- *Corresponding Author:
- N. Mallikarjunarao
Department of Pharmaceutical Sciences, Jawaharlal Nehru Technological University Kakinada, Kakinada, Andhra Pradesh 533003,India
E-mail: mallimpharmmba@gmail.com
| Date of Received | 26 January 2021 |
| Date of Revision | 13 October 2022 |
| Date of Acceptance | 01 February 2023 |
|
Indian J Pharm Sci 2023;85(1):83-92 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The goal of this work was to create and verify a simple reversed phase-high performance liquid chromatography-photodiode array detector-based method for quantifying cinacalcet in rat plasma. Six albino Wistar rats were given cinacalcet orally by oral gavage. Blood was obtained at regular intervals using retro-orbital vein puncture and processed to get plasma. Cinacalcet was extracted from plasma using a 70:30 v/v ethanol:dichloromethane combination, with an 82.80 %-104.08 % recovery rate. The reversed phase-high performance liquid chromatography-photodiode array detector technique was used to determine cinacalcet plasma concentrations. The mobile phase was acetonitrile:0.1 M dipotassium phosphate buffer (pH 6.8) (50:50 v/v) at a flow rate of 1.0 ml/min. 241.0 nm was used as the detecting wavelength. The method's linearity for cinacalcet was proven by a correlation coefficient (R2) of more than 0.9996 and a linear range of 1 μg/ml to 24 μg/ml for cinacalcet. The lower and upper limits of quantification were found to be 1 μg/ml and 24 μg/ml respectively. At the cinacalcets retention time, no interfering peaks were seen. This reversed phase-high performance liquid chromatography-photodiode array detector approach is a straightforward and effective way to determine pharmacokinetic parameters by measuring plasma cinacalcet levels.
Keywords
Reversed phase-high performance liquid chromatography, validation, cinacalcet, rat plasma, pharmacokinetic studies
Calcium-Sensing Receptor (CaSR) is a G-protein coupled receptor that controls the production and release of Parathyroid Hormone (PTH) and is responsible for calcium signaling in PTH cells[1]. Cinacalcet (CIN) (N-(1-(R)-(-)-(1-naphthyl) ethyl)- 3-(3-(trifluoromethyl) phenyl)-1-aminopropane HCL)[2,3] is a type II calcimimetic that modulates CaSR signaling allosterically. CIN modulates bone metabolism and decreases PTH production by raising the CaSR's sensitivity to extracellular calcium[4]. CIN was recently licensed for the treatment of secondary hyperthyroidism in dialysis patients with chronic renal disease and the treatment of hypercalcemia in parathyroid cancer patients[5]. Too far, only two approaches have been used to distinguish CIN enantiomers in laboratory-made racemic mixtures; thin-layer and liquid chromatography[6-8].
Interestingly, numerous approaches for determining plasma CIN levels have been documented[9-13]. However, these methodologies have inherent flaws that must be addressed before they can be considered as a reliable method for determining CIN pharmacokinetic parameters in human individuals. We created an isocratic elution mode reverse-phase chromatographic separation. The proposed approach has the extra benefit of a 5 min total run time for each sample, which may be used for routine CIN measurement in plasma.
Materials and Methods
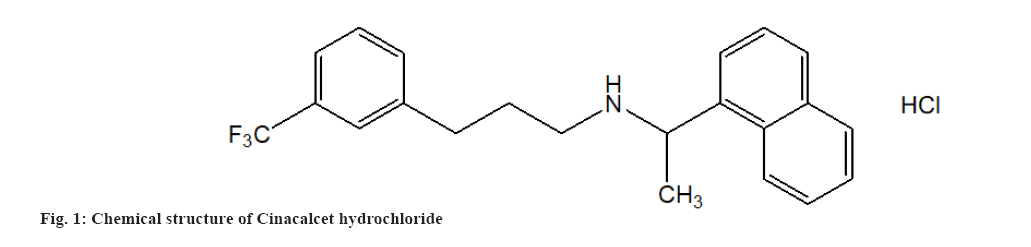
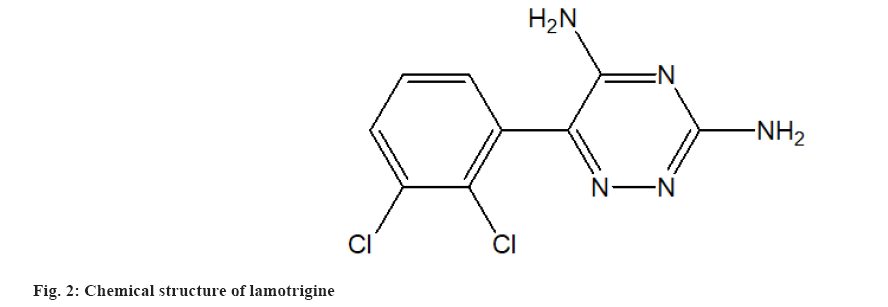
MSN Laboratories Pvt. Ltd., Hyderabad, India, provided pure CIN powder (fig. 1). Lamotrigine powder was obtained as an Internal Standard (ISTD) from Torrent Pharmaceutical in Ahmedabad, India. The chemical structure of lamotrigine is shown in fig. 2. According to the certificate of analysis, the purity of CIN and ISTD was 100.00 % and 99.99 %, respectively. Merck, Mumbai, provided High Performance Liquid Chromatography (HPLC) grade Acetonitrile (ACN), Dichloromethane (CH2Cl2), Diethyl Ether (Et2O), Methanol (MeOH) and Cyclohexane (C6H12). Distilling deionized water generated by a Milli-Q Millipore water system yielded HPLC grade water (Milford, Massachusetts (MA), United States of America (USA)). All of the additional reagents and components were purchased from commercial sources and were of analytical grade. The mobile phase was made by mixing the aqueous and organic phases and then degassing the HPLC under vacuum. The mobile phase was filtered using cellulose acetate membrane filters with a 0.2 mm pore size (Sartorius Stedim Biotech S.A., Aubagne Cedex, France).
Chromatographic conditions:
An LC waters HPLC was utilized in this investigation (Waters, Milford, MA, USA). A quaternary gradient system (600 controller), auto sampler (Waters, model 717 plus), in-line degasser (Waters, model AF) and photodiode array detector are included in this setup (Waters, 2998 model). Empower 3 software was used to process the data (Waters, USA). The chromatographic separation was performed using a Waters C18 analytical column (250 mm×4.6 mm inner diameter, 5 mm particle size, Waters, Dublin, Ireland) kept at 45°. The detection wavelength was set at 241.0 nm, and the mobile phase flow rate was 1.0 ml/min.
Mobile phase:
A 50:50 v/v combination of 0.1 M dipotassium phosphate buffer (pH 6.8, adjusted with orthophosphoric acid) and ACN makes up the mobile phase. With a solvent filtration system, the produced mobile phase was filtered over a 0.2 µm cellulose acetate membrane (Sartorius Stedim Biotech, France) and degassed. For the manufacture of medication solutions, the same was utilized as a diluent.
Standard solutions:
In the mobile phase, CIN hydrochloride (1 mg/ml concentration) was produced in a volumetric flask and kept at 4°. A suitable dilution of the stock standard solution was made by diluting 1 to 10 ml with the mobile phase to reach a final concentration of 0.1 mg/ml. This CIN solution was successfully diluted with diluent in plastic tubes (2 ml) to achieve final concentrations of 0.5 mg/ml and 10 mg/ml (Stock solutions). At 4°, all stock solutions were kept before the experiment; working solutions (50 µg/ml to 1100 µg/ml) were made from the relevant stock solutions. The working solutions were stored in screw-capped tubes that were wrapped with aluminium foil on the outside.
Animal treatment and sampling:
To establish standard care and management of the animals, the Committee for Control and Supervision of Experiments on Animals recommendations were followed. The experimental protocol was approved by the Institutional Animal Ethical Committee. In this investigation, healthy albino Wistar rats of either sex (n=6) weighing 150-200 g were employed. Rats were given a commercial pellet meal and water before the experiment. The rats were fasted overnight before being given CIN hydrochloride (1 mg/kg, per oral) through oral gavage, with free access to water. Blood samples (0.2 ml) were taken through retro-orbital vein puncture into centrifuge tubes containing Dipotassium (K2) Ethylene Diamine Tetraacetic Acid (EDTA) at predetermined time intervals (0, 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h). The samples were centrifuged at 3000 rpm for 10 min to extract supernatant plasma and kept at -20° within 3 min of blood collection. Before the analysis, the samples were thawed at room temperature. Blank plasma was also created by collecting blood from healthy rats and using it to make multiple batches of blank matrices and calibration curves.
Sample extraction:
In a 1.5 ml polypropylene snap cap tube, a 0.05 ml aliquot of ISTD solution (0.500 mg/ml) was added to 0.5 ml of plasma (Sarsedt, Verona, Italy). The tube was then filled with 0.4 ml of extraction solvent (Ethanol:CH2Cl2 70:30, v/v), vortexed (30 s), shaken (5 min) and centrifuged (5 min) at 3000 rpm. The organic phase was separated after centrifugation and transferred to a glass tube to evaporate under a stream of nitrogen at 50° for 5 min using a TurboVap LV Evaporator (Caliper Life Sciences, Hopkinton, MA, USA). The dried residue was then reconstituted and vortexed for 1 min in 50 ml of the mobile phase.
Bio analytical method validation:
To construct a thorough method validation, United States Food and Drug Administration criteria were followed. The proposed approach assessed selectivity, sensitivity, linearity of response, accuracy, precision, recovery, matrix effect and analyte stability throughout both short-term sample processing and long-term storage.
Selectivity:
The method's selectivity for endogenous plasma matrix components and concurrent medicines was tested.
The suggested extraction methodology was used to prepare and analyze six samples of blank rat plasma (four K2 EDTA, one haemolysed and one lipemic). The Lower Limit of Quantitation (LLOQ) level was used to define the chromatographic parameters for CIN. Blank interference peak regions should not exceed 20 % of the mean peak area of CIN LLOQ and should not exceed 5 % of the mean peak area of CIN samples.
Linearity (calibration curve):
The connection between instrument response and known analyte concentrations is depicted by a calibration (standard) curve. The linearity of CIN to Lamotrigine (y) was established by graphing the peak response (area) ratio of CIN vs. CIN concentration in plasma over the concentration range of 1 µg/ml to 24 µg/ml (x).
Detection and quantification limit:
The lowest concentration of a chemical that produces a peak area three times the baseline noise is known as the Limit of Detection (LOD). The ratio of signal size to noise size was calculated using equation 2 H/h. In a chromatogram obtained with the required reference solution, H is the peak height and h is the noise in the blank chromatogram. The lowest concentration with a signal-to-noise ratio greater than 5, an accuracy of 80 %-120 % and a precision of 20 % to its nominal value was established as the LLOQ.
Recovery:
The mean peak areas of three extracted Low Quality Control (LQC), Medium Quality Control (MQC) and High-Quality Control (HQC) samples were compared to the mean peak areas of three non-extracted solutions to measure CIN recovery (neat reference). The mean peak area of extracted samples was compared to the mean peak areas of neat reference solutions at a concentration of 20 g/ml. The analytes recovery does not have to be 100 %, but it should be consistent and repeatable for both the analyte (CIN) and the internal standard (Lamotrigine). The formula for calculating recovery is as follows:
Recovery=Mean peak area response of extracted samples at LQC; MQC; HQC/Mean peak area response of unextracted samples at LQC; MQC and HQC
Accuracy and precision:
To evaluate intra-day and inter-day accuracy and precision, the different concentrations, including lower and upper limits of each QC sample (LOQ-QC, LQC, MQC and HQC), were evaluated on the same day and 3 separate d. Six duplicates of each measurement were obtained.
The accuracy (% bias) was calculated as follows:
Accuracy (% bias)=(Concentration found)/(Nominal concentration)×100
The percent Coefficient of Variation (% CV) was calculated as follows:
% CV=(Standard deviation)/Mean×100
The accuracy calculated at each concentration level must be within 15 % of the associated nominal value, except for LOQ-QC, when it must not exceed 20 %. Except for LOQ-QC, which must be within 20 % of the percent CV, the accuracy around the mean value cannot exceed 15 %.
Matrix effect:
LLOQ-QC and HQC concentrations were used to determine the matrix impact. Six blank plasma samples (4 normal, 1 haemolyzed, 1 lipemic) were chosen and spiked with the LQC and HQC working concentrations because they were free of any substantial interference during the Retention time (Rt) of CIN and ISTD. All spiking samples were processed twice and quantified using a newly spiked calibration curve. If the accuracy and precision do not depart by 715.0 % for HQC and 720.0 % for LLOQ-QC of the nominal concentration, the matrix effect is deemed to be neutralized.
Stability studies:
Stock solution stability: The stability of stock solutions was tested under various storage circumstances, including room temperature for 8 h and 2°-8° for 32 h. The stability of stock solutions was determined by comparing newly produced CIN and ISTD samples to stability samples at the MQC level after five injections of each. For both CIN and ISTD, the mean percentage change was computed. If the mean percentage change of ISTD and CIN was less than 10 %, the stock solution of CIN and ISTD was considered stable.
Bench top stability: Six duplicates of LQC and HQC in the biological matrix were removed, thawed at room temperature without assistance and left unprocessed for 8 h (stability samples). Later, with one set of low and high QC samples, a new calibration was recorded (comparison samples).
Freeze and thaw stability: After four freeze and thaw cycles, plasma freeze and thaw stability were determined by evaluating LQC and HQC samples (six duplicates). Samples were frozen for 24 h at -28°±5° and then thawed at room temperature without assistance. Two further freeze-thaw cycles were performed. Following the completion of the third cycle, samples were examined.
Long term stability: After 30 d in the deep freezer (-70°), six duplicates of LQC and HQC in the biological matrix were removed and thawed at room temperature (stability samples). Six duplicates of low and high QC samples were used to create a new calibration (comparison samples). If the mean percentage change in concentration was <15 %, CIN was considered stable in the matrix.
Mean percentage change=Calculated concentration of stability samples/Calculated concentration of comparison samples×100
Application to preclinical pharmacokinetics:
The method's applicability was determined by measuring the oral pharmacokinetics of CIN in rat plasma. Wistar albino rats measuring 150-200 g, of either sex, were confined with free access to food and water. The rats in the group (n=6) were given a commercially available CIN uncoated tablet formulation (1 mg/kg) to take orally. At 0, 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h, 0.2 ml of blood was obtained from the retro-orbital vein and placed in K2EDTA-containing centrifuge tubes. Cold centrifugation (Sigma, Germany) at 10 000 rpm for 10 min separated the plasma, which was then kept at -20° until analysis.
Results and Discussion
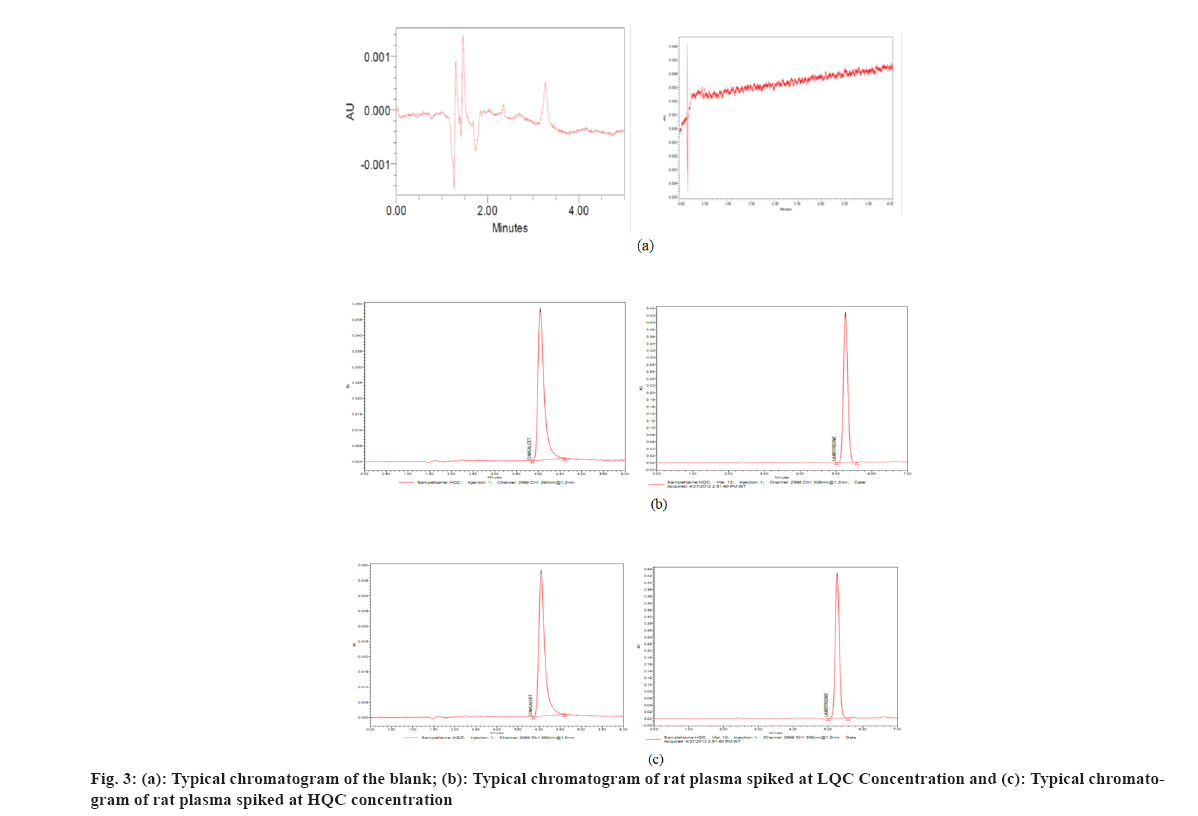
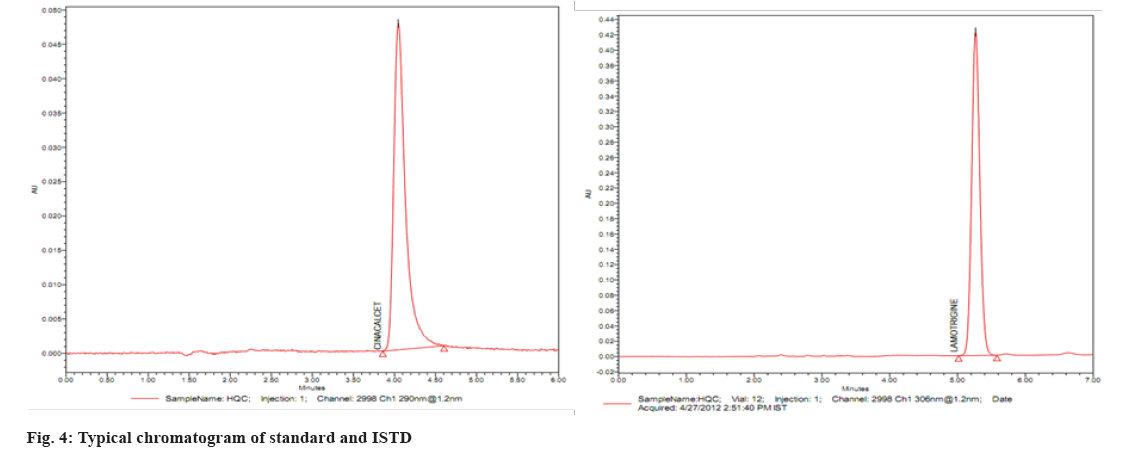
Several reversed-phase columns were tested for optimum selectivity, retention time, efficiency and symmetric peak shape for the CIN and ISTD, including Hypersil-C18, Waters C18 Column, Zorbax SB-CN and Phenomenex Luna. The Waters C18 column with (250 mm×4.6 mm i.d.) and 5 µm particle size was employed in this investigation because it had a greater Signal-to-Noise (S/N) ratio, better sensitivity and a better peak shape. Fast mass transfers were made possible by the Waters C18 columns, which reduced the overall assay, run time to 5.0 min. The mobile phase (50:50 v/v of 0.1 M dipotassium phosphate buffer (pH 6.8):ACN) also contributed to improved signal intensity, detector responsiveness and background noise reduction. The amount of ACN in the mobile phase was shown to have a significant impact on peak form and retention time. In this experiment, a mobile phase of ACN:0.1 M dipotassium phosphate buffer (pH 6.8) (50:50 v/v) was used. CIN and lamotrigine eluted in 3.23 and 3.28 min, respectively, at these ideal circumstances, as shown in fig. 3 and fig. 4.
Liquid-liquid extraction produces the cleanest sample when compared to protein precipitation or solid-phase extraction. To clean up the sample utilized in the investigation, liquid-liquid extraction was employed. For liquid-liquid extraction, several solvents were used, but ethanol:CH2Cl2 (70:30 v/v) removed the ISTD and CIN adequately. Furthermore, the introduction of lamotrigine, a stable and structurally comparable ISTD, compensated for any putative matrix impact and increased the method's resilience. These circumstances aided in the rapid isolation of CIN and ISTD from the plasma matrix.
Selectivity, linearity CaSRryover, the LOD, the LOQ, recovery, accuracy and precision and stability were all tested.
At the retention durations of CIN and ISTD, no interference peaks were identified, suggesting that the devised technique was highly selective for both CIN and ISTD. Fig. 3 show typical chromatograms of blank extracted rat plasma and spiked rat plasma at LQC and HQC concentrations, respectively.
To measure CIN levels in plasma samples, the ratio of CIN peak regions to ISTD was computed. In the concentration range of 1 µg/ml-24 µg/ml, calibration curves were linear, with a correlation value (r2) of 0.9996 (Table 1). The mean regression equation was y=1.0151x+00008, where x is the plasma concentration of CIN and y is the peak area ratio.
| STD ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Slope | Intercept | r2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal concentration (μg/ml) | 1 | 4 | 8 | 12 | 16 | 18 | 20 | 24 | |||
| P and A No. | Back calculated concentration (μg/ml) | ||||||||||
| P and A I | 1.06 | 4.011 | 7.803 | 12.127 | 16.294 | 17.849 | 19.802 | 23.946 | 3.2555 | 1.7883 | 0.9996 |
| P and A II | 0.98 | 3.993 | 8.104 | 11.918 | 16.461 | 18.006 | 19.914 | 23.989 | 3.2652 | 1.7843 | 0.99957 |
| P and A III | 1.03 | 4.068 | 7.995 | 12.336 | 16.475 | 17.952 | 20.197 | 24.494 | 3.320 | 1.8729 | 0.9997 |
| Mean | 1.02 | 4.0240 | 7.967 | 12.1270 | 16.4100 | 17.9357 | 19.9710 | 24.1430 | |||
| SD | 0.04 | 0.039 | 0.152 | 0.209 | 0.100 | 0.079 | 0.203 | 0.304 | NA | ||
| % CV | 3.95 | 0.97 | 1.910 | 1.72 | 0.61 | 0.44 | 1.02 | 1.26 | |||
| % Mean accuracy | 102.67 | 98.98 | 98.91 | 101.53 | 100.08 | 100.50 | 102.21 | 99.28 | |||
Table 1: Back Calculated Calibration Curve Standard for CIN
The r2 value should be less than 0.98; the percentage mean accuracy for all Column Chromatography (CC) standards except the LLOQ standard should be in the range of 85 %-115 %; the % mean accuracy for the LLOQ standard should be in the range of 80 %-120 %; at least 75 % of the CC standards in each P and A batch, including the LLOQ and highest CC standard, should meet the acceptance criteria; except for the LLOQ standard, all CC standards should have a % CV of less than 15 %; the LLOQ standard should have a percent CV of less than 20 %.
The lower LOQ and upper limit of quantification for CIN were determined to be 8 µg/ml and 24 µg/ml, respectively, indicating that this approach is most efficient in detecting CIN plasma concentrations in the range of 8 µg/ml-24 µg/ml. The HQC level was 19.2 µg/ml, the MQC level was 13 µg/ml and the LQC level was 8 µg/ml in quality control samples. With a signal-to-noise ratio of 3:1, the LOD was calculated to be 3 g/ml.
The recovery of CIN extraction at LQC, MQC and HQC is 80.54 %, 79.82 % and 76.32 % respectively, as shown in Table 2. The extraction recovery was found to be efficient and consistent.
| Replicate no. | HQC | MQC | LQC | |||
|---|---|---|---|---|---|---|
| Aqueous response | Extracted response | Aqueous response | Extracted response | Aqueous response | Extracted response | |
| Mean | 244421.5 | 186549.8 | 137622.3 | 109846.2 | 1957.5 | 1576.5 |
| SD | 14969.2 | 5688.4 | 8998.2 | 3997.3 | 135.4 | 138.9 |
| % CV | 6.12 | 3.05 | 6.54 | 3.64 | 6.92 | 8.81 |
| % Mean recovery | 76.32 | 79.82 | 80.54 | |||
| Overall % mean recovery | 78.721 | |||||
| Overall SD | 1.87 | |||||
| Overall % CV | 2.38 | |||||
Table 2: Recovery of Cinacalcet from Biological Matrix
The accuracy and precision values of the plasma concentrations of CIN were well within the acceptable limits (Table 3). The within-batch and between batch precision (% CV) values for CIN were 1.05 % to 8.08 %. Within batch and between batches accuracies were within 82.80 % to 104.08 % and 95.49 % to 103.27 %, respectively, as acceptable per guidelines.
| P and A batch | HQC | MQC | LQC | LLOQ-QC |
|---|---|---|---|---|
| Calculated concentration (μg/ml) | ||||
| Nominal concentration (μg/ml) | 19.2 | 13.0 | 8.0 | 1.0 |
| P and A I | ||||
| Mean | 19.2145 | 13.0168 | 8.0158 | 1.0083 |
| SD | 0.24787 | 0.22129 | 0.0529 | 0.125 |
| % CV | 1.29 | 1.70 | 0.66 | 1.24 |
| % Mean accuracy | 100.32 | 103.54 | 101.97 | 104.30 |
| n | 6 | 6 | 6 | 6 |
| P and A-II | ||||
| Mean | 19.2105 | 13.0637 | 8.1203 | 1.0092 |
| SD | 0.26126 | 0.2469 | 0.13805 | 0.001282 |
| % CV | 1.36 | 1.89 | 1.70 | 1.27 |
| % Mean accuracy | 89.12 | 97.42 | 99.59 | 92.66 |
| n | 6 | 6 | 6 | 6 |
| P and A III | ||||
| Mean | 18.9810 | 13.0653 | 8.0522 | 1.0167 |
| SD | 0.35494 | 0.15548 | 0.08455 | 0.01261 |
| % CV | 1.81 | 1.19 | 1.05 | 1.24 |
| % Mean accuracy | 91.16 | 99.35 | 97.58 | 82.80 |
| n | 6 | 6 | 6 | 6 |
| P&A IV | ||||
| Mean | 19.2497 | 13.3658 | 8.3540 | 1.1072 |
| SD | 0.3003 | 0.26331 | 0.13533 | 0.01295 |
| % CV | 1.56 | 1.97 | 1.62 | 1.17 |
| % Mean accuracy | 101.36 | 104.08 | 103.96 | 102.68 |
| n | 6 | 6 | 6 | 6 |
| Between batch precision and accuracy | ||||
| Mean | 19.1639 | 13.1279 | 8.13558 | 1.03535 |
| SD | 1.40088 | 0.72202 | 0.65735 | 0.05487 |
| % CV | 7.31 | 5.50 | 8.08 | 5.30 |
| % Mean accuracy | 95.49 | 101.10 | 103.27 | 98.11 |
| n | 24 | 24 | 24 | 24 |
Table 3: Accuracy and Precision Data for CIN at Different Concentration Levels
Due to the co-eluting of the endogenous component of the sample matrix with the CIN or ISTD during the creation of the HPLC assay, a matrix effect may impair the analytical method's accuracy and chromatography. The matrix influence on the current approach was determined to assure the process selectivity. Table 4 shows a summary of the findings. Despite using several plasma lots, the results indicated that CIN plasma concentrations were consistent. As a result, the current approach is the most reliable for measuring plasma CIN concentrations.
| S. No. | QC | HQC | LQC |
|---|---|---|---|
| Nominal concentration (μg/Ml) | 19.2 | 8 | |
| Plasma lot No. | Calculated concentration (μg/ml) | Calculated concentration (μg/ml) | |
| 1 | P-1 | 19.501 | 1.701 |
| 19.187 | 1.31 | ||
| 19.671 | 1.093 | ||
| 2 | P-2 | 19.675 | 1.134 |
| 19.449 | 1.006 | ||
| 19.306 | 1.317 | ||
| 3 | P-3 | 19.531 | 1.26 |
| 19.04 | 1.267 | ||
| 19.842 | 1.409 | ||
| 4 | P-4 | 19.603 | 1.257 |
| 19.531 | 1.338 | ||
| 19.901 | 1.485 | ||
| 5 | P-5 | 19.53 | 1.291 |
| 19.977 | 1.391 | ||
| 19.904 | 1.28 | ||
| 6 | P-6 | 19.976 | 1.096 |
| 19.494 | 1.13 | ||
| 19.809 | 1.265 | ||
| Mean | 19.60705556 | 1.279444444 | |
| SD | 0.266503608 | 0.161430452 | |
| % CV | 1.36 | 12.62 | |
| % Mean accuracy | 102.41 | 98.56 | |
Table 4: Matrix Effect for Estimation of CINACALCET
The % mean accuracy of LQC and HQC samples prepared from different biological matrix lots should be within 85 % to 115 %. The % CV of LQC and HQC samples prepared from different lots should be ≤15 %.
The stability experiments were designed to test all the possible conditions that the samples kept stored after collection and before the analysis. All stability results were summarized in Table 5. Stock solutions of CIN (1 µg/ml) and ISTD (1 µg/ml) were separately prepared and exposed to different temperatures. At 4°, both solutions were found to be stable for at least 1 mo under light-protected conditions. Besides, CIN spiked quality control samples were stable for at least 30 d if stored in the freezer at -28°±5°. Furthermore, three freeze-thaw cycles and bench top stability testing of the spiked samples for 7 h at room temperature indicated that CIN was stable in rat plasma. Interestingly, auto sampler stability of quality control samples also demonstrated that CIN was stable up to 54 h when kept at 5°±3° as shown in Table 6.
| QC | HQC | LQC | ||
|---|---|---|---|---|
| Nominal concentration (μg/ml) | Comparison samples | Stability samples | Comparison samples | Stability samples |
| 19.2 | 19.2 | 8 | 8 | |
| Replicate No. | Calculated concentration (μg/ml) | Calculated concentration (μg/ml) | ||
| 1 | 19.15 | 19.23 | 7.89 | 8.01 |
| 2 | 19.21 | 19.22 | 7.95 | 7.99 |
| 3 | 19.25 | 19.24 | 7.48 | 7.69 |
| 4 | 19.35 | 19.3 | 7.88 | 8.05 |
| 5 | 19.14 | 19.12 | 7.59 | 8.03 |
| 6 | 19.3 | 19.33 | 8.04 | 7.98 |
| Mean | 19.2333 | 19.24 | 7.805 | 7.958 |
| SD | 0.08311 | 0.0729 | 0.22 | 0.134 |
| % CV | 0.4321 | 0.3791 | 2.813 | 1.683 |
| % Mean accuracy | 102.28 | 98.64 | 103.4 | 98.12 |
| % Mean stability | 100.46 | 100.56 | ||
| n | 6 | 6 | 6 | 6 |
Table 5: Freeze and Thaw Stability Studies of CINACALCET
| QC | HQC | LQC | ||
|---|---|---|---|---|
| Nominal concentration (μg/ml) | Comparison sample | Stability sample | Comparison sample | Stability sample |
| 19.2 | 19.2 | 8 | 8 | |
| Replicate no. | Calculated concentration (μg/ml) | Calculated concentration (μg/ml) | Calculated concentration (μg/ml) | Calculated concentration (μg/ml) |
| 1 | 19.1 | 19.11 | 8.24 | 8.335 |
| 2 | 19.2 | 19.22 | 8.07 | 8.105 |
| 3 | 19.3 | 19.26 | 8.755 | 9.04 |
| 4 | 19.5 | 19.52 | 7.705 | 7.617 |
| 5 | 19.1 | 19.04 | 8.26 | 8.412 |
| 6 | 19.4 | 19.4 | 8.265 | 8.338 |
| Mean | 19.3 | 19.26 | 8.21583 | 8.3078 |
| SD | 0.13 | 0.181 | 0.33996 | 0.4619 |
| % CV | 0.7 | 0.939 | 4.13789 | 5.5603 |
| % Mean accuracy | 102 | 102.4 | 103.41 | 99.9 |
| % Mean stability | 102.35 | 101.655 | ||
| n | 6 | |||
Table 6: Auto Sampler Stability Studies of CINACALCET
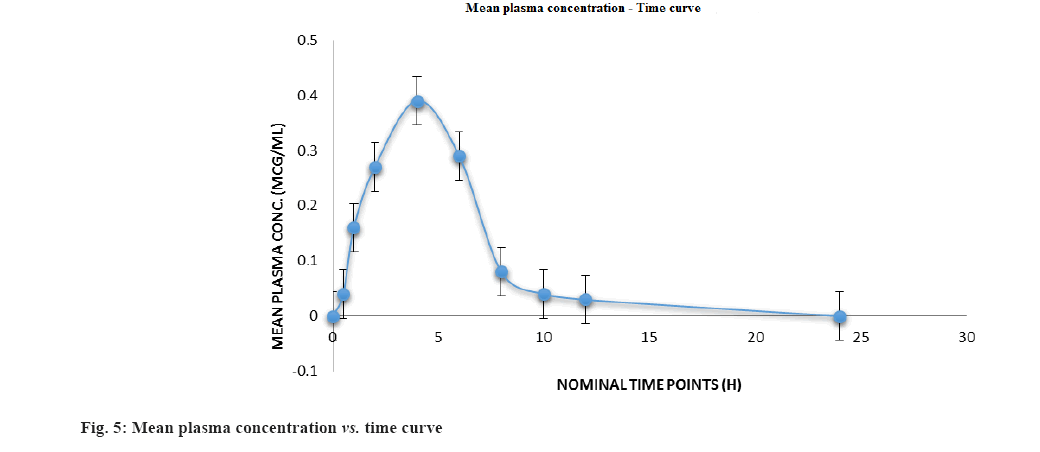
Following oral administration of 100 mg of CIN tablet (1 mg/kg) to Wistar rats, this devised approach was used to estimate preclinical pharmacokinetics of CIN. Fig. 5 depicts the mean plasma concentration profile; whereas Table 7 lists the mean predicted pharmacokinetic parameters based on the plasma concentration profiles.
| Pharmacokinetic parameter | CIN |
|---|---|
| Cmax (μg/ml) | 0.3855±0.0927 |
| Tmax (h) | 4.0±7.3456 |
| AUC(0-t) (μg.h/ml) | 1.2988±0.1390 |
| AUC(0-∞) (μg.h/ml) | 1.3413±2.3468 |
| T1/2 (h) | 13.378 |
Note: All values are expressed as mean±SD, n=6; Cmax: Maximum plasma concentration; Tmax: Time for maximum plasma concentration; AUC(0-t): Area under the curve from pre-dose to the last sampling time; AUC(0-t): Area under the curve from pre-dose extrapolated to infinity and T1/2: Elimination half-life
Table 7: Pharmacokinetic Parameters of CIN in RAT Plasma
For CIN in rat plasma, a simple, sensitive, repeatable, accurate and precise HPLC-photo diode array approach was devised. Over a concentration range of 1-24 µg/ml, the procedure was verified (r2=0.9996). It was discovered to have high accuracy and precision when used to estimate the oral pharmacokinetic profile of CIN in preclinical animals such as rats. Without jeopardizing sensitivity, this approach only required a tiny processing volume for extraction. This one-of-a-kind benefit of sample preparation approaches a straightforward analytical procedure. The method’s low LOQ suggests that it might be beneficial for determining drug concentrations even at low dosages. Furthermore, because the approach involves a tiny amount of plasma, it is simple to estimate the pharmacokinetic profile of CIN in animals (such as rats and rabbits) and people.
Acknowledgments:
The authors would like to thank JNTU Kakinada and Andhra University for their technical assistance.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sundararaman SS, van der Vorst EP. Calcium-Sensing receptor (CaSR), its impact on inflammation and the consequences on CaSR diovascular health. Int J Mol Sci 2021;22(5):2478.
[Crossref] [Google Scholar] [PubMed]
- Alsodani MH, Merza KH, Ali SH. A prospective randomized study on intermittent post-dialysis dose regimen of Cinacalcet, single-center experience. Psychol Edu J 2021;58(5):558-70.
- Balfour JA, Scott LJ. Cinacalcet hydrochloride. Drugs 2005;65(2):271-81.
- Papadopoulou A, Bountouvi E, Karachaliou FE. The molecular basis of calcium and phosphorus inherited metabolic disorders. Genes 2021;12(5):734.
[Crossref] [Google Scholar] [PubMed]
- Alvarado L, Sharma N, Lerma R, Dwivedi A, Ahmad A, Hechanova A, et al. Parathyroidectomy vs. cinacalcet for the treatment of secondary hyperparathyroidism in hemodialysis patients. World J Surg 2022;46(4):813-9.
[Crossref] [Google Scholar] [PubMed]
- Darwish IA, Al-Shehri MM, El-Gendy MA. Novel spectrophotometric method for determination of cinacalcet hydrochloride in its tablets via derivatization with 1,2-naphthoquinone-4-sulphonate. Chem Cent J 2012;6(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- A Darwish I, M AlShehri M, A Al-Gendy M. A highly sensitive fluorimetric method for determination of cinacalcet hydrochloride in tablets and plasma via derivatization with 7-chloro-4-nitrobenzoxadiazole. Curr Anal Chem 2013;9(3):504-12.
- Darwish IA, Al-Shehri MM, Al-Gendy MA. A highly sensitive HPLC method with non-extractive sample preparation and UV detection for trace determination of cinacalcet in human plasma. Digest J Nanomat Biostruct 2013;8(4):1563-70.
- Yang F, Wang H, Zhao Q, Liu H, Hu P, Jiang J. Determination of cinacalcet hydrochloride in human plasma by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 2012;61:237-41.
[Crossref] [Google Scholar] [PubMed]
- Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, van Wagenen BC, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 2004;308(2):627-35.
[Crossref] [Google Scholar] [PubMed]
- Li LL, Chen CL, Cai NF, Yi JL, Zheng C, Feng Y, et al. An improved LC–MS/MS method for determination of cinacalcet in human plasma and its application to the evaluation of food intake effect on the pharmacokinetics of cinacalcet in healthy volunteers. Biomed Chromatogr 2019;33(10):e4631.
[Crossref] [Google Scholar] [PubMed]
- Nirogi R, Kandikere V, Komarneni P, Aleti R, Padala N, Kalaikadiban I. Quantification of cinacalcet by LC–MS/MS using liquid–liquid extraction from 50 μl of plasma. J Pharm Biomed Anal 2011;56(2):373-81.
[Crossref] [Google Scholar] [PubMed]
- A Wani T, Y Khalil N, Darwish I, Iqbal M, H Bakheit A. Highly sensitive and simple validated ultra-performance liquid chromatography/tandem mass spectrometry method for the determination of cinacalcet in human plasma. Curr Pharm Anal 2014;10(1):51-7.