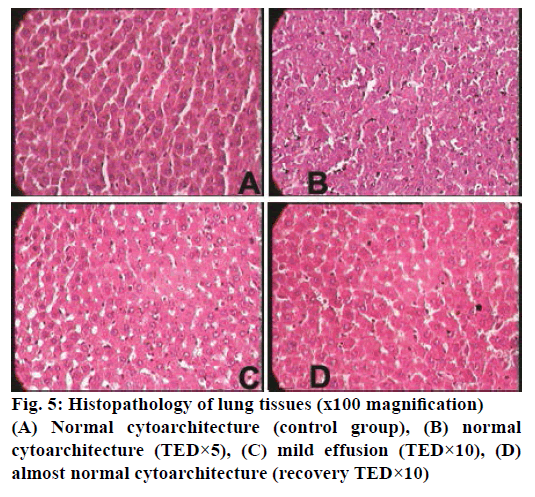
The histopathological studies of twenty organs showed that Rasasindura along with adjuvant at highest dose level exhibited mild to moderate changes in kidney, liver, intestine, stomach, and lung in comparison to the control group. Rasasindura produced mild pigment deposition, fatty changes in epithelium, and oedematous changes in kidney tubule, whereas recovery group showed normal cytoarchitecture (Figure 1). Rasasindura TED×10 and TED×5 treated groups exhibited pigment deposition, mild necrosis, and fatty changes in the liver in comparison the control group, while the recovery group showed almost normal cytoarchitecture (Figure 2). Test drug at highest-dose level exhibited mild to moderate loss of villi and cell infiltration in intestine (Figure 3) and displayed mild to moderate sub-mucosal inflammation in the stomach (Figure 4). Mild effusion was seen in cytoarchitecture of lung of one of the rat at higher dose of TED×10 dose level in comparison to the control group; whereas, in other dose levels the changes were almost normal (Figure 5). Bone marrow smear and the other organs exhibited normal cytoarchitecture in the Rasasindura-preparation treated groups, both in the main study as well as in the recovery study, in comparison to the control groups.
The observed histopathological changes were not seen at therapeutic dose level and not in the recovery study. The result of bio-chemical parameters reveal that the drugs do not seem to produce any drastic changes in the liver and kidney function parameters in rats; which suggests that the organ damage as mentioned above in chronic toxicity is of mild intensity at higher dose level, however drug is relatively safe at therapeutic dose level. In previous 28-d toxicity study also demonstrated that Rasasindura (50-100 mg/kg) in Wistar-albino rats did not have any adverse effect on kidney [31]. Further, feeding on Rasasindura supplemented food did not elicit any evidence of heavy metal toxicity in larvae or flies, since there was neither any evidence of lethality, nor of any developmental defects in the emerging flies [32].
The results reiterates the fact that Bhasmas, despite their trace heavy metal content, are safe when appropriately manufactured and consumed as per directed instructions [33]. Toxic effects of mercury were said to be neutralized in the presence of sulphur [6].
In Ayurvedic system of medicine, Anupana (called vehicle, as a medium of administration) improves acceptability and palatability and helps in absorption of the main drug; additionally, it may also act as early antidote [32]. Guduchi used as adjuvant in current study is having antioxidant property acts as hepatoprotective drug and has potential against aflatoxins and heavy metal toxicity [34,35].
From the present study, it is concluded that Rasasindura along with adjuvant is not toxic on acute administration at a maximum oral dose level of 2000 mg/kg in female rats. However, on chronic administration of test drug for 90 d produced mild to moderate adverse changes in the kidney, liver, intestine, and stomach of rats at TED×10 dose level, equivalent of which are not likely to be ever employed in clinical conditions, conversely Rasasindura at TED dose has no toxic potential. Rasasindura prepared as per customary method and administered with appropriate adjuvant is safe to consume at therapeutic dose level.
Acknowledgments
The authors wish to thank the staff of Pharmacology laboratory and R. S and B. K Department, Institute of Postgraduate Teaching and Research in Ayurveda for their support.
Financial assistance
None.
Conflict of interests
None declared.
References
- Krishnamachary B, Rajendran N, Pemiah B, Krishnaswamy S, Krishnan UM, Sethuraman S, et al. Scientific validation of different purification steps involved in the preparation of an Indian Ayurvedic medicine, Lauha Bhasma. J Ethnopharmacol 2012;142:98-104.
- Kohli KR. Ayurvedic medicines and heavy metals issue. Ayurveda Herit 2005;1:5-6.
- http://apps.who.int/medicinedocs/en/d/Jh2946e/.
- Gokarn RA, Patgiri B. An approach towards pharmaceutical standardization of Shadguna rasasindura. J Res Edu Indian Med 2013;19:97-102.
- Patil S, Chaudhary AK. Quantitative estimation of Guduchi Ghana obtained from different amount of water used for kwath. Int J Pharma Arch 2013;2:160-4.
- Kumar A, Nair AGC, Reddy AVR, Garg AN. Availability of essential elements in bhasmas: analysis of Ayurvedic metallic preparations by INAA. J Radioanal Nucl Chem 2006;270:173-80.
- Shastry K. Sadananda Sharma’s Rasa Tarangini. 11th ed. New Delhi: Motilal Banarasidas; 2004. p. 140-1.
- Dhundi SN, Yadav P, Patgiri BJ, Prajapati PK. Pharmaceutical standardization of Guduchi Ghana (solidified aqueous extract of Tinospora cordifolia Moerrs.). Int Res J Pharm 2011;2:102-4.
- http://naturalingredient.org/wp/wp-content/uploads/API-Vol-7.pdf.
- Paget GE, Barnes JM. Toxicity tests. In: Laurance DR, Bacharach AL, editors. Evaluation of drug activities: pharmacometrics. New York: Academic Press; 1964. p. 205-10.
- https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl425-508.pdf.
- https://www.oecd.org/chemicalsafety/testing/Revision-OECD-TG408-repeated-dose-90-day-oral-toxicity-study-in-rodents.pdf.
- http://ayush.gov.in/sites/default/files/File779%20%20%204.pdf.
- Pennock CA, Murphy D, Sellers J, Longdon KJ. A comparison auto analyzer method for the estimation of glucose in blood. Clin Chim Acta 1973;48:193-201.
- Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in Warburg optical test. Klin Wochenschr 1965;42:174-5.
- Slot C. Plasma creatinine determination: a new and specific Jaffe reaction method. Scand J Clin Lab Invest 1965;17:381-7.
- Roeschlau P, Bernt E, Gruber WA. Enzymatic determination of total cholesterol in serum. J Clin Chem Clin Biochem 1974;12:226.
- Dominiczak M, McNamara J, Nauk M, Wiebe D, Warnick G. Measurement of high-density-lipoprotein cholesterol. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. 2nd ed. Washington DC: AACC Press; 2000. p. 819.
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 1982; 28:2077-80.
- Tietz NW. Text book of Clinical Chemistry. Philadelphia (PA): WB Saunders; 1986. p. 579.
- Doumas BT, Arends RL, Pinto PC. In standard methods of clinical chemistry, vol. VII. Chicago: Academic Press; 1972. p. 175-89.
- Wilkinson JH, Boutwell JH, Winsten S. Evaluation of a new system for kinetic measurement of serum alkaline phosphatase. Clin Chem 1969;15:487-95.
- Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia (PA): WB Saunders; 1995. p. 76.
- Burtis CA, Ashwood ER. Tietz textbook of Clinical Chemistry. 3rd ed. Philadelphia (PA): WB Saunders; 1999. p. 652, 1136.
- Kabasakalian P, Kalliney S, Wescott A. Determination of uric acid in serum, with use of uricase and tribromophenol-aminoantipyrine chromogen. Clin Chem 1973;19:522.
- Pearlman PC, Lee RT. Detection and measurement of total bilirubin in serum with use of surfactants as solubilizing agents. Clin Chem 1974;20:447.
- Biggs HG, Moorehead WR. 2-Amino-2-methyl-1-propanol as the alkalizing agent in an improved continuous-flow cresolphthalein complexone procedure for calcium in serum. Clin Chem 1974;20:1458-60.
- https://www.unece.org/trans/danger/publi/unrec/12_e.html.
- Timbrell JA. Principles of biochemical toxicology. London: Taylor and Francis Limited; 1982. p. 446.
- Gad SC. The rat: pathology. In: Gad SC, Chengellis CP editors. Animal Models in Toxicology. Boca Raton: CRC press; 2007. p. 147-217.
- Anita K, Sharma A, Venkateshwaralu U, Gotecha VK. Toxicological evaluation of Rasasindoor in albino rats. Int Ayur Med J 2013;1:1-6.
- Dwivedi V, Anandan EM, Mony RS, Muraleedharan TS, Valiathan MS, Mutsuddi M, et al. In vivo effects of traditional ayurvedic formulations in Drosophila melanogaster model relate with therapeutic applications. PLoS ONE 2012;7:e37113.
- Sathya T, Murthy B, Vardhini N. Genotoxicity evaluation of certain bhasmas using micronucleus and Comet assays. Int J Alt Med 2009;7:1.
- Sharma V, Gupta R, Sharma S. Preventive effects of Tinospora cordifolia extract against aflatoxin-B1 induced oxidative stress in swiss albino mice. Asian J Pharma Clin Res 2011;4:49-55.
- Sharma V, Pandey D. Beneficial Effects of Tinospora cordifolia on blood profile in male mice exposed to lead. Toxicol Int 2010;17:8-11.