- *Corresponding Author:
- S. K. Kashaw
Pharmaceutical Chemistry Division, Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar - 470 003, India
E-mail: sushilkashaw@gmail.com
| Date of Submission | 17-Feb-2010 |
| Date of Revision | 23-Oct-2010 |
| Date of Acceptance | 14-Jan-2011 |
| Indian J Pharm Sci, 2011, 73 (1): 74-76 |
Abstract
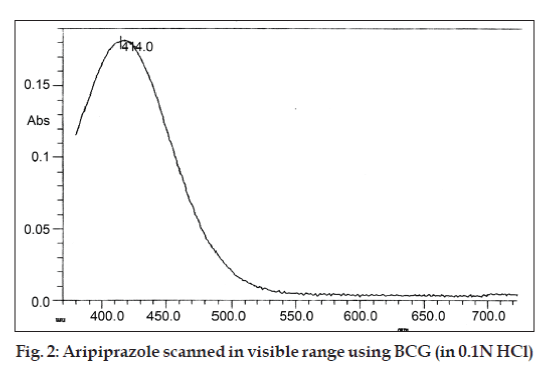
A simple, accurate and economic spectrophotometric method for the determination of aripiprazole in tablet formulation is proposed. In the present method acidic solution of the aripiprazole formed colored ion-association complexes with bromocresol green, soluble in chloroform. Yellowish orange chromogen showed λmax at 414 nm and obeyed Beer’s law in the concentration range of 10-60 µg/ml. Statistical analysis and recovery studies validated the method. The proposed method is rapid, precise and accurate and can be applied for the routine estimation of aripiprazole in the laboratory.
Keywords
Aripiprazole, UV/Vis spectrophotometry
Introduction
Aripiprazole (C23H27Cl2N3O2; molecular weight=448.39) is chemically 7-4-[4-(2,3-dichlorophenyl)-1-piprazenyl] butoxy]-3,4-dihydro-(1H)-quinolinone (fig. 1). It is an atypical antipsychotic drug with serotonin 5- HT1A-receptor partial agonist and 5HT2A-receptor antagonist properties as well as being a partial agonist at dopamine D2 receptors. It is used in the management of schizophrenia and is also under investigation for bipolar disorder[1,2]. Literature survey revealed that several methods including HPLC with column switching and spectrophotometric detection[3] and reverse phase HPLC methods have been reported for the estimation of aripiprazole. Till date no UV/ Vis spectrophotometric method for the estimation of aripiprazole is reported. Therefore the present paper reports a simple and sensitive spectrophotometric method for the estimation of aripiprazole in tablets.
A GBC Cintra-10 double beam UV/Vis spectrophotometer (Australia) equipped with 10 mm matched quartz cells was used in the present investigation. The spectrophotometer was run at a spectral band width of 5 nm with a scan speed of 24~1400 nm/min. Methanol AR grade, Bromocresol green dye and chloroform AR grade (Qualigens, Mumbai) was used in the present study. Pure aripiprazole as gift sample was obtained from M/s Torrent Pharmaceuticals Ltd, Ahmdebad. All other chemicals used were of analytical grade.
A stock solution (S) of aripiprazole was prepared by dissolving 10 mg (accurately weighed) of the drug in 1 ml of concentrated sulphuric acid and finally the volume was made up to 10 ml with distilled water (1000 μg/ml). Stock solution (S) was further diluted with distilled water to get a working standard solution (100 μg/ml) for spectrophotometric estimation. The stock solution was suitably diluted to produce solution of concentration 10 μg/ml, this working solution was scanned in the entire UV/Vis range (200-800 nm) to determine the λmax (fig. 2). Different aliquots (1.0, 2.0…6.0 ml) of working standard solution was taken in to a series of 10 ml volumetric flasks, and to this 2 ml of buffer solution and 2 ml of 0.5% bromocresol green dye solution were added and volume were made up with distilled water in each volumetric flask to prepare a series of concentration between 10-60 μg/ml. Poured in different separating funnels and the solutions were extracted with chloroform (successively) 4´2 ml. Separated organic layer were collected in 10 ml volumetric flask and volume was made up with chloroform. The absorbance of the yellowish chromogen was measured at 414 nm, against a reagent blank and calibration curve was plotted in 10 to 60 μg/ml concentration range.
Three commercial formulations, Arip-MT 15 (M/s Torrent Pharmaceuticals Ltd.) Asprito (Intas Pharmaceuticals Ltd.) and Arive (M/s Alkem Laboratory Ltd.) were purchased from local pharmacy. Average weight of a tablet was calculated by weighing 20 tablets. Tablets were powdered finely and powder equivalent to 100 mg of aripiprazole was extracted with 10 ml portion of concentrated sulphuric acid, filtered and the volume made up to 100 ml with water (1000 mg/ml). Aliquots of this solution were treated as mentioned for standard. The above tablet powder solution was then suitably diluted to obtain concentration range of 10-60 μg/ml of aripiprazole. Absorbances were taken and concentrations of aripiprazole were determined using the calibration curve. Finally calculations were made with the dilution factor to find out the concentration of the drug in tablets. The experiments were repeated six times to check its reproducibility. To study accuracy, reproducibility and precision of the method, recovery studies were carried out by adding known amount of pure drugs to the analyzed sample of tablet powder and mixture was reanalyzed for the drug content using the proposed method. Results of recovery were found to be satisfactory. The proposed method for determination of aripiprazole showed molar absorptivity of 6.5018´104 l/mol´cm. Linear regression of absorbance on concentration obtained the equation y = 0.0125x + 0.0539 with a correlation coefficient (r) of 0.9968 (Table 1). Statistical analysis of commercial formulations is presented in Table 2. The recovery experiments indicated the absence of interference from the commonly encountered pharmaceutical additives and excipients (Table 3).
| Parameters | Values |
|---|---|
| max (nm) | 414 |
| Beer’s Law Limit (µg/ml) | 10-60 |
| Molar absorptivity (L/mol.cm)* | 6.5018´104 |
| Sandell’s sensitivity | 0.0689 |
| (µg/cm2x0.001 absorbance unit) | |
| Regression equation | y = 0.0125x +0.0539 |
| Slope (a) | 0.0125 |
| Intercept (b) | 0.0539 |
| Correlation coefficient (r) | 0.9968 |
| *Average of six determinations. |
*Average of six determinations.
Table 1: Optical characteristics and regression analysis of aripiprazole
| Brand name | Label claim | Amount Found | SD* | COV* | SE* | ‘t’ cal* | ‘t’ the* |
|---|---|---|---|---|---|---|---|
| (mg/tab) | (mg/tab)* | ||||||
| ARIP-MT 15 | 15 | 15.01±0.01 | 0.0182 | 0.1214 | 0.0074 | 0.6856 | 3.365 |
| ASPRITO | 10 | 10.01±0.01 | 0.0126 | 0.1257 | 0.0051 | 1.7712 | |
| ARIVE | 20 | 20.03±0.02 | 0.0279 | 0.1397 | 0.0114 | 2.7391 |
*Average of six determinations. *Theoretical‘t’ values were calculated at 95% confidence level with (n-1) degrees of freedom ‘t’ (0.025, 5) = 3.365. SD = Standard deviation, COV = Coefficient of variance and SE = Standard error.
Table 2: Statistical analysis of aripiprazole
| Brand name | % Recovery ± S.D* |
|---|---|
| ARIP-MT 15 | 101.62±0.16 |
| ASPRITO | 100.75±0.02 |
| ARIVE | 100.38±0.01 |
| *Average of six determinations |
*Average of six determinations
Table 3: Recovery studies of aripiprazole
Significantly low values of standard deviation, standard error and coefficient of variation indicates the precision of the proposed method. These values are compared with the theoretical values of 100 percent by means of unpaired students ‘t’ test (Table 2). As the calculated ‘t’ values were less than theoretical ‘t’ values, it is calculated that the results of analysis were in good agreement for each brand of tablet. To test the accuracy and reproducibility of the proposed method, recovery experiments were performed by adding known amount of pure drug to previously analyzed samples, and these samples were reanalyzed by the proposed methods. The percentage recovery was close to 100% for both methods. The results are summarized in Table 2. The reproducibility, repeatability, and accuracy of this method were found to be good evidenced by low standard deviation.
Acknowledgements
The authors wish to thank Torrent pharmaceuticals ltd., Ahemdebad, for providing gift sample of aripiprazole. One of the authors (RJ) thanks UGC, New Delhi for providing financial assistance.
References
- Sweetman SC. Martindale: The Complete Drug Reference. 34th ed. London: Royal Pharmaceutical Society of Great Britain; 2005. p. 671.1.
- Budavari S. The Merck Index. 13th ed. Whitehouse Station, NJ: Merck and Co., Inc.; 2001. p. 791.
- Kirchherr H, Kuhn-Velten WN. Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: A multi-level, single-sample approach. J Chromatogr B AnalytTechnol Biomed Life Sci 2005;51:1718.