- *Corresponding Author:
- N. Mohammadpour Dounighi
Departments of Human Vaccines and Sera, and Serum Research Institute, Karaj, Zipcode 31976197, Iran
E-mail: nasser_mohammadpour@yahoo.com
| Date of Submission | 07 November 2012 |
| Date of Revision | 30 April 2013 |
| Date of Acceptance | 20 May 2013 |
| Indian J Pharm Sci, 2013;75(4):442-449 |
Abstract
During last decades, diphtheria has remained as a serious disease that still outbreaks and can occur worldwide. Recently, new vaccine delivery systems have been developed by using the biodegradable and biocompatible polymers such as alginate. Alginate nanoparticles as a carrier with adjuvant and prolong release properties that enhance the immunogenicity of vaccines. In this study diphtheria toxoid loaded nanoparticles were prepared by ionic gelation technique and characterized with respect to size, zeta potential, morphology, encapsulation efficiency, release profile, and immunogenicity. Appropriate parameters (calcium chloride and sodium alginate concentration, homogenization rate and homogenization time) redounded to the formation of suitable nanoparticles with a mean diameter of 70±0.5 nm. The loading studies of the nanoparticles resulted in high loading capacities (>90%) and subsequent release studies showed prolong profile. The stability and antigenicity of toxoid were evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and ouchterlony test and proved that the encapsulation process did not affect the antigenic integrity and activity. Guinea pigs immunized with the diphtheria toxoid-loaded alginate nanoparticles showed highest humoral immune response than conventional vaccine. It is concluded that, with regard to the desirable properties of nanoparticles and high immunogenicity, alginate nanoparticles could be considered as a new promising vaccine delivery and adjuvant system.
Keywords
Alginate, diphtheria toxoid, nanoparticle, vaccine delivery system
Diphtheria is a contagious, acute and bacterial disease, which is caused by Corynebacterium diphtheriae. Most of the clinical symptoms of the disease are pertain to the release of potent diphtheria toxin from lysogenized strains bacteria. Current diphtheria vaccines are prepared by diphtheria toxoid, which is nontoxic but antigenic and is mostly combined with alum or Al(OH)3 as an adjuvant. In the 1970s, extended vaccination almost led to the eradication of this disease, but in recent decades, diphtheria has been resurgence and, in spite of widespread vaccination outbreaks, it can still occur worldwide. It seems that inadequate immunogenicity which leads to using boosters is probably due in part to adjuvants deficiency [1]. The currently approved vaccine adjuvants are not always sufficiently potent to induce an efficient protective immune response against different target pathogens, particularly in immunologically hyporesponsive populations such as the elderly and immunocompromised populations, where there is a decreased antibody response to T cell dependant antigens, owing to the compromised T cell function. Aluminum salt‑based adjuvants (alum) have been the most widely used adjuvants in humans. Some adverse effects such as muscle soreness and occasional fever have been observed by alum. Moreover, alum can induce granulomas at the injection site, a concern for vaccines requiring frequent boosts. Risk of granulomas becomes particularly high when alum‑based vaccines are injected subcutaneously or intradermally [2]. In this regard, the new generation vaccines consisting of particulate systems have been suggested and developed to be used as a potent adjuvant to overcome and extirpate these kinds of fatal diseases [3‑7].
The particulate systems made from biopolymers have promising properties and safety to be used as carriers and adjuvant for vaccine delivery. These systems can offer three different mechanisms: Targeting of antigens to the relevant antigen‑presenting cells of the immune system, i.e., macrophages, direct activation of cells in the immune system (e.g., cytokines), and providing controlled‑release properties for encapsulated protein and leading to the increase of the immune response in the body [8‑11]. Various types of mucoadhesive, biocompatible, and biodegradable polymers such as chitosan [12,13], dextran [14], and sodium alginate have been used to prepare micro or nanoparticles as new vaccine delivery systems [15‑17]. Due to its unique properties, sodium alginate has been used as a carrier for different biological agents such as genes [18], antigens, and drugs [19].
Alginate is derived from marine brown algae cell walls. It is anionic polysaccharide consisting of a chain of (1-4)‑linked β‑D‑mannuronic acid and α‑L‑guluronic acid in different arrangements of residues. Alginate is a natural, biodegradable, and mucoadhesive polymer that does not produce toxicity in administration [20,21]. Alginate nanoparticles (NPs) as hydrophilic carriers prolong the antigen release and enhance the immunogenicity greater than traditional vaccines which is because of their adjuvant properties. Moreover, in nasal and oral administration because of mucoadhesive properties and enhancing permeability, they can reduce degradation in acidic environment. Also, alginate particles have not been observed to agglomerate in any of the major organs. Recent reports have revealed the importance of size and advantages of NPs over microparticles [22,23]. It is well known that the size of the particles should be proportionate to the loaded protein and administration rout; for example, nasal [24], oral [25,26], and subcutaneous [27,28]. While compared with the microparticles, the advantages of NPs are their ability to improve drug encapsulation, pharmacokinetics, bioavailability as well as therapeutic efficacy [29]. Moreover, the preservation of antigen and stability during entrapment are also essential for successful controlled release and immunogenicity of vaccines. Alginate NPs can be prepared easily by inducing gelation with divalent cations such as calcium ions [15,30]. Ionic gelation method is one of the more simple techniques that lead to the formation of calcium alginate complexes. In this matter, the interaction between the calcium ions and the glucuronic sequences of alginate polymer leads to egg‑box structures [31]. This technique offers benefits such as a single‑step process which can be readily scaled up, less toxic reagents, simplification of the procedure that does not require specialized equipments, low cost, narrow particle size distribution and simple optimization to improve yield and entrapment efficiency. The aim of the present study was to develop a new vaccine formulation based on alginate NPs containing diphtheria toxoid (DT) as a novel and promising adjuvant and vaccine delivery system.
Materials and Methods
DT (5600 Lf/ml), diphtheria toxin, and antitoxin of DT were obtained from Razi Vaccine and Serum Research Institute, Karaj, Iran. Low molecular weight sodium alginate (medium viscosity of 3500 cps for a 2% w/v solution) and poly-L-lysine (4000-15000 Mw) were purchased from Sigma–Aldrich, St. Louis, USA. Also, calcium chloride dihydrate, Coomassie brilliant blue G‑250, methanol 95%, and phosphoric acid 85% (w/v) were purchased from Merck, Germany. All the aqueous solutions were prepared with double distilled water. All the other materials used in this study were in analytical grade.
Investigating diphtheria toxoid characteristics
The toxoid titer and protein concentration of DT were determined by Ramon flocculation test [32] and micro‑Kjeldahl assay [33], respectively. The molecular weight of the DT was also evaluated by the sodium dodecyl sulfate poly acrylamide gel electrophoresis (SDS‑PAGE) with the standard protein markers. Briefly, DT was mixed with buffer solution, boiled for 10 min, and subjected to SDS‑PAGE under standard operating conditions (a constant current of 1200 mA for 6 h at room temperature). Gels were stained with 0.1% Coomassie brilliant blue in 10% of acetic acid in a solution of methanol:water (1:1).
Preparing alginate nanoparticles
Alginate NPs were synthesized by ionic gelation method [34]. In brief, CaCl2 solution was added to sodium alginate solution dropwise under a constant homogenization rate 1300 rpm (Velp Scientifica, Usmate (MB), Italy) at room temperature. Subsequently, the suspension was centrifuged at 30000×g for 30 min (Sorvall RC 26 Plus, Sorvall, USA) and the supernatant was discarded. Pellet of NPs was freeze‑dried (Christ Alpha 1‑2, Martin Christ Gefriertrocknungsanlagen GmbH, Germany) without using any cryoprotectant. The structure and properties of NPs changed markedly with slight alterations in polymer, cross‑linking agent concentration, and some physical conditions. Hence, to find suitable concentrations and evaluate the effects of CaCl2 and sodium alginate concentrations on the NPs’ properties, various concentrations of CaCl2 as a cross‑linking agent (0.025, 0.05, 0.075, 0.1, 0.15% w/v) were added to different concentrations of sodium alginate (0.07, 0.15, 0.3, 0.5, 0.75% w/v) dropwise at the flow rate of 0.01 ml/s using burette under continuous homogenization. The suspension of NPs was maintained under constant homogenization (1300 rpm) for 45 min at room temperature to improve curing and then it was centrifuged at 30 000×g and 4° for 30 min. After obtaining suitable concentration, the effect of varying homogenization rate (600, 800, 1000, 1100, 1300 rpm) and homogenization time (15 to 60 min) was investigated on particle characteristics. In order to study one of the above‑mentioned parameters, other parameters were kept constant.
Characterization of nanoparticles
Morphological characteristics and surface appearance of alginate NPs were examined by scanning electron microscopy (SEM, INCAWave, Oxford Instruments, UK). The particle size distribution and zeta potential of NPs were determined by means of dynamic light scattering (DLS) (zen 3600 Laser Particle Size Analyzer, Malvern Instruments, UK). These measurements were performed at least three times with independent particle batches.
Loading diphtheria toxoid in alginate nanoparticles
To prepare DT‑loaded NPs, calcium chloride aqueous solution (0.1% w/v) was added dropwise to the sodium alginate solution (0.3% w/v) with ratio of 1:3 which contained various concentrations of DT (0.1, 0.2, 0.4, 0.8, 1, 1.5, 2, 2.5, 3 mg/ml) under the homogenization rate of 1300 rpm. Then NPs were coated with poly‑L‑lysine (PLL) [34]. Finally, NPs were freeze‑dried and stored at 4-8°.
Evaluating loading efficiency and loading capacity
To assess the ability of alginate NPs to DT entrapment, loading efficiency (LE) and loading capacity (LC) of NPs were determined indirectly by determining the free DT in the supernatant. For this purpose, NPs’ suspension was centrifuged at 30 000×g for 30 min and supernatant was recovered. The concentration of DT in the supernatant was measured by Bradford protein assay [35]. The concentration of DT in the supernatant was calculated using the standard curve prepared at the same time and the LE and LC values were calculated.
Fourier transform infra‑red measurements
In order to analyze the interactions between polymer, CaCl2, and DT in alginate NPs and DT‑loaded NPs, the samples were evaluated by Fourier transform‑infrared spectroscopy (FT‑IR; Jasco FTIR‑410; Jasco, Colchester, UK) at room temperature [36].
Gel electrophoretic analysis of the released DT
The molecular weight and integrity of encapsulated DT were determined by SDS‑PAGE. Encapsulated DT released from alginate NPs in PBS and the stability of encapsulated DT were evaluated using SDS‑PAGE (as mentioned in investigating diphtheria toxoid characteristics).
Ouchterlony double immunodiffusion
The antigen activity (before and after encapsulation) was evaluated by double immunodiffusion test. For this purpose, an equine DT antitoxin was used. The precipitin lines were visualized with staining by Coomassie brilliant blue solution [37].
In vitro release of diphtheria toxoid
The In vitro DT release profile was performed in PBS at 37°. Briefly, freeze‑dried DT‑loaded NPs and DT‑adsorbed Al3PO4 adjuvant (traditional adjuvant), both containing equal amount of DT, were accurately weighed and suspended within several enclosed test tubes containing 1 ml pH 7.4 PBS solution (1 mg in each tube) and incubated in a shaker incubator (100 cycles/min) with the temperature adjusted at 37°. At scheduled time intervals, the samples were centrifuged at 13000 rpm for 20 min at 4° and the supernatant was collected. The amount of DT released in the supernatant was determined and considered as a percent of total DT encapsulated in alginate NPs and adsorbed in traditional adjuvant [35].
In vivo studies
In this study, healthy white guinea pigs with 300-350 g weight were used for immunogenicity test [38]. The animals were distributed to six groups (n=5). The samples were injected subcutaneously with 0.5 ml dose of the following formulations twice with 4 weeks of time interval. The first group received only normal saline; the second one received DT‑loaded NPs with 10 Lf/ml DT; the third, alginate NPs (12.5 μg/ml); the fourth, 10 Lf DT adsorbed to Al3PO4; the fifth, free DT (10 Lf/ml) and the sixth received alginate NPs (12.5 μg/ml) and free DT (10 Lf/ml) suspension. On 28th day after the second immunization, the guinea pigs were bled and serums were collected and stored at −20º until being analyzed for antibody titers. According to World Health Organization technical report series 800 (WHO TRS 800), the intradermal challenge method was used for potency test [39]. Different concentrations of the standard diphtheria toxin were incubated with suitable dilution of animal’s sera for 1 h at ambient temperature. Then from each tube, 0.5 ml was intradermally injected at the six separate sites in both shaved flanks of rabbits and observed after 72 h. The erythema score of different sites were examined and used for calculation of diphtheria antitoxin titers in guinea pig sera. The intradermal challenge scores for all the animals receiving the same sample mixture were tabulated together and used for the calculation of the potency for each of the test preparations by probit analysis.
Results and Discussion
Before loading the DT within alginate NPs, the properties of toxoid such as concentration, antigenic titer, and molecular weight were estimated. The total concentration and antigenic titer of DT were obtained as 6.8±0.1 mg/ml and 5600 Lf/ml, respectively. In addition, based on the SDS‑PAGE pattern, the molecular weight of DT was 66 KDa. DT purity was confirmed since no extra band was observed in the gel electrophoresis.
To find optimum parameters, preparation of alginate NPs was carried out using various concentrations of alginate and CaCl2 solutions. The effect of CaCl2 concentration on the formation of NPs was studied by fixing the alginate solution at 0.3% w/v. In the present study, 0.3% w/v of sodium alginate and 0.1% w/v of CaCl2 were observed as a suitable concentration for preparing optimized NPs (Table 1). Subsequently, the effect of other processing parameters like homogenization time and homogenization rate on the characteristics of NPs was evaluated. In the present study, with the increase of homogenization time from 15 to 60 min, marginal decrease in particle size was observed; however, after 45 min, no significant change was observed in particle size. Increase in homogenization rate also revealed the decrease in particle size and brought good sphericity. Hence, this observation illustrated that homogenization rate of 1300 rpm at 45 min for homogenization time can be the optimized conditions (Table 1).
| Formulations code | Physicochemical factors | Yield of nanoparticles (% w/v) | Nanoparticle formation | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | |||
| F 1 | 0.07 | 0.10 | 45 | 1300 | 0.055±0.001 | Sd |
| F2 | 0.15 | 0.10 | 45 | 1300 | 0.091±0.008 | S |
| F 3 | 0.30 | 0.10 | 45 | 1300 | 0.182±0.005 | SNd |
| F 4 | 0.50 | 0.10 | 45 | 1300 | 0.300±0.0005 | Gd |
| F5 | 0.75 | 0.10 | 45 | 1300 | 0.021±0.0009 | G |
| F6 | 0.30 | 0.025 | 45 | 1300 | 0.004±0.0006 | S |
| F7 | 0.30 | 0.05 | 45 | 1300 | 0.013±0.0005 | S |
| F8 | 0.30 | 0.075 | 45 | 1300 | 0.180±0.0011 | S |
| F9 | 0.30 | 0.10 | 45 | 1300 | 0.183±0.0052 | SN |
| F 10 | 0.30 | 0.15 | 45 | 1300 | 0.219±0.0005 | MGd |
| F11 | 0.30 | 0.10 | 0 | 1300 | 0.178±0.0038 | MG |
| F12 | 0.30 | 0.10 | 15 | 1300 | 0.183±0.0015 | MG |
| F 13 | 0.30 | 0.10 | 30 | 1300 | 0.179±0.0021 | NSd |
| F14 | 0.30 | 0.10 | 45 | 1300 | 0.180±0.0052 | SN |
| F15 | 0.30 | 0.10 | 60 | 1300 | 0.180±0.0019 | SN |
| F16 | 0.30 | 0.10 | 45 | 600 | 0.225±0.0019 | G |
| F17 | 0.30 | 0.10 | 45 | 800 | 0.214±0.0040 | G |
| F18 | 0.30 | 0.10 | 45 | 1000 | 0.168±0.0012 | MG |
| F19 | 0.30 | 0.10 | 45 | 1100 | 0.175±0.0012 | NS |
| F20 | 0.30 | 0.10 | 45 | 1300 | 0.182±0.0052 | SN |
X1=Sodium alginate concentration (% w/v), X2=CaCl2 concentration (% w/v), X3=homogenization time (min), X4=homogenization rate (rpm), dG=continuous gel, SN=suitable nanoparticles, S=solution, MG=microgel gel, NS=non spherical
Table 1: Effet of Physicochemical Factors on Nanoparticle Formation
As mentioned before, due to various desirable characteristics, sodium alginate has been recognized as a suitable biopolymer for antigen delivery. These properties include suitable biodegradability, easy preparation of micro and nanoparticles, mucoadhesive nature, and high loading capacity for proteins. While preparing NPs, size as well as morphology is important for quality control and biodistribution [40]. Hence, in the present study, in order to prepare suitable NPs, the influence of processing parameters on the features of alginate NPs was investigated. The optimum formulation was selected according to good morphology (in terms of particle size, poly dispersity index, and sphericity), high protein loading and suitable sustained release. As shown in Table 1, the use of concentration higher than 0.3% w/v of sodium alginate and 0.1% w/v concentrations of CaCl2 led to the formation of macroscopic and microscopic gel aggregates, respectively. It is also worth noting that high concentration of alginate led to the increase of viscosity and subsequent decrease of shear stress. Use of the lower mentioned concentration of sodium alginate and CaCl2 could not produce desirable NPs. In contrast, in the presence of high CaCl2 concentrations, calcium divalent cations allowed for the formation of microdomains with high local concentrations of alginate. In other respects, this effect was presumably related to the fact that, with the increase in the number of Ca2+ per unit solution volume, the equilibrium between Ca2+ and alginate binding sites was disordered and could extend the cross‑linking between the glucoronic acid residues and the Ca2+ therefore lead to microscopic gel aggregates. The present results correlated with the report of Gonzalez Ferreiro et al. [16], which suggested that using lower than 0.1% w/v concentrations of CaCl2, the numbers of binding sites on alginate were higher than Ca2+ ions and the Ca2+ could not induce the appearance of very well‑organized and desirable NPs with good density.
In order to study the ability of alginate NPs for encapsulating antigens and the effect of the amount of antigen on LE and LC, different concentrations of DT were loaded in NPs and the DT‑loaded NPs were prepared as mentioned above. The studies demonstrated that with the increase of DT concentration, LE and LC increased, too. More importantly, NPs with high concentration of DT showed considerable LE (>80%) and LC (>90%) (fig. 1). Finally, 2.5 mg/ml concentration of DT was adopted as the suitable concentration for preparing optimum NPs. It was observed that the large amount of DT could be incorporated into the NPs and the use of appropriate concentration of DT could lead to the increase in the LC up to 90% (fig. 1). This phenomenon can be explained by the fact that, association of antigens with particles can be due to different linkage or physical entrapment and strongly depends on unsaturated sites to the formation of hydrogen and electrostatic bounds on alginate chains. Hence, DT interacts with hydroxyl groups of alginate chains via electrostatic and hydrogen bounds. So, with increasing the DT concentration, free binding sites within polymer chains saturated with antigen molecules and LE did not increase in higher than 2.5 mg/ml concentration of DT. It could be suggested that the quantity of calcium alginate become insufficient to entrap more amount of antigen.
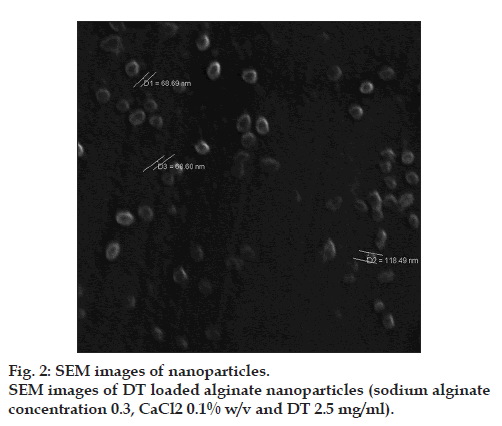
The results determined by DLS demonstrated that both alginate and DT‑loaded NPs exhibited relatively narrow particle size distribution by a relatively low polydispersity index (PDI) value. The optimized alginate NPs had the particle size distribution of <100 nm with PDI=0.46. The zeta potential of alginate NPs was −46.7. The DT‑loaded NPs with PDI=0.32 were smaller than the alginate NPs and zeta potential of these NPs decreased from −46.7 to −36.1. Poly‑L‑lysine, as a cationic poly‑amino acid that is likely to have more affinity for the mannuronic residues [41], was used to further crosslink the micro domains to the NPs. After adding poly‑L‑lysine, the electrostatic interaction between the positively charged –NH3 + of poly‑L‑lysine and negatively charged –COO− of alginate redounded to smaller size and lower negative charge of DT‑loaded NPs compared with alginate NPs. SEM photograph indicated that NPs were spherical and discrete without any aggregation (fig. 2).
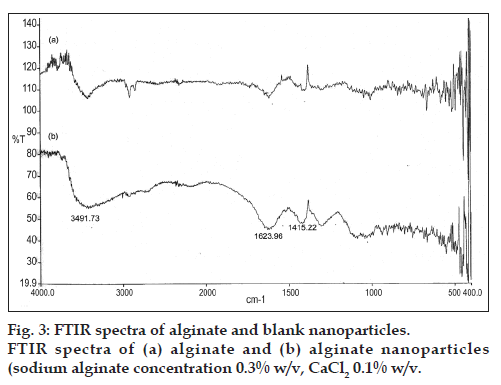
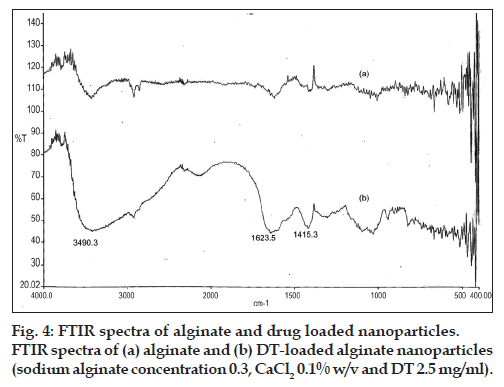
Based on interpretation of the FTIR spectra, the slight difference can be observed in the width and frequency of the peaks. In three spectra, the strong and broad peaks in the 3500-3300 cm−1 ranged corresponding to O-H stretching and intermolecular hydrogen bonding. Around the wave numbers of 1700-1400 cm−1, the observed peaks belonged to the C=O stretching (amide). Carboxyl peaks were seen near 1613 cm–1 (symmetric –COO− stretching vibration) and 1415 cm–1 (asymmetric COO– stretching vibration) which were broad after interaction with DT. To compare the spectra of free and loaded NPs, changes were observed in the amino groups, carboxyl groups, and amide peaks, which revealed an ionic interaction between the carbonyl group of alginate and the amino group of DT and it also proved that DT was loaded in NPs (figs. 3 and 4). The FTIR results confirmed the encapsulation of DT in NPs. These findings were similar to the previous reports [16,42].
Evaluation and comparison of DT (before and after entrapment within the alginate NPs) were performed using the SDS‑PAGE analysis as well as the Ouchterlony double immunodiffusion method. These results confirmed that the integrity and antigen activity of the encapsulated DT remained intact.
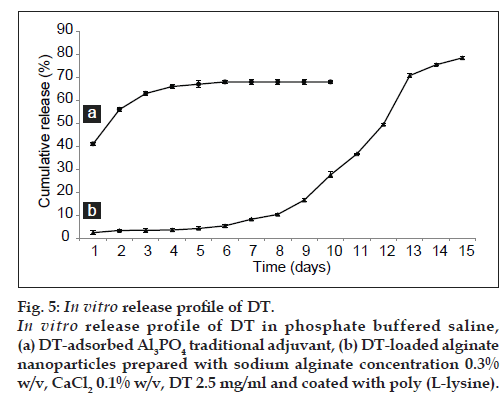
In vitro release study of DT was performed by phosphate buffer (PBS, pH 7.4) at 37°. The In vitro DT release from DT‑loaded nanoparticles was continuous and slow profile, as depicted in these results (fig. 4). Prolong release of DT was observed over a period of 15 days. About 80% of DT released in 336 h. The sustained release of antigen without any burst release probably came from the fact that DT macromolecules were bind to NPs by strong interactions. Additionally, the presence of PLL membrane on the surface of particles could increase the stability of NPs and bring about an extended release profile. The In vitro release profile of DT adsorbed to Al3PO4 shown in fig. 5. The release profile of DT adsorbed to Al3PO4 exhibit an initial burst release of about 41% in the first 24 h and the slow release of 27% during the following 96 h (fig. 5). The observed high burst effect was due to dissociation of protein molecules that were loosely bound to the surface of traditional adjuvant crystals. Importantly, the DT‑loaded NPs prepared in the present work can be a promising candidate for antigen delivery due to the good sustained release profile and the absence of burst release (fig. 5). These results have been confirmed by other reports [43]. There are few reports on bovine serum albumin (BSA) and DT release profiles, the observations of which are different when compared with the present results. In those cases, the release was a biphasic linear profile. The first phase was very fast, which was followed by a slow release [44]. It seems that this difference can be pertaining to the use of different procedures for preparing particles.
Figure 5: In vitro release profile of DT. In vitro release profile of DT in phosphate buffered saline, (a) DT‑adsorbed Al3PO4 traditional adjuvant, (b) DT‑loaded alginate nanoparticles prepared with sodium alginate concentration 0.3% w/v, CaCl2 0.1% w/v, DT 2.5 mg/ml and coated with poly (L‑lysine).
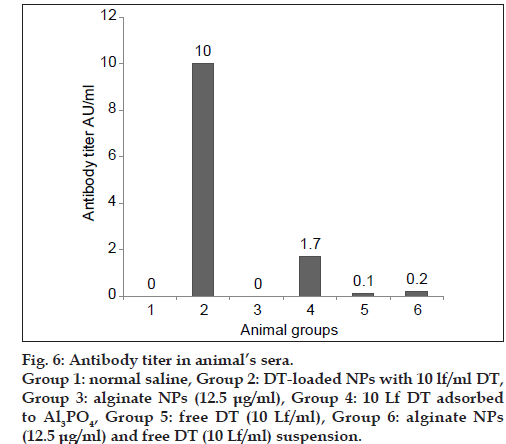
The immunogenicity of the NPs containing DT was compared with traditional adjuvant (Al3PO4) and positive and negative controls. These results illustrated that the level of antibody was significantly high in the animal vaccinated with DT‑loaded NPs. DT‑loaded NPs enhanced the humoral immune response about ten times more than the DT adsorbed to Al3PO4, as the conventional adjuvant (fig. 6). Thus, guinea pigs vaccinated with DT‑loaded NPs produced significantly more anti‑DT antibodies than guinea pigs vaccinated with DT adsorbed to Al3PO4. From the present study, it was evident that the antibody titer was significantly elevated in the vaccinated animals with DT‑loaded NPs and DT‑loaded NPs were able to induce strong humoral immune response after being administered in guinea pigs. Therefore, it can be suggested that this antigen delivery system would be more efficient in the enhancement of humoral immune response when compared with traditional adjuvants. In contrast to the conventional chemical adjuvant, prepared alginate NPs can presumably decrease the side effects besides increasing humoral immune response, which can be due to the use of biodegradable and biocompatible polymer.
As a matter of fact, important matter in the preparation of a carrier system for antigens is the preservation of their native features such as stability and antigenic activity [45‑47]. During the preparation of NPs, harsh conditions such as shear force and freeze drying may affect the structure and decrease antigenicity of protein. In the present study, the important point to emphasize was that the DT released from the NPs was in an active form and the process of encapsulation had no adverse effect on the integrity and activity of DT.
Our results indicated that features of NPs (morphology, mean particle size) strongly depended on physicochemical conditions of NPs’ formation process. The optimized DT‑loaded alginate NPs of this study showed high entrapment efficiency and extended sustained In vitro release profile and remarkably high humoral immune response. It is suggested that the DT‑loaded NPs prepared in this work could be a good alternative system for DT antigen delivery.
Acknowledgements
This work was supported by Razi Vaccine and Serum Research Institute Karaj, Iran. The authors also would like to thank of Dr. Seyed Vafa Maljaei for his help in this research.
References
- Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. Biodegradable and biocompatible poly (DL‑lactide‑co‑glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin‑neutralizing antibodies. Infect Immun 1991;59:2978‑86.
- Montomoli E, Piccirella S, Khadang B, Mennitto E, Camerini R, Rosa AD. Current adjuvants and new perspectives in vaccine formulation. Expert Rev Vaccines 2011;10:1053‑61.
- Malyala P, Singh M. Micro/nanoparticle adjuvants: Preparation and formulation with antigens. Methods MolBiol 2010;626:91‑101.
- Oyewumi MO, Kumar A, Cui Z. Nano‑microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev Vaccines 2010;9:1095‑107.
- Callender DP, Jayaprakash N, Bell A, Petraitis V, Petraitiene R, Candelario M, et al. Pharmacokinetics of oral zidovudine entrapped in biodegradable nanospheres in rabbits. Antimicrob Agents Chemother 1999;43:972‑4.
- Cui Z, Mumper RJ. Microparticles and nanoparticles as delivery systems for DNA vaccines. Crit Rev Ther Drug Carrier Syst 2003;20:103‑37.
- Vila A, Sanchez A, Evora C, Soriano I, Vila Jato JL, Alonso MJ. PEG‑PLA nanoparticles as carriers for nasal vaccine delivery. J Aerosol Med 2004;17:174‑85.
- Galindo‑Rodriguez SA, Allemann E, Fessi H, Doelker E. Polymeric nanoparticles for oral delivery of drugs and vaccines: A critical evaluation of in vivo studies. Crit Rev Ther Drug Carrier Syst 2005;22:419‑64.
- Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly (amino acid) derivatives. Biomaterials 2007;28:3427‑36.
- Raghuvanshi RJ, Mistra A, Talwar GP, Levy RJ, Labhasetwar V. Enhanced immune response with a combination of alum and biodegradable nanoparticles containing tetanus toxoid. J Microencapsul 2001;18:723‑32.
- SajadiTabassi SA, Tafaghodi M, Jaafari MR. Induction of high antitoxin titers against tetanus toxoid in rabbits by intranasal immunization with dextran microspheres. Int J Pharm 2008;360:12‑7.
- Illum L, Farraj NF, Davis SS. Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res 1994;11:1186‑9.
- Vila A, Sanchez A, Janes K, Behrens I, Kissel T, Vila Jato JL, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm 2004;57:123‑31.
- Sharma S, Mukkur TK, Benson HA, Chen Y, Enhanced immune response against pertussis toxoid by IgA‑loaded chitosan‑dextran sulfate nanoparticles. J Pharm Sci 2012;101:233‑44.
- Li X, Kong X, Shi S, Zheng X, Guo G, Wei Y, et al. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol 2008;8;89‑99.
- Gonzalez Ferreiro M, Tillman L, Hardee G, Bodmeier R. Characterization of alginate/poly‑L‑lysine particles as antisense oligonucleotide carriers. Int J Pharm 2002;239:47‑59.
- Ghiasi Z, SajadiTabasi A, Tafaghodi M. Preparation and in vitro characterization of alginate microspheres encapsulated with Autoclaved Leishmania major (ALM) and CpG‑ODN. Iran J Basic Med Sci 2007;10:90‑8.
- Jiang G, Min SH, Kim MN, Lee DC, Lim MJ, Yeom YI. Alginate/ PEI/DNA polyplexes: A new gene delivery system. Yao XueXueBao 2006;41:439‑45.
- Chretien C, Chaumeil JC. Release of a macromolecular drug from alginate‑impregnated microspheres. Int J Pharm 2005;304:18‑28.
- Draget KI, Tylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll 2011;25:251‑6.
- Downs EC, Robertson NE, Riss TL, Plunkett ML. Calcium alginate beads as a slow‑release system for delivering angiogenic molecules in vivo and in vitro. J Cell Physiol 1992;152:422‑9.
- Fundueanu G, Nastruzzi C, Carpov A, Desbrieres J, Rinaudo M. Physico‑chemical characterization of Ca‑alginate microparticles produced with different methods. Biomaterials 1999;20:1427‑35.
- Jani P, Halbert GW, Langridge J, Florence AT. The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J Pharm Pharmacol 1989;41:809‑12.
- Borges O, Cordeiro‑da‑Silva A, Tavares J, Santarém N, de Sousa A, Borchard G, et al. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur J Pharm Biopharm 2008;69:405‑16.
- Prego C, Torres D, Fernandez‑Megia E, Novoa‑Carballal R, Quiñoá E, Alonso MJ. Chitosan‑PEG nanocapsules as new carriers for oral peptide delivery: Effect of chitosan pegylation degree. J Control Release 2006;111:299‑308.
- Qurratul A, Sharma S, Khuller GK, Garg SK, Alginate‑based oral drug delivery system for tuberculosis: Pharmacokinetics and therapeutic effects. J AntimicrobChemother 2003;51:931‑8.
- Kohli AK, Alpar HO. Potential use of nanoparticles for transcutaneous vaccine delivery: Effect of particle size and charge. Int J Pharm 2004;275:13‑7.
- Borges O, Silva M, de Sousa A, Borchard G, Junginger HE, Cordeiro‑da‑Silva A. Alginate coated chitosan nanoparticles are an effective subcutaneous adjuvant for hepatitis B surface antigen. IntImmunopharmacol 2008;8:1773‑80.
- Pandey R, Khuller GK. Polymer based drug delivery systems for mycobacterial infections. Curr Drug Deliv 2004;1:195‑201.
- Machado AH, Lundberg D, Ribeiro AJ, Veiga FJ, Lindman B, Miguel MG, et al. Preparation of calcium alginate nanoparticles using water‑in‑oil (W/O) nanoemulsions. Langmuir 2012;28:4131‑41.
- Fang Y, Al‑Assaf S, Phillips GO, Nishinari K, Funami T, Williams PA,et al. Multiple steps and critical behaviors of the binding of calcium toalginate. J PhysChem B 2007;111:2456‑62.
- Moloney PJ, Beecher Weld C. Diphtheria toxin‑antitoxin flocculation (Ramon test). J Pathol 1925;28:655‑72.
- Ma T, Zuazaga G. Micro‑Kjeldahl determination of nitrogen. A new indicator and an improved rapid method. IndEngChem Anal Ed 1942;14:280‑2.
- Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci 1993;82:912‑7.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‑dye binding. Anal Biochem 1976;72:248‑54.
- Sarmento B, Ferreira D, Veiga F, Ribeiro A. Characterization of insulin‑loaded alginate nanoparticles produced by ionotropic pre‑gelation through DSC and FTIR studies. CarbohydrPolym 2006;66:1‑7.
- Kindt TJ, Goldsby RA, Osborne BA, Kuby J. Kuby immunology. New York: W.H. Freeman; 2007.
- Amidi M, Pellikaan HC, Hirschberg H, de Boer AH, Crommelin DJ, Hennink WE, et al. Diphtheria toxoid‑containing microparticulate powder formulations for pulmonary vaccination: Preparation, characterization and evaluation in guinea pigs. Vaccine 2007;25:6818‑29.
- World Health organization. Requirements for DTP. World Health Organ Tech Rep Ser 1990;800:87‑179.
- Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst 1987;3:233‑61.
- Ma X, Vacek I, Sun A. Generation of alginate-poly‑l-lysine-alginate (APA) biomicrocapsules: The relationship between the membrane strength and the reaction conditions. Artif Cells Blood SubstitImmobilBiotechnol 1994;22:43‑69.
- Ahmad Z, Pandey R, Sharma S, Khuller GK. Alginate nanoparticles as antituberculosis drug carriers: Formulation development, pharmacokinetics and therapeutic potentia. Indian J Chest Dis Allied Sci 2006;48:171‑6.
- Anal AK, Bhopatkar D, Tokura S, Tamura H, Stevens WF. Chitosan‑alginate multilayer beads for gastric passage and controlled intestinal release of protein. Drug DevInd Pharm 2003;29:713‑24.
- Coppi G, Iannuccelli V, Leo E, Bernabei MT, Cameroni R. Chitosan‑alginate microparticles as a protein carrier. Drug DevInd Pharm 2001;27:393‑400.
- Kahl LP, Lelchuk R, Scott CA, Beesley J. Characterization of Leishmania major antigen‑liposomes that protect BALB/c mice against cutaneous leishmaniasis. Infect Immun 1990;58:3233‑41.
- Bowersock TL, HogenEsch H, Suckow M, Guimond P, Martin S, Borie D, et al. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine 1999;17:1804‑11.
- Tafaghodi M, SajadiTabassi SA, Jaafari MR. Induction of systemic and mucosal immune responses by intranasal administration of alginate microspheres encapsulated with tetanus toxoid and CpG‑ODN. Int J Pharm 2006;319:37‑43
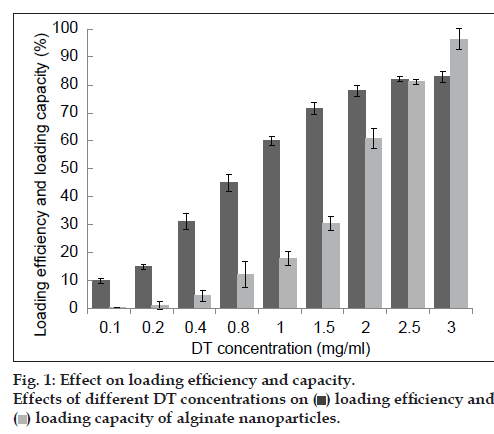
 ) loading efficiency and (
) loading efficiency and ( ) loading capacity of alginate nanoparticles.
) loading capacity of alginate nanoparticles.