N. D. Rawool* and A. Venkatchalam
Department of Chemistry, Bhavan’s College, Andheri (W), Mumbai-400 058, India
- Corresponding Author:
- N. D. Rawool
Department of Chemistry, Bhavan’s College, Andheri (W), Mumbai-400 058, India
E-mail: nitin_rawool@yahoo.co.in
| Date of Submission | 8 May 2010 |
| Date of Revision | 20 January 2011 |
| Date of Acceptance | 5 March 2011 |
| Indian J. Pharm. Sci., 2011, 73 (1): 219-223 |
Abstract
The present study deals with the estimation by RP-HPLC of two different drug components hydrochlorothiazide and metoprolol tartrate present in a tablet formulation. It is a simple, fast, precise and accurate high performance liquid chromatographic method. It is performed using phosphate buffer along with methanol as mobile phase, in the proportion of 60:40. The separation is done on a C18 column and it is estimated at a λmax of 226 nm with a flow of 1 ml/min. The detection limits range from a 0.013 to 0.075 mg/ml for hydrochlorothiazide and 0.10 to 0.60 mg/ml for metoprolol tartrate, respectively. The specificity for interference of any peak with main peak of interest is checked. A scan of the individual drug was taken for assuring the λmax. The system suitability by precision is also checked to ensure the analytical method. The method was found to be accurate and precise for estimation of the two drugs simultaneously.
Keywords
Hydrochlorothiazide, metoprolol tartrate, RP-HPLC
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H- 1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide and metoprolol tartrate is 1,[4-(2-methoxyethyl) phenoxy]-3-[(1-methylethyl)amino]-2-propan-ol tartrate. The molecular mass of hydrochlorothiazide and metoprolol tartrate is 297.74 g/mol and 684.81 g/ mol, respectively.
Few methods for simultaneous estimation of hydrochlorothiazide and metoprolol tartrate by reverse phase chromatography have been reported [1,2]. There are also some methods used for estimating individual hydrochlorothiazide, and metoprolol tartrate [3-5]. Some pharmacopoeia methods are also available for estimating individual hydrochlorothiazide [6] and metoprolol tartrate [7]. The HPLC methods using the most commonly available columns and detectors like the UV detectors are preferred. The present study describes the determination of hydrochlorothiazide and metoprolol tartrate by using reverse phase chromatography, a C18 column with a UV detector.
The use of HPLC is very much preferred nowa- days for routine analysis. It is important that well validated HPLC methods are to be developed for simultaneously estimating hydrochlorothiazide and metoprolol tartrate. The aim of this study is development of a simple, precise, rapid and accurate reverse phase HPLC method for the simultaneous estimation of hydrochlorothiazide and metoprolol tartrate in pharmaceutical tablet dosage form.
Hydrochlorothiazide and metoprolol tartrate obtained as commercial samples were used for the analysis. Solvent methanol HPLC grade was procured from Rankem, Mumbai, India. Dibasic potassium phosphate was procured from S. D. Fine Chemical, Mumbai, India, which were of AR grade. The water used for analysis was also HPLC grade.
A liquid chromatography system consisted of a Shimadzu, class VP LC-10AT equipped with a binary solvent delivery pump, manual injector, column thermostat and UV detector. The system was controlled by Class VP software. The column used was Inertsil ODS-3, 250 mm, 4.6 mm ID, packed with 5 μ particle size and the detection was done at a wavelength of 226 nm. The flow rate was 1.0 ml per min and run time was 16 min. The column temperature was kept ambient. The mobile phase with a mixture of dibasic potassium phosphate buffer, and methanol in the ratio of 60:40 v/v was prepared. The mobile phase was filtered through 0.45 μ Nylon 6,6 membrane filter and degassed in ultrasonic water bath.
A mixture of dibasic potassium phosphate buffer and methanol was used as mobile phase. Buffer was prepared by weighing accurately an amount of 7.7 g of dibasic potassium phosphate in to a 1000 ml volumetric flask and diluted to volume with water.
Weighed quantity of 12.5 mg of hydrochlorothiazide in to a 100 ml volumetric flask and about 50 ml of methanol is added to it. Sonicated to dissolve completely and diluted to 100 ml with methanol. Weighed quantity of 25 mg of metoprolol tartrate in to a 50 ml volumetric flask and add 10 ml of methanol to it, and the contents were sonicated to dissolve. Then using a graduated pipette added 25 ml of above hydrochlorothiazide stock solution to it. Mixed and diluted to 50 ml with the same methanol. The final concentration was 62.5 ppm of hydrochlorothiazide and 500 ppm for metoprolol tartrate.
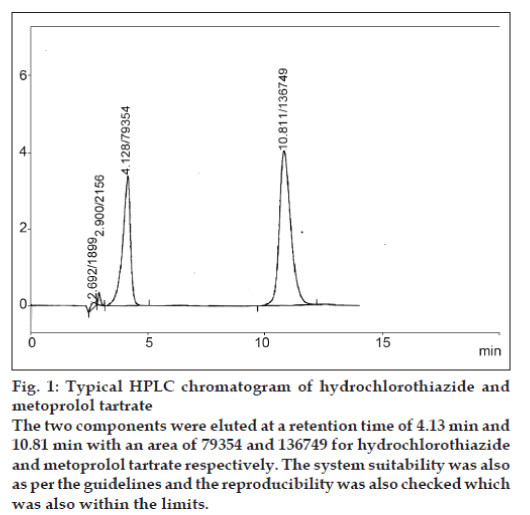
Twenty microlitres of the above standard solution of hydrochlorothiazide and metoprolol tartrate was injected each time in to the stream of mobile system at a flow rate of 1 ml/min. The solution was injected 7 times in to the column and the corresponding chromatograms were obtained. From these chromatograms the area under the peaks and respective retention time of the drug were noted. The retention time of hydrochlorothiazide and metoprolol tartrate observed was 4.13 and 10.81 min, respectively. A model chromatogram is shown in fig. 1. Using these values of the two drug substances the precision was checked for the area and retention time of both the drugs.
A commercial brand of Metolar H tablets was chosen for testing suitability of the proposed method to estimate hydrochlorothiazide and metoprolol tartrate in tablet formulation. The label claim was 100 mg and 12.5 mg for metoprolol tartrate and hydrochlorothiazide, respectively. Twenty tablets were weighed and average weight was determined. The tablets were crushed to a fine powder, and weighed quantity of powder equal to half of the average weight, in each of the three 100 ml volumetric flasks. Then 50 ml of methanol was added in each of the volumetric flasks. The contents of the flask were allowed to dissolve with intermittent sonication to ensure complete solubility of the drug. The mixture was diluted to the volume with methanol, thoroughly mixed and then filtered through 0.45 μ nylon filter. The final concentration of the solution was 62.5 ppm for hydrochlorothiazide and 500 ppm for metoprolol tartrate. From each of these solutions 20 μl was injected in to the system. The drug content in the test preparation was quantified by comparing with the known amount of standard injected. The results obtained are as shown in Table 1.
To achieve sharp peaks with good resolution under isocratic conditions, mixture of methanol and dibasic potassium phosphate salt solution in different proportion were tested as mobile phase on a C18 stationary phase. The mixture of buffer and methanol in the proportion (60:40 v/v) proportions was proved to be the most suitable for estimation. Since the chromatographic peaks were better defined, resolved, and free from tailing with this system, under the above mentioned chromatographic conditions, the retention time obtained for hydrochlorothiazide and metoprolol tartrate were 4.13 and 10.81 min, respectively. The method was validated for accuracy, precision, specificity, linearity, as per ICH guidelines [8,9].
The recovery studies were carried out by adding known amounts of hydrochlorothiazide and metoprolol tartrate and then analyzing them by the proposed HPLC method. Subsequent dilutions of the solutions ranging from 12.5 to 75.0 ppm for hydrochlorothiazide and 100 to 600 ppm for metoprolol tartrate were made and linearity was checked. Twenty microlitres were injected each time in the stream of mobile phase, at a flow rate of 1 ml/ min. Each of these dilutions of different concentration was injected in duplicate in to the column and the corresponding chromatograms were obtained. From these chromatograms the area under the peak of the drug were noted. Using these values, the mean ratio of the drug was calculated. The regression of the drug concentration over these ratios was completed. The % RSD was less than 0.44%, which shows high precision. The percent recovery is in between 98 to 102% which indicates specificity and accuracy of the method.
The system suitability was carried out by injecting standard solution of hydrochlorothiazide (62.5 ppm) and metoprolol tartrate (500 ppm) in to the chromatographic system to check the reproducibility of peak areas (% RSD). The % RSD observed was 0.33 and 0.44 for hydrochlorothiazide and metoprolol tartrate, respectively. The results of precision are shown in Table 2.
The two components were eluted at a retention time of 4.13 min and 10.81 min with an area of 79354 and 136749 for hydrochlorothiazide and metoprolol tartrate respectively. The system suitability was also as per the guidelines and the reproducibility was also checked which was also within the limits.
Figure 1:Typical HPLC chromatogram of hydrochlorothiazide and metoprolol tartrate
| Hydrochlorothiazide | Metoprolol Tartrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean Standard Area: 76879.86 | Mean Standard Area: 136934 | |||||||
| Standard concentration (ppm): 62.25 | Standard concentration (ppm): 502.2 | |||||||
| Standard % Purity: 100.02 | Standard % Purity: 99.97 | |||||||
| Mean Area | % Assay | Mean Area | % Assay | |||||
| Sample 1. | Inj. 1 | 77085 | 77450.5 | 99.56 | Inj. 1 | 138139 | 138493.5 | 100.15 |
| Inj. 2 | 77816 | Inj. 2 | 138848 | |||||
| Sample 2. | Inj. 1 | 76985 | 77114 | 99.60 | Inj. 1 | 138799 | 138580 | 100.51 |
| Inj. 2 | 77243 | Inj. 2 | 138361 | |||||
| Sample 3. | Inj. 1 | 77121 | 77204.5 | 99.40 | Inj. 1 | 138155 | 138504 | 100.16 |
| Inj. 2 | 77288 | Inj. 2 | 138853 | |||||
Table 1: Assay of commercial sample
Placebo solutions (mixture of excepients), diluent used for preparation of standard solution and sample solution were injected in the chromatographic system and checked for interference at retention time corresponding to the retention time of hydrochlorothiazide and metoprolol tartrate. The experiment was carried out at three different levels i.e. 110%, 120%, and 130% of the working concentration of hydrochlorothiazide (62.5 ppm) and metoprolol tartrate (500 ppm). The pure standards at these three levels were added to the sample. From the amount found, the percentage recovery was calculated. The percentage recovery found was 99.4% to 100.61% for hydrochlorothiazide and 99.27 to 100.83% for metoprolol tartrate, respectively. The results of recovery are shown in Table 3.
| Hydrochlorothiazide-62.5 ppm | Metoprolol Tartrate-500 ppm | ||
|---|---|---|---|
| Injection No. | Area count | Injection No. | Area count |
| 1 | 76712 | 1 | 136762 |
| 2 | 76651 | 2 | 137029 |
| 3 | 76667 | 3 | 136092 |
| 4 | 77039 | 4 | 137556 |
| 5 | 76737 | 5 | 137115 |
| 6 | 77325 | 6 | 137694 |
| 7 | 77028 | 7 | 136290 |
| Average | 76880 | 136934 | |
| SD | 256 | 600 | |
| % RSD | 0.33 | 0.44 | |
Table 2: Precision data
The linear working range was selected depending upon the nature of application. The linear working range selected corresponding to the concentrations range of 12.5 to 75.0 ppm of hydrochlorothiazide and 100 to 600 ppm of metoprolol tartrate. Six levels were prepared and each level was injected in duplicate in to the chromatographic system. Mean peak area of each level was calculated. Graph of mean peak area vs. concentration was plotted and the best-fit line was determined by regression. % intercept and correlation coefficient was obtained. The results of linearity are shown in Table 4 and Table 5.
In the present study an attempt has been made to develop a simple, sensitive, accurate, HPLC method for the simultaneous estimation of hydrochlorothiazide and metoprolol tartrate. The proposed method for determination of hydrochlorothiazide and metoprolol tartrate from oral dosage form is specific, accurate, precise, and rapid. It can be used for routine quality control analysis of oral dosage form containing hydrochlorothiazide and metoprolol tartrate.
| Hydrochlorothiazide | Metoprolol tartrate | |||||
|---|---|---|---|---|---|---|
| Level-% | Result | % Recovery | Level-% | Result | % Recovery | |
| 110 | 110.30 | 100.27 | 110 | 110.45 | 100.41 | |
| 110 | 110.43 | 100.39 | 110 | 110.69 | 100.63 | |
| 110 | 109.34 | 99.40 | 110 | 109.20 | 99.27 | |
| 120 | 119.86 | 99.88 | 120 | 120.99 | 100.83 | |
| 120 | 120.13 | 100.11 | 120 | 120.23 | 100.19 | |
| 120 | 120.09 | 100.08 | 120 | 119.85 | 99.88 | |
| 130 | 130.79 | 100.61 | 130 | 129.32 | 99.48 | |
| 130 | 129.61 | 99.70 | 130 | 129.18 | 99.37 | |
| 130 | 129.29 | 99.45 | 130 | 129.64 | 99.72 | |
Table 3: Recovery data
| Concentration in ppm | Area count | Area count | Average |
|---|---|---|---|
| Injection-1 | Injection-2 | ||
| 12.5 | 16279 | 16289 | 16284 |
| 25 | 32790 | 33000 | 32895 |
| 37.5 | 47827 | 47978 | 47903 |
| 50 | 64298 | 64252 | 64275 |
| 62.5 | 79354 | 79680 | 79517 |
| 75 | 94657 | 94829 | 94743 |
| Correlation | 0.9998 |
Table 4: Linearity hydrochlorothiazide
| Concentration in ppm | Area count | Area count | Average |
|---|---|---|---|
| Injection-1 | Injection-2 | ||
| 100 | 30025 | 29450 | 29738 |
| 200 | 57957 | 57863 | 57910 |
| 300 | 83956 | 84443 | 84200 |
| 400 | 110408 | 109552 | 109980 |
| 500 | 136749 | 136985 | 136867 |
| 600 | 160554 | 160858 | 160706 |
| Correlation | 0.9995 |
Table 5: Linearity metoprolol tartrate
Acknowledgements
The authors are grateful to Bhavan’s college, Andheri, for providing the necessary facilities for the research work.
References
- Garg G, Saraf S. Spectrophotometric and column high-performance liquid chromatographic methods for simultaneous estimation of metoprolol tartrate and hydrochlorothiazide in tablets. JAOAC Int 2008;91:1045-50.
- Gupta KR, Tajne MR, Wadodkar SG. New Spectrophotometric method for simultaneous determination of metoprolol tartrate and hydrochlorothiazide in tablets. Indian J Pharm Sci 2008;70:511-3.
- KannaRao KV, Rao ME, Nagoji KE, Rao SS. Determination of Metoprolol Tartrate by reverse phase HPLC. Indian J Pharm Sci 2003;65;204-6.
- Badulescu M, Balala UD, Cacovean I, Ilie M, Baconi DL. UV-Visiblespectrophotometeric assay of metoprolol. Note-2. Method validation. Farmacia 2008;LVI(4):363-70.
- Rahman N, Rahman H, Aami SN. Validated Kinetic Spectrophotometric method for the determination of metoprolol tartrate in pharmaceutical formulations.Chem Pharm Bull 2005;53:942-8.
- United States Pharmacopeia. 32nd ed. Rockville MD: US Pharmacopoel Convention Inc.; 2009. p. 2566
- United States Pharmacopeia. 32nd ed. Rockville MD: US Pharmacopoel Convention Inc.; 2009. p. 2965
- ICH-Q2A, Guidelines for industry: Text on validation of analytical methods, March-1995.
- ICH, Q2B, Validation of Analytical Procedures: Methodology, International Conference on Harmonization, Nov-1996.