- *Corresponding Author:
- H. Askin
Ataturk University, Science Faculty, Molecular Biology and Genetics Department, 05100-Amasya, Turkey
E-mail: hakanbiyolog@gmail.com
| Date of Submission | 28 July 2017 |
| Date of Revision | 21 December 2017 |
| Date of Acceptance | 14 July 2018 |
| Indian J Pharm Sci 2018;80(5): 802-812 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, phytochemical components and antioxidant capacity of extracts obtained from Iris taochia plant, which is endemic to Caucasus, were investigated. Fifteen new compounds were detected and identified using a GC-MS Sytem, which were isolated from Iris taochia for the first time. From the calibration curve, the amount of α-amyrin in dry powder of Iris taochia was calculated. The mean amount of α-amyrin found in whole plant powder of Iris taochia was 4.598 μg/10 mg with a percent recovery of 0.045 % for α-amyrin. Antioxidant properties of Iris taochia, in vitro using Fe3+-Fe2+, Cu2+-Cu+ and Fe3+-2,4,6-tripyridyl-s-triazine reducing, 1,1-diphenyl-2-picrylhydrazyl scavenging, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) scavenging, N,N-dimethyl-p-phenylenediamine scavenging and Fe2+ chelating activities were measured. The antioxidative effects of Iris taochia have not been reported earlier. These results shown that crude ethanol and water extracts of Iris taochia had effective reducing effect, radical scavenging activity and metal chelating effects when compared to standard antioxidants. The results indicated that the ethanol and water extracts of Iris taochia could be used as a potential source of natural antioxidants by the pharmaceutical industry or as a conceivable food supplement.
Keywords
Antioxidant activity, reducing power, α-amyrin, GC-MS, Iris taochia
Reactive oxygen species (ROS) including free radicals such as hydroxyl radicals (OH•), singlet oxygen (1O2), superoxide anion radicals (O2•-), and non-free radical species such as hydrogen peroxide (H2O2) are diverse kinds of activated oxygen and mostly produced by exogenous factors or oxidation product of biological reactions [1-3]. ROS have created considerable interest among scientists in the recent years [4-6]. Their wide range of effects that are of medicinal and biological interest have led to several experimental studies [7-9]. Additionally, it is well-known that ROS induce oxidative damage to biomolecules such as lipids, proteins, carbohydrates and deoxyribonucleic acids [10-12], which might induce cancer, ageing, and other multitude of diseases [13-16]. In addition, ROS has also been implicated in more than 100 diseases such as heart disease, acquired immunodeficiency syndrome, malaria, stroke, diabetes, arteriosclerosis, and cancer [17-20].
The plant kingdom, including medicinal and dietary plants, offers many natural phytochemicals that include phenolic diterpenes, triterpenes, flavonoids, phenolic acids and sterols [21-23]. Antiinflammatory, antioxidant and anticancer activities reported to be possessed by these compounds might have resulted from prevention of oxidative damage [24,25]. There is rising interest in endemic species because these can be used for preparing phytopharmaceuticals with considerable antioxidant potential or for the production of raw materials and health confidants [26-28]. Antioxidant compounds are molecules that can inhibit or delay the oxidation of lipids or other biomolecules by inhibiting the initiation and propagation of oxidative chain reactions [29]. Some plants synthesize large amounts of vitamin C, vitamin E, and carotenoids, which are well-known antioxidants [30-33]. Phenolic compounds are also widely distributed in plants and have been found to possess antioxidative potential as well [34]. Natural antioxidant compounds can retard the progression of chronic diseases and protect human cells from free radicals as well as prevent oxidative rancidity of lipid containing foods [35-37].
Iris taochia is endemic to the Caucasus. This plant is quite rich in triterpenes and sterols [38]. Terpenes play significant role as growth regulators (phytohormones) of plants and signal compounds, secondary pigments pending photosynthesis and also with antimicrobial activities [39]. Terpene compounds include cucurbitacins, cardenolides, bufadienolides, phytosterols, sesquiterpenoids, iridoids, diterpenoids, sesquiterpene lactones, triterpenoid saponins, steroid saponins, carotenoids, nortriterpenoids, other triterpenoids, and monoterpenoids [40]. Triterpene saponins are the most valued in terms of pharmacology [41]. These compounds have been reported to possess antiinflammatory, hypoglycemic and anticancer activities [42]. The amyrins are three closely related natural compounds of the triterpene class [43]. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin [42,43]. In this investigation chemical constituents and antioxidant potential of dried whole plant of Iris taochia was studied.
It appeared from the literature reports, the phenolic constituents and the oxidative potential of I. taochia has not been reported. The antioxidant potential of I. taochia was investigated using seven different antioxidant procedures including ferric reducing antioxidant power (FRAP), cupric ion reducing antioxidant capacity (CUPRAC), Fe3+-Fe2+ reducing, 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS•+) scavenging, N,N-dimethyl-pphenylenediamine (DMPD•+) scavenging, Fe2+ chelating, and 1,1-diphenyl-2-picrylhydrazyl (DPPH•) assays. On the other hand, the antioxidant effect of the extract was decisively related to the total flavonoid and phenolic contents of the extract that considered in this paper.
Materials and Methods
Collection and extraction of Iris taochia
Aerial parts and rhizomes of I. taochia were collected from Tortum in the province of Erzurum in April to June 2016 and were authenticated as per the characteristics described by Davis et al. and Richardson [44,45]. Specimens were air-dried at room temperature for 7 d and stored for analysis at a later date. The drying process of the plant and all the remaining studies were carried out in the Genetics Laboratory, Science Faculty, Ataturk University. The air-dried and powdered aerial parts and rhizomes of the plant were extracted thrice with ethanol in a mantle heater at 40°. Ethanol extraction was also carried out according to the method of Kotan et al. [46]. In this method, 100 g dried plant powder was shaken with 2 times the volume of ethanol on a shaker for 3 days at room temperature. At the end of d 3, the extract was filtered, 1 μl of the filtrate was employed for GC-MS analysis. This sample was also used in antioxidant capacity assays.
GC-MS system
Chromatographic analysis was carried out on an Agilent 7820A gas chromatography system equipped with 5977 series Mass Selective Detector, 7673 series Autosampler and Chemstation (Agilent Technologies, Palo Alto, CA). HP-5 MS column with 0.25 μm film thickness (30 m×0.25 mm I.D., USA) was used for separation. The temperatures of the inlet, transfer line and detector were 250, 250 and 300°, respectively.
GC-MS conditions
Diverse temperature programs were evaluated for GCMS procedure. Towards the end of this evaluation, the temperature program chosen was as follows, initial temperature was 50° held for 1 min, increased to 100° at a rate of 20°/min, held for 1 min, increased to 180° at a rate of 10°/min, held for 1 min, increased to 220° at a rate of 5°/min, held for 5 min, and finally to 300° at a rate of 10°/min and held for 5.5 min. The injector volume was 1 ml in splitless mode and the carrier gas was helium at a flow rate of 1 ml/min.
Antioxidant capacity assays
The in vitro antioxidant potential of I. taochia was evaluated using various procedures that included, cupric ion (Cu2+) reducing power (CUPRAC method), FRAP reduction of Fe3+-2,4,6-Tris(2-pyridyl)-s-triazine (Fe3+-TPTZ) complex, DPPH· scavenging, ferrous ions (Fe2+) chelating, DMPD radical scavenging and ABTS·+ scavenging activities. Additionally, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), α-tocopherol and trolox (6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid) were used as standard antioxidants.
Fe3+ reducing power assay
Reducing power was determined by the straight reduction of Fe3+(CN−)6 to Fe2+(CN−)6, which was obtained by evaluating absorbance resulted from the organization of the Perl’s Prussian blue complex following the addition of excess ferric ions (Fe3+). This method is dependent on the reduction of (Fe3+) ferricyanide in stoichiometric overplus relative to the antioxidants. Briefly, different concentrations of this plant extract (10-30 μg/ml) in 0.75 ml of deionized H2O were added with 1.25 ml of phosphate buffer (0.2 M, pH 6.6) and 1.25 ml of potassium ferricyanide (K3Fe(CN)6, 1 %). Then, the solution was incubated at 50° for 20 min. After the incubation, 1.25 ml of trichloroacetic acid (TCA, 10 %) was added. Lastly, 0.5 ml of FeCl3 (0.1 %) was transferred to this mixture and the absorbance was measured at 700 nm in a spectrophotometer [36].
CUPRAC assay
The reducing capacity of the extract was determined by the CUPRAC procedure [4]. In this method, 0.25 ml of CH3COONH4 buffer solution (1.0 M), 0.25 ml of neocuproine solution in ethanol (7.5×10-3 M), 0.25 ml CuCl2 solution (0.01 M) were mixed and the sample extract at various concentrations (10-50 g/ml) was added to this mixture. The final volume was adjusted to 2 ml with distilled water and 30 min later absorbances of the samples were measured at 450 nm. Increase in absorbance was defined as increase in reducing capacity.
FRAP assay
FRAP assay was based on reduction of Fe3+-TPTZ complex under acidic conditions. Increased absorbance of blue-coloured ferrous form (Fe2+-TPTZ complex) was recorded at 593 nm. TPTZ solution (2.25 ml, 10 mM TPTZ in 40 mM HCl) was freshly prepared, then transferred to acetate buffer (25 ml, 0.3 M, pH 3.6), and FeCl3 solution (2.25 ml, 20 mM) in water. Then, different concentrations of the plant extract (10-50 μg/ml) were dissolved in 5 ml of appropriate buffer, stirred and incubated at 37° for 30 min. Finally the absorbance of the mixture was measured at 593 nm [36].
DPPH• scavenging activity
Electron or hydrogen donating capabilities of the samples was determined using DPPH method [47]. Accordingly, purple-colored 1 mM DPPH solution in ethanol was added to samples at varying concentrations (10-50 μg/ml). Finally, the mixture was incubated in a dark room for 30 min and the radical scavenging ability was determined spectrophotometrically at 517 nm against a blank [46]. Reduced absorbance of the sample indicated the DPPH free radical scavenging capability.
ABTS•+ scavenging activity
ABTS radical cation was generated by the interaction of ABTS (7 mM) and K2S2O8 (2.45 mM) as described previously [47]. One milliliter of ABTS•+ solution was added to 3 ml of novel symmetric sulfamides and control solutions. ABTS+ was produced by the reaction of 2 mM ABTS in H2O with 2.45 mM potassium persulfate (K2S2O8) stored in the dark at room temperature for 4 h. Before usage, the ABTS+ solution was diluted to get an absorbance of 0.750±0.025 at 734 nm with sodium phosphate buffer (0.1 M, pH 7.4). Then, to 1 ml of ABTS+ solution, 3 ml of I. taochia solution in ethanol at different concentrations (10-50 μg/ml) was added. After 30 min, the percent inhibition of ABTS at 734 nm was calculated for each concentration relative to a blank absorbance [47]. The extent of decoloration was calculated as percent reduction of absorbance.
Metal chelating assay
Ferrous ions (Fe2+) chelating activities of I. taochia were investigated conforming to the method described previously [48]. Different concentrations (10-50 μg/ml) of this plant extract in 0.25 ml FeSO4 solution (2 mM), 0.25 ml ethanol, 1 ml ferrozine solution (0.2 % in 0.2 M HCl), 1 ml Tris-HCl buffer solution (pH 7.4), and 2.5 ml ethanol were placed into a test tube. The absorbance was measured at 562 nm.
DMPD•+ scavenging activity
DMPD•+ scavenging capability of I. taochia extract was obtained conforming to Fogliano et al. [49], as previously reported [26]. DMPD (100 mM) was prepared by dissolving 209 mg of DMPD in 10 ml of deionized water and 1 ml of this solution was added to 100 ml of 0.1 M acetate buffer (pH 5.3) and the colored radical cation (DMPD•+) was obtained by adding 0.2 ml of a solution to 0.05 M ferric chloride (FeCl3). The absorbance of this solution, which was freshly prepared daily, is constant up to 12 h at room temperature. Different concentrations of standard antioxidants or resveratrol (10-50 μg/ml) were added in test tubes and the total volume was adjusted with distilled water to 0.5 ml [26]. Ten minutes later, the scavenging capability of DMPD•+ radical of the sample was spectrophotometrically measured at 505 nm.
Determination of total phenolic content by the Folin-Ciocalteu assay
The total phenolic contents of I. taochia was obtained using the Folin-Ciocalteu method [50], as previously described [51]. Initial stock solutions of I. taochia at 2 mg/ml were prepared. Absorbance (λ760) = 0.0016×total phenols (gallic acid equivalent, GAE; μg). The content of total phenolics in I. taochia was determined by employing this graph (R2: 0.9872), which was prepared using gallic acid and expressed as μg of GAE. Briefly, 1 ml of extract containing 1 g extract was mixed with 45 ml distilled water. One millilitre of Folin-Ciocalteu reagent was added and the content of the flask mixed thoroughly. After 3 min, 3 ml of 2 % Na2CO3 was added, the mixture was allowed to stand for 2 h with intermittent shaking and the absorbance was measured at 760 nm.
Determination of total flavonoid contents
The total flavonoid content of I. taochia was approximately obtained by a colorimetric method, which was reported previously [52]. One milligram of the sample was added into a test tube. Then 0.1 ml CH3COOK (1.0 M) and 0.1 ml of 10 % Al(NO3)3 in 4.3 ml ethanol were added and the samples were vortexed. The vortexed samples were kept at room temperature for 40 min. The absorbance of the samples was recorded at 415 nm. Results were reported as μg quercetin equivalents (QE) per mg extract. A quercetin calibration curve was prepared, and the amount of flavonoid was calculated using the linear regression equation that was determined from the calibration curve. Finally, the results were expressed as QEs/mg extract. Absorbance (λ415) = 0.011×total phenols (QE μg). The total flavonoid content in I. taochia was calculated using the graph, which was prepared using quercetin and determined as QE μg.
Results and Discussion
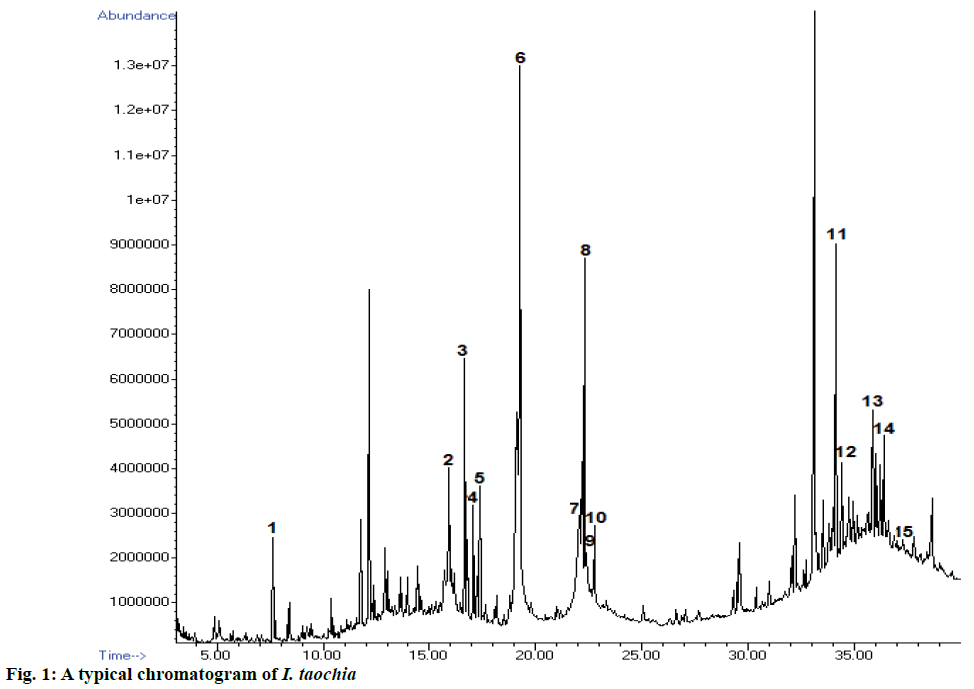
In this investigation, fifteen compounds were obtained and identified as L-α-terpineol, tetradecanoic acid, neophytadiene, 2-pentadecanone, 6,10,14-trimethyl phthalic acid, n-hexadecanoic acid, linoleic acid, linolenic acid, oleic acid, octadecanoic acid (stearic acid), hexacosanoic acid, docosanoic acid 1,2,3-propanetriylester, β-sitosterol, α-tocopherol and α-amyrin (Figure 1 and Table 1).
| Compound Name | Chemical Structure | Retention Time (min) |
|---|---|---|
| L-a-terpineol | 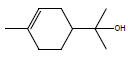 |
7.72 |
| Tetradecanoic acid ethyl ester | 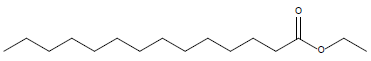 |
15.92 |
| Neophytadiene | 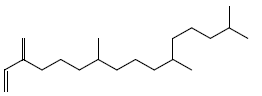 |
16.66 |
| 2-Pentadecanone, 6,10,14-trymethyl | 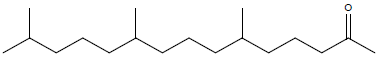 |
16.78 |
| Phthalic acid, butyl tetradecyl ester | 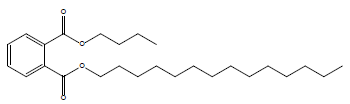 |
17.24 |
| n-Hexadecanoic acid | 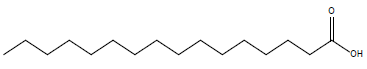 |
19.1 |
| Linoleic acid ethyl ester | 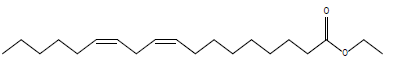 |
22.15 |
| Linolenic acid ethyl ester | 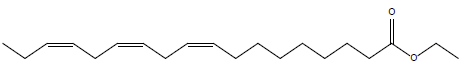 |
22.29 |
| Oleic acid ethyl ester | 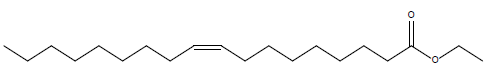 |
22.37 |
| Octadecanoic acid ethyl ester | 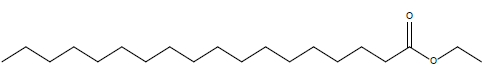 |
22.75 |
| Hexacosanoic acid methyl ester |  |
34.4 |
| Docosanoic acid 1,2,3-propanetriyl ester |  |
34.9 |
| b-sitosterol |  |
36 |
| a-tocopherol |  |
36.4 |
| a-amyrin | 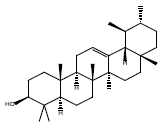 |
37 |
Table 1: Structural formulae of compunds detected in whole plant of I. taochia
To validate the present method, parameters such as linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ) and recovery were investigated according to International Conference on Harmonization validation guidelines (ICH, 1996). The linear range of α-amyrin in this developed method was 1-100 μg/ml. The intra- and inter-day precisions, expressed as the relative standard deviation (RSD), were less than 4.97 and 4.99 %, determined from quality control samples for α-amyrin, and accuracy was within 2.00 and 4.60 % in terms of relative error, respectively. The percent recovery obtained for α-amyrin was 99.7 %. LOD and quantification for α-amyrin were 5 and 15 ng/ml, respectively.
Also, the developed method can be used for routine quality control analysis of α-amyrin in dried whole plant of I. taochia. The sample working solution (1 μl) was injected and the height of α-amyrin peak was measured. From the calibration curve, the amount of α-amyrin in dry powder of I. taochia was calculated. The mean amount of α-amyrin found in the whole plant powder of I. taochia was 4.598 μg/10 mg with percent recoveries 0.045 % for α-amyrin.
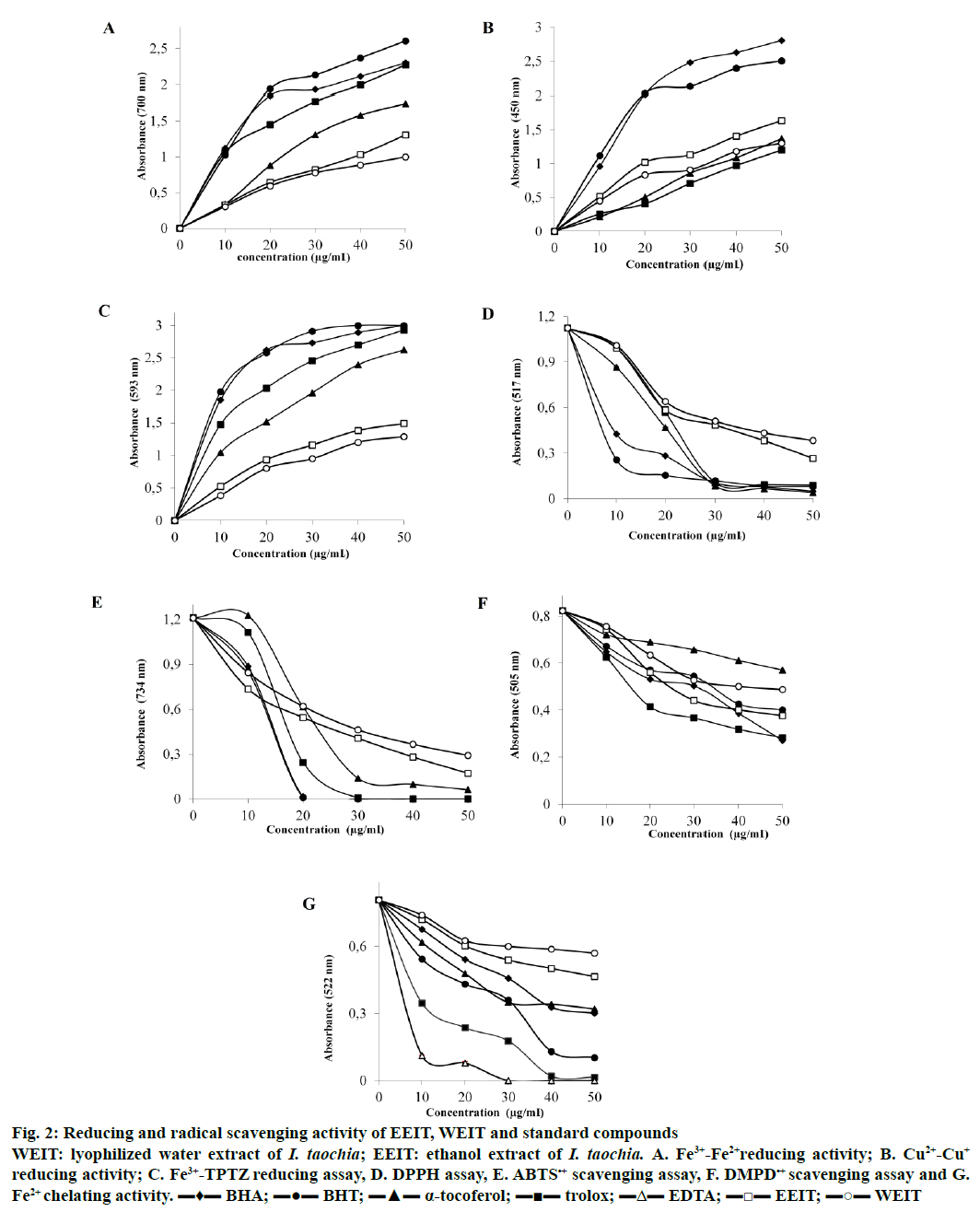
Reducing ability of I. taochia and standard compounds was given in (Table 2 and Figure 2). These results demonstrated that crude ethanol extract (EEIT) exhibited reducing effect when compared to standard antioxidants. Also, radical scavenging activity and metal chelating effects of I. taochia and standard compounds were summarized in (Table 3 and Figure 2). The IC50 values of the EEIT and lyophilized water extract of I. taochia (WEIT) in the DPPH•, ABTS•+, DMPD•+ and metal chelating assays were 24.75, 18.23, 40.76, 57.75, and 30.13, 23.10, 57.75, 86.62 μg/ml, respectively. In addition to these, 47.50 μg GAE phenolic compounds and 10.72 μg QE flavonoids were detected in 1 mg dried EEIT (Table 4).
| Antioxidants | Fe3+-Fe2+reducing | Cu2+-Cu+ reducing | Fe3+-TPTZ reducing | |||
|---|---|---|---|---|---|---|
| λ700 | r2 | λ450 | r2 | λ593 | r2 | |
| BHA | 2.304±0.013 | 0.9733 | 2.803±0.020 | 0.9424 | 3.000±0.017 | 0.9711 |
| BHT | 2.607±0.012 | 0.9818 | 2.506±0.024 | 0.9616 | 3.000±0.012 | 0.9636 |
| a-Tocopherol | 1.744±0.017 | 0.9602 | 1.371±0.019 | 0.9381 | 2.627±0.001 | 0.9844 |
| Trolox | 2.277±0.013 | 0.9722 | 1.202±0.005 | 0.9783 | 2.932±0.015 | 0.9744 |
| EEIT | 1.308±0.012 | 0.9889 | 1.638±0.011 | 0.9905 | 1.490±0.005 | 0.9550 |
| WEIT | 1.001±0.017 | 0.9582 | 1.303±0.009 | 0.9690 | 1.288±0.006 | 0.9937 |
Table 2: Reducing Power of EEIT, WEIT and Standard Compounds
Figure 2: Reducing and radical scavenging activity of EEIT, WEIT and standard compounds
WEIT: lyophilized water extract of I. taochia; EEIT: ethanol extract of I. taochia. A. Fe3+-Fe2+ reducing activity; B. Cu2+-Cu+ reducing activity; C. Fe3+-TPTZ reducing assay, D. DPPH assay, E. ABTS•+ scavenging assay, F. DMPD•+ scavenging assay and G. Fe2+ chelating activity. ▬♦▬ BHA; ▬●▬ BHT; ▬▲▬ α-tocoferol; ▬■▬ trolox; ▬Δ▬ EDTA; ▬□▬ EEIT; ▬○▬ WEIT
| Antioxidants | DPPH· Scavenging | ABTS•+ Scavenging | DMPD•+ Scavenging |
Fe2+ Chelating |
|---|---|---|---|---|
| BHA | 10.34 | 1.86 | 34.65 | 34.65 |
| BHT | 10.65 | 1.75 | 46.20 | 22.35 |
| α-Tocopherol | 9.86 | 11.55 | 86.62 | 31.50 |
| Trolox | 12.51 | 2.88 | 28.87 | 14.14 |
| EDTA | - | - | - | 1.96 |
| EEIT | 24.75 | 18.23 | 40.76 | 57.75 |
| WEIT | 30.13 | 23.10 | 57.75 | 86.62 |
Table 3: IC50 of EEIT, WEIT and Standards In DPPH•, ABTS•+, DMPD•+ Radical Scavenging and Fe2+ Chelating Assays
| EEIT | WEIT | |
|---|---|---|
| Total phenols* | 47.50 | 39.26 |
| Total flavonoids** | 10.72 | 9.12 |
Table 4: Total Phenolic and Flavonoid Content of EEIT and WEIT
The IC50 values for DPPH scavenging by standard antioxidants and both extracts were compared. The IC50 of WEIT was 30.13 μg/ml, which was higher than that of EEIT, 24.75 μg/ml, but both these values were much higher than those of Trolox (12.51 μg/ml), BHT (10.65 μg/ ml), BHA (10.34 μg/ml), and α-tocopherol (9.86 μg/ ml). The lower the IC50 value the higher the DPPH radical scavenging effect. These results indicated that both EEIT and WEIT possessed DPPH radical scavenging activities. In this investigation, DMPD scavenging, ABTS radical cation scavenging and Fe chelating effects were also evaluated to detect radical scavenging activity. In these procedures, EEIT and WEIT exhibited similar reduction in free radicals when compared to those obtained in the DPPH reaction. The IC50 values for ABTS radical scavenging of EEIT, WEIT, and standard antioxidants were as follows, WEIT with an IC50 value of 23.10 μg/ml and EEIT with an IC50 value of 18.23 μg/ml were slightly higher than those of α-tocopherol, 11.55 μg/ml, Trolox, 2.88 μg/ml, BHA, 1.86 μg/ml, and BHT, 1.75 μg/ml. In the DPPH, ABTS, and metal chelating tests the antioxidant activities by these two extracts although were less than the standard antioxidants, both EEIT and WEIT could be considered to have effective radical scavenging activity. Additionally, the DMPD scavenging method was also used to estimate detect radical scavenging activity. In this procedure, the IC50 values obtained were in the following order; α-tocopherol (86.62 μg/ml)>WEIT (57.75 μg/ml)>BHT (46.20 μg/ml)>EEIT (40.76 μg/ml)>BHA (34.65 μg/ ml)>trolox (28.87 μg/ml). These values indicated that the plant extracts exhibited direct radical scavenging activity within the range of activities exhibited by the standard antioxidants.
Antioxidant molecules induced reduction of the Fe3+/ ferricyanide complex to its ferrous (Fe2+/ferricyanide) form due to their reducing potential. As shown in Table 2, the ferric reducing power increased with increasing concentration of both WETT and EETT, which however were lower than the standard antioxidants. The reducing power of the standard antioxidants and the extracts at 50 μg/ml were as follows, BHT (2.607, R2: 0.9818) = BHA (2.304, R2: 0.9733)>trolox (2.277, R2: 0.9722)>α-tocopherol (1.744, R2: 0.9602)>EEIT (1.308, R2: 0.9889)>WEIT (1.001, R2: 0.9582).
In this investigation the CUPRAC procedure was also used to get an estimate of the reducing power of the extracts. This procedure is based on the reduction of Cu2+ to Cu+ by antioxidants in the presence of neocuproine [53]. In this method, a higher absorbance indicated a higher cupric ion (Cu2+) reducing power. The cupric ion reducing powers of 50 μg/ml of EEIT, WEIT, and the standard molecules were as follows, BHA (2.803, R2: 0.9424)>BHT (2. 506, R2: 0.9616)>EEIT (1.638, R2: 0.9905)>α-tocopherol (1.371, R2: 0.9381)>WEIT (1.303, R2: 0.9690)>trolox (1.202, R2: 0.9783; Table 2). In addition, the FRAP procedure was also used to further understand the reducing power of the molecules. The reducing power of 50 μg/ml of EEIT, WEIT, and the standards were as follows, BHA (3.000, R2: 0.9711)=BHT (3.000, R2: 0.9636)>trolox (2.932, R2: 0.9744)>α-tocopherol (2.627, R2: 0.9844)>EEIT (1.490, R2: 0.9550)>WEIT (1.288, R2: 0.9937; Table 2).
The compounds listed in Table 1 were identified in I. taochia for the first time. There is no work about the chemical composition and antioxidative activity of I. taochia in literature. Therefore, the current study became very significant. These findings might shed light on the design of new drugs. These results indicated that EEIT and WEIT could be very useful to pharmaceutical industry or as a food supplement and as an easily attainable source of natural antioxidants. Additionally, this study demonstrated that I. taochia has powerful antioxidant, reducing power, free radical scavenging and metal chelating activities comparable to those possessed by diverse standards such as α-tocopherol, trolox, BHA, BHT. More recently research in food science and nutrition has focused on plant products that possessed antimicrobial and potential antioxidant activities [54-56]. Antioxidant properties, particularly radical scavenging are of high value due to the detrimental role of free radicals in biological systems and in foods [57-61]. DPPH• assay provided data on the reactivity of test molecules with constant free radicals [62]. Due to the odd electron quantity, in visible spectroscopy, DPPH• and ABTS• gave powerful absorption bands at 517 and 734 nm, respectively. If the electron pairs off in the presence of a free radical scavenger, the absorption vanishes, and the resulting decolorization is stoichiometric in respect to the number of electrons taken up [63-66].
Metal chelating capacity of I. taochia was considerable, since it decreased the concentration of the hydrolysing transition metal. It was recorded that chelating factors are efficacious as secondary antioxidants due to the decrease the redox potential, finally stabilizing the oxidized form of the metal ion. Fe3+-Fe2+ transformation was evaluated to obtain the measurements of the reductive capability of I. taochia by using the procedure of Oyaiz [67]. Its results on the reducing power show the electron donor confidants of I. taochia, finally neutralizing free radicals by forming fixed products. The effect of the reducing reaction is to finish the radical chain reactions that can otherwise be very harmful [68,69]. Free radicals are significantly accessible in living systems; although, high contents of free radicals can oxidise biomolecules, leading to tissue harm, degenerative factors or cell death, inflammation containing cancer, aspects of ageing, skin irritations arteriosclerosis, cardiovascular diseases, and neural disorders [70,71]. Also, the antioxidant activity of the extract was decisively dependent on the total phenolic and flavonoid contents of the extract that determined in this study.
Previous studies were designed to investigate antioxidant and anticholinesterase potential of I. germanica var. florentina. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory potential of plant samples were investigated using the Ellman’s assay. Antioxidant activity was performed using DPPH, H2O2 and ABTS free radical scavenging assays. Total phenolics and flavonoids contents were expressed in mg GAE/g dry weight and mg RTE/g, respectively [72]. And also, Basgedik et al. [73] investigated the antimicrobial, antioxidant and antimutagenic properties of ethanolic extracts of the aerial parts and rhizomes of I. albicans Lange. While previous studies have examined the antimicrobial and antioxidant properties of I. albicans, to our knowledge, this is the first study to report on the antimutagenic activity of this plant. Both aerial part and rhizome extracts exhibited limited antimicrobial activity against Bacillus subtilis ATCC 6633. IC50 values for the radical scavenging activity of the extracts were 8.8 and 11.1 mg/ml, respectively. Total antioxidant activity of the extracts (at 3.15 mg/ml) was 96.6±0.07 and 97.2±0.7 %, respectively [73].
Conforming to data of this paper, when compared to standard antioxidant molecules such as BHT and BHA, the natural antioxidant trolox (a water-soluble analogue of tocopherol) and α-tocopherol, WEIT and EEIT were shown to be effective antioxidants in various in vitro methods, including reducing power, DPPH• radical, ABTS•+ radical scavenging; DMPD scavenging; and metal chelating activities. Also, this study revealed that WEIT and EEIT appear to be excellent sources of antioxidants for medicines, food, and pharmaceuticals.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol 2012;86:345-91.
- Gülçin İ, Elias R, Gepdiremen A, Taoubi K, Köksal E. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195-212.
- Gülçin İ, Elmastas M, Aboul-Enein HY. Antioxidant activity of clove oil - A powerful antioxidant source. Arab J Chem 2012;5:489-99.
- Kose LP, Gülçin İ, Goren AC, Namiesnik J, Martinez-Ayala AL, Gorinstein S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crops Prod 2015;74:712-21.
- Bursal E, Koksal E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res Int 2011;44:2217-21.
- Gülçin I, Bursal E, Sehitoğlu MH, Bilsel M, Gören AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227-38.
- Gülçin I, Beydemir Ş. Phenolic Compounds as Antioxidants: Carbonic Anhydrase Isoenzymes Inhibitors. Mini Rev Med Chem 2013;13:408-30.
- Gülçin I, Beydemir S, Topal F, Gagua N, Bakuridze A, Bayram R, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J Enzyme Inhib Med Chem 2012;27:587-94.
- Elmastaş M, Turkekul I, Oztürk L, Gülçin I, Isildak O, Aboul-Enein HY. Antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta) from North Turkey. Comb Chem High T Scr 2006;9:443-8.
- Topal M, Gocer H, Topal F, Kalin P, Köse LP, Gülçin İ, et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem 2016;31:266-75.
- Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 2004;90:205-15.
- Topal M, Gülçin I. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894-902.
- Gülçin I, Sat IG, Beydemir S, Elmastas M, Kufrevioglu OI. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 2004;87:393-400.
- Gülçin I, Büyükokuroglu ME, Oktay M, Küfrevioglu OI. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp pallsiana (Lamb.) Holmboe. J Ethnopharmacol 2003;86:51-8.
- Koksal E, Gülçin I. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk J Agric For 2008;32:65-78.
- Gülçin I, Topal F, Sarikaya SBO, Bursal E, Bilsel G, Goren AC. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158-75.
- Bursal E, Gülçin I. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482-9.
- Bursal E, Koksal E, Gülçin I, Bilsel G, Goren AC. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res Int 2013;51:66-74.
- Göçer H, Akıncıoğlu A, Öztaşkın N, Göksu S, Gülçin İ. Synthesis, Antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine-related compounds. Arch Pharm 2013;346:783-92.
- Gülçin I, Topal F, Çakmakçı R, Bilsel M, Gören AC, Erdogan U. Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:C585-93.
- Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci 2006;78:803-11.
- Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213-20.
- Gülçin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007;32:431-38.
- Lee JY, Hwang WI, Lim ST. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J Ethnopharmacol 2004;93:409-15.
- Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 2008;110:76-82.
- Gülçin I. Antioxidant properties of resveratrol: A structure-activity insight. Innov Food Sci Emerg Technol 2010;11:210-18.
- Gülçin I. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J Enzym Inhib Med Chem 2008;23:871-76.
- Cetinkaya Y, Göçer H, Menzek A, Gülçin I. Synthesis and antioxidant properties of (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch Pharm 2012;345:323-34.
- Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem 2003;83:547-50.
- Gülçin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 2010;3:43-53.
- Gülçin I. Antioxidant activity of L-adrenaline: A structure-activity insight. Chem Biol Interact 2009;179:71-80.
- Köksal E, Gülçin I, Beyza S, Sarikaya O, Bursal E. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395-405.
- Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27-37.
- Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidative properties of Cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J Agric Food Chem 2002;50:4989-93.
- Göçer H, Gülçin I. Caffeic acid phenethyl ester (CAPE): Correlation of structure and antioxidant properties. Int J Food Sci Nutr 2011;62:821-5.
- Gülçin İ, Kireçci E, Akkemik E, Topal F, Hisar O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: duckweed (Lemna minor L., Lemnaceae). Turk J Biol 2010;34:175-88.
- Tohma HS, Gülçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Prop 2010;13:657-71.
- Kandemir N. An investigation on the autecological endemic Iris taochia Woronow Ex Grossh. (Iridaceae) distributed in the North East Anatolia region. Pak J Biol Sci 2006;9:2753-60.
- Islam AKMN, Ali MA, Sayeed A, Salam SM, Islam A, Rahman M, et al. An Antimicrobial terpenoid from Caesalpinia pulcherrima Swartz.: Its Characterization, antimicrobial and cytotoxic activities. Asian J Plant Sci 2003;2:1162-5.
- Lai SM, Chen IW, Tsai MJ. Preparative isolation of terpene trilactones from Ginkgo biloba leaves. J Chromatogr A 2005;1092:125-34.
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, et al. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol 2001;127:1256-65.
- Thompson A, Cooper J, Ingram LL. Distribution of terpenes in heartwood and sapwood of loblolly pine. Forest Prod J 2006;56:46-48.
- Trapp SC, Croteau RB. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001;158:811-32.
- Davis PH, Cullen J, Coode MJE. Flora of Turkey and the East Aegean Islands. Edinburgh: University Press; 1965.
- Richardson IBK. A revision of the genus Centranthus DC. (Valerianaceae). Bot J Linnean Soc 1975;71:211-34.
- Kotan R, Cakir A, Dadasoglu F, Aydin T, Cakmakci R, Ozer H, et al. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J Sci Food Agric 2010;90:145-60.
- Sujayev A, Garibov E, Taslimi P, Gulçin İ, Gojayeva S, Farzaliyev V, et al. Synthesis of some tetrahydropyrimidine-5-carboxylates, determination of their metal chelating effects and inhibition profiles against acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:1531-9.
- Ghous T, Aziz N, Mehmood Z, Andleeb S. Comparative study of antioxidant, metal chelating and antiglycation activities of Momordica charantia flesh and pulp fractions. Pak J Pharm Sci 2015;28:1217-23.
- Fogliano V, Verde V, Randazzo G, Ritieni A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem 1999;47:1035-40.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 1999;299:152-78.
- Elmastas M, Gülçin İ, Beydemir S, Kufrevioglu OI, Aboul-Enein HY. A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) fruit extracts. Anal Lett 2006;39:47-65.
- Gülçin İ, Elias R, Gepdiremen A, Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 2006;223:759-67.
- Gülçin I, Mshvildadze V, Gepdiremen A, Elias R. Screening of antiradical and antioxidant activity of monodesmosides and crude extract from Leontice smirnowii tuber. Phytomedicine 2006;13:343-51.
- Gülçin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nutr 2005;56:491-9.
- Gülçin I, Berashvili D, Gepdiremen A. Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J Ethnopharmacol 2005;101:287-93.
- Koksal Z, Kalin R, Gulcin I, Ozdemir H, Atasever A. Impact of some avermectins on lactoperoxidase in bovine milk. Int J Food Prop 2016;19:1207-16.
- Öztaşkın N, Çetinkaya Y, Taslimi P, Göksu S, Gülçin İ. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49-57.
- Gülçin I, Mshvildadze V, Gepdiremen A, Elias R. The antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(beta-D-glucopyranosyl)-hederagenin. Phytother Res 2006;20:130-4.
- Gülçin I, Oktay M, Kirecci E, Kufrevioglu OI. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem 2003;83:371-82.
- Garibov E, Taslimi P, Sujayev A, Bingol Z, Çetinkaya S, Gulçin İ, et al. Synthesis of 4,5-disubstituted-2-thioxo-1,2,3,4-tetrahydropyrimidines and investigation of their acetylcholinesterase, butyrylcholinesterase, carbonic anhydrase I/II inhibitory and antioxidant activities. J Enzyme Inhib Med Chem 2016;31:1-9.
- Gülçin I, Beydemir S, Sat IG, Kufrevioglu OI. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment Hung 2005;34:193-202.
- Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A, Hansen UP. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 2007;7:2080-95.
- Koksal E, Bursal E, Dikici E, Tozoglu F, Gülçin I. Antioxidant activity of Melissa officinalis leaves. J Med Plants Res 2011;5:217-22.
- Gülçin I, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005;53:281-5.
- Cakmakci S, Topdas EF, Kalin P, Han H, Sekerci P, Kose LP, et al. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int J Food Sci Tech 2015;50:472-81.
- Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu OI. On the in vitro antioxidative properties of melatonin. J Pineal Res 2002;33:167-71.
- Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 1986;44:307-15.
- Aksu K, Özgeriş B, Taslimi P, Naderi A, Gülçin İ, Göksu S. Antioxidant activity, acetylcholinesterase, and carbonic anhydrase inhibitory properties of novel ureas derived from phenethylamines. Arch Pharm 2016;349:944-54.
- Kalin P, Gülçin İ, Goren AC. Antioxidant Activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec Nat Prod 2015;9:496-502.
- Sehitoglu MH, Han H, Kalin P, Gülçin İ, Ozkan A, Aboul-Enein HY. Pistachio (Pistacia vera L.) gum: A potent inhibitor of reactive oxygen species. J Enzyme Inhib Med Chem 2015;30:264-9.
- Koksal E, Bursal E, Gülçin İ, Korkmaz M, Caglayan C, Goren AC, et al. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int J Food Propert 2017;20:514-25.
- Ullah F, Ayaz M, Sadiq A, Hussain A, Ahmad S, Imran M, et al. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina. Nat Prod Res 2016;30:1440-4.
- Basgedik B, Ugur A, Sarac N. Antimicrobial, antioxidant and antimutagenic properties of Iris albicans. Ind Crops Prod 2015;69:480-84.