- Corresponding Author:
- M. Vijayakumar
Pharmacognosy and Ethnopharmacology Division, National Botanical Research Institute, Rana Partap Marg,Lucknow-226 001, India
E-mail: herbalvijay@yahoo.co.in
| Date of Submission | 10 January 2011 |
| Date of Revision | 28 July 2011 |
| Date of Acceptance | 29 August 2011 |
| Indian J Pharm Sci, 2011, 73 (5): 572-577 |
Abstract
The present study was carried out to evaluate antiulcer activity of hydroalcohol extract of Momordica dioica Roxb. fruit. Momordica dioica Roxb. fruit extract (100, 200 and 400 mg/kg body weight) was administered orally, twice daily for 5 days for prevention from ethanol, cold-restraint stress and pylorus ligation-induced ulcers. Estimation of H+-K+ ATPase activity and gastric wall mucous were performed in ethanol-induced ulcer model, antioxidant enzyme activities was carried out in cold-restraint stress-induced ulcer model, and various gastric secretion parameters like volume of gastric juice, acid output, and pH value were estimated in pylorus ligation-induced ulcer model. A significant reduction in lesion index was observed in ulcer-induced animals pre treated with extract at different doses when compared with ulcerated rats in all models. A significant decrease occurred in the level of H+-K+ ATPase, volume of gastric juice, and acid output. Gastric wall mucus and pH were increased significantly. These showed dose-dependent action of extract. LPO and antioxidant enzyme levels of SOD were decreased, but CAT enzyme showed significant increase. Thus the results indicate that the Momordica dioica extract possess antiulcerogenic effect, that attributable to augmentation of gastric defense mechanisms.
Keywords
Ethanol, gastroprotective, Momordica dioica, pylorus ligation, stress
Peptic ulcer disease is a deep gastrointestinal erosion disorder that involves the entire mucosal thickness and can even penetrate the muscular mucosa. Gastric hyperacidity and gastroduodenal ulcer is a very common global problem today because of unhealthy eating habits, and stress. Stress, both psychological and physical is common in everyday life and is known to induce circulatory diseases and ulceration of the digestive tract[1]. Gastric lesions develop when the delicate balance between some gastroprotective and aggressive factors is lost or damaged like acidpepsin secretion, mucosal barrier, mucus secretion, blood flow, cellular regeneration, prostaglandins and epidermal growth factors[2]. Moreover gastric and duodenal ulcers may be induced by a variety of factors, such as stress, nutritional deficiencies and noxious agents, including non-steroidal antiinflammatory drugs[3]. A recent review emphasizes that role of reactive oxygen species in several diseases and the potential antioxidant protective effect of natural compounds on affected tissues are topics of high current interest[4].
Momordica dioica Roxb. (Family: Cucurbitaceae) is commonly known as spine gourd or teasle gourd is an annual or perennial climber. It has native of tropical regions on Asia with extensive distribution in China, Japan, parts of South East Asia, and India. It is used as a vegetable in India, tender fruits and deseeded mature and ripe fruits cooked as vegetable[5]. It is known as Kakora in Hindi. Many research literatures including ancient Ayurveda an Indian system of traditional medicine demonstrate its medicinal values. The roots are used in head trouble, treating urinary calculi. The leaves having aphrodisiac and anthelmintic properties. The fruit was used as stomachic; treating constipation, and the powder or infusion of the dried fruits, when introduced into the nostrils produces a powerful errhine effect and provokes a copious discharge from the schneiderian mucous membrane[6]. The leaves have reported for having strong antioxidant, hepatoprotective action[7]. M. dioica fruits proved to be effective in controlling drug induced nephrotoxicity, and curing renal damages[8]. A recent study concludes that the ethanolic extract of M. dioica seeds possesses marked nephroprotective and curative activities and facilitates the treatment of acute renal injury induced by gentamicin a potent nephrotoxin[9]. The extract of the dried roots of this plant was successfully evaluated for its abortifacient and estrogenic activity[10]. Diversified therapeutic benefits of this plant made us initiation to conduct this work. According to our literature survey none of the recently validated scientific claims available for the antiulcer property of this plant. Thus the present study was undertaken from the above mentioned leads focused us to investigate the antiulcer activity of M. dioica fruits extract.
The Momordica dioica fruits were procured from Madurai market, Tamil Nadu, India in the month of May 2010. The plant material was identified and authenticated by a taxonomist of the institute, and voucher specimen of the collected sample was deposited in the departmental herbarium (NAB 68017M) for future reference. Fruits were removed, dried, powdered and passed through a 10-mesh sieve. The coarsely powdered material was exhaustively extracted thrice with 50% aqueous ethanol. The extracts were filtered, pooled and concentrated at reduced in sub zero temperature (-5º) on a rotary evaporator and then freeze-dried. Yield of M. dioica fruit extract (MDFE) was 13.2% w/w. All the chemicals, kits used in this study were of analytical grade and procured from Sigma chemicals Co., USA and Qualigens Fine Chemicals, Mumbai.
Male Sprague–Dawley rats (140–180 g) procured from CDRI, Lucknow were maintained in a 12 h light/dark cycle at a constant temperature of 25°. Rats were allowed to access standard rodent feed (Dayal, India) and water. Food was withdrawn 18–24 h before the experiment though water was allowed ad libitum and animals were fasted, allocated to different experimental groups each of 6 rats. All studies were performed in accordance with the guide for the care and use of laboratory animals, as adopted and promulgated by the Institutional Animal Care Committee, CPCSEA, India (Reg. No. 222/2000/ CPCSEA). The rats were administered with MDFE, suspended in 1% carboxy methyl cellulose (CMC) in distilled water in doses of 100, 200 and 400 mg/kg and ranitidine, the reference drug (RAN), in the dose of 50 mg/kg orally twice daily at 10:00 and 16:00 h respectively for 5 days for this ulcer protective study in all the models. Ranitidine was used over other drugs because of its superior ulcer treating property[11]. Control group of animals received suspension of 1% CMC in distilled water for the same administration period.
Ethanol-induced ulcers in rats were induced by administering ethanol orally (1 ml/200 g, 1 h). After 1 h animals were sacrificed by cervical dislocation and stomach was incised along the greater curvature and examined for ulcers[12]. Ulcer index was calculated by adding the total number of ulcers per stomach and the total severity of ulcers per stomach. The pooled group ulcer score was calculated[13]. The H+-K+ ATPase activity was assayed by the method of Nagaya et al. [14] Gastric wall mucus was determined according to the method of Corne et al.[15].
Cold restraint stress was performed by following the method of Gupta et al.[16] by strapping the rats on a wooden plank and keeping them for 2 h at 4–6° after five days of treatment. The animals were then sacrificed by cervical dislocation and ulcers were scored on the dissected stomachs as described above. The fundic part of the stomach was homogenized (5%) in ice-cold 0.9% NaCl with a Potter–Elvehjem glass homogenizer for 30 s. The homogenate was centrifuged at 800 rpm for 10 min and the supernatant was again centrifuged at 12,000 rpm for 15 min and the obtained mitochondrial fraction was used for the following estimations[17]. The catalase (CAT) enzyme was estimated by the method[18]. The superoxide dismutase (SOD) activity was estimated by the inhibition of nicotinamide adenine dinucleotide (reduced)-phenazine methosulphatenitrobluetetrazolium reaction system as described by Nishikimi et al.[19] as adapted by Kakkar et al.[20] Lipid peroxidation (LPO) inhibition was measured by estimating MDA the method[21].
On day 6 after the last dose, the pyloric ligation was performed by the method of Shay et al.[22] rats were kept for 18 h fasting and care was taken to avoid coprophagy. Animals were anaesthetized using pento-barbitone (35 mg/kg, i.p.), the abdomen was opened and pylorus ligation was done without causing any damage to its blood supply. The stomach was replaced carefully and the abdomen wall was closed in two layers with interrupted sutures. The animals were deprived of water during the post-operative period. After 4 h, stomachs were dissected out and contents were collected into tubes and gastric secretion volume, pH and HCl concentration were estimated. The ulcers were scored as described under ethanol-induced ulcers. Gastric contents were analyzed for total acidity by titrating against 0.01N NaOH using phenolphthalein as indicator. The pH of gastric juice was measured by using pH paper strips of varying ranges. The color of the pH paper after the procedure was matched with standard scale and the pH was recorded for different groups of animals.
The values were represented as mean±S.E.M. for 6 rats. Analysis of variance (ANOVA) test was followed by individual comparison by Newman– Keuls test using Prism Pad software for the determination of level of significance. The P-values of <0.05 was considered statistically significant for the experiment.
Results of this study clearly reveal MDFE at doses of 100, 200, and 400 mg/kg twice a day for 5 days prevented the acute gastric ulcers in a dose-related manner. Rat gastric mucosal damage induced by high concentrations of ethanol has widely been used to investigate gastroprotective effect of medicinal plants[23]. Ethanol destroys the protective factors of the mucosa, such as the mucus barrier, also gastric wall mucus depletion induced by ethanol is one of the pathogenic mechanisms responsible for gastric lesion Koo et al.[24] Findings of our study shows, pre-treatment with 50% ethanolic MDFE (100, 200 and 400 mg/kg, p.o.) caused significant decrease in the intensity of gastric mucosal damages induced by ethanol, as compared with control group. The gastric lesions index witnessed significant reduction at MDFE 400 mg/kg dose as 9.83±0.41, 15.24±0.34 and 10.86±0.41 from their control values of 12.67±0.61, 17.14±0.46 and 13.59±0.62 respectively in ethanol, cold resistant stress, and pylorus-ligated ulcer models (fig. 1). It indicates MDFE could act as a good ulcer protective agent and reduced the ulcer index significantly. Parietal cells secrete acid into stomach lumen. This is achieved by a unique H+-K+ATPase (proton pump) that catalyses the exchange of intercellular H+ for extracellular K+. Hyper acidity is a prevalent pathological condition caused due to uncontrolled hyper secretion of HCl from parietal cells of gastric mucosa mediated through H+-K+ATPase[25]. In this study H+-K+ATPase activity was elevated to 1.46±0.08 (P<0.05) after ethanol administration, but MDFE at 200, 400 mg/kg doses significantly brought down H+K+ATPase activity to 1.09±0.05, 0.95±0.07 respectively (P<0.05). Mucus layer forms a physical barrier on the surface of the stomach and proximal duodenum, and consists of a mucus gel into which HCO3- is secreted. Within the gel matrix the HCO3- neutralizes acid diffusing from lumen. This creates a pH gradient and the gastric mucosa is maintained at neutral pH. The ethanol administration depleted the gastric wall mucus drastically to 182.94±6.21 (P<0.05) as compared with normal group rats value of 239.15±6.40, but MDFE 400 mg/kg restored the gastric wall mucus to 211.32±7.96 (P<0.05).
In cold restraint stress, incidence of ulcers is mainly due to increased acid secretion and generation of free radicals. Ulcers occur because of stress are due to both physiological and psychological factors[26]. Generally stress-induced ulcers also involve damage by reactive oxygen species (ROS) apart from acid and pepsin related factors. Increased level of LPO is due to increase in generation of ROS during stress leading to oxidative damage. SOD converts the reactive superoxide radical to H2O2, which if not scavenged by CAT can by itself cause lipid peroxidation by generation of hydroxyl radicals. Consequently decrease in CAT levels has led to increase in accumulation of these ROS and thus, has caused increased lipid peroxidation and tissue damage[27]. Preventive antioxidants, such as superoxide dismutase, catalase are the first line of defence against reactive oxygen species. The experimental data stated that the cold-resistant stress aggravated the ulcer severity and lipid peroxidation as compared to unstressed rats. After pre-treatment of MDFE 100, 200 and 400 mg/kg doses, the LPO, SOD levels fell significantly (P<0.05) and SOD levels showed decreasing trend at 100, 200, 400 (P<0.05) mg/kg dose levels as compared with CRS-induced group, On contrary CAT values showed gradual, significant increase at 100, 200 and 400 mg/kg dose levels (Table 1). These data satisfying the efficacy of MDFE in this model shows potent antioxidant activity by virtue of which it decreased the susceptibility of gastric mucosal damage through its the free radical scavenging capacity.
| Treatment dose(mg/kgb.w.) | LPO (nmol of MDA SOD (nmol/g CAT (units/mg | ||
|---|---|---|---|
| formed/h/100mgprotein) | tissue) | protein) | |
| Normal control | 0.43±0.03 | 110.69±4.07 | 25.09±1.55 |
| CRS-induced ulcer | 0.54±0.02† | 200.43±4.70† | 16.62±1.08† |
| control | |||
| MDFE (100)+CRS | 0.41±0.01* | 189.66±3.60* | 19.59±0.44 |
| MDFE (200)+CRS | 0.37±0.01* | 179.31±3.77* | 20.83±0.79* |
| MDFE (400)+CRS | 0.26±0.01* | 162.05±2.56* | 25.42±0.94* |
| Ranitidine (50) | 0.37±0.02* | 163.00±4.22* | 28.09±1.24* |
Values are expressed as Mean±SEM of 6 rats in each group. †P<0.05 are compared to respective normal control group. *P<0.05 are compared to respective CRS-induced ulcer control group
Table 1: Effect of 50% Ethanolic Mdfe on lpo, Sod and Cat Activity In Rat Gastric Mucosa In Cold Restraint Stress (crs)-Induced Gastric Ulcers In Rats
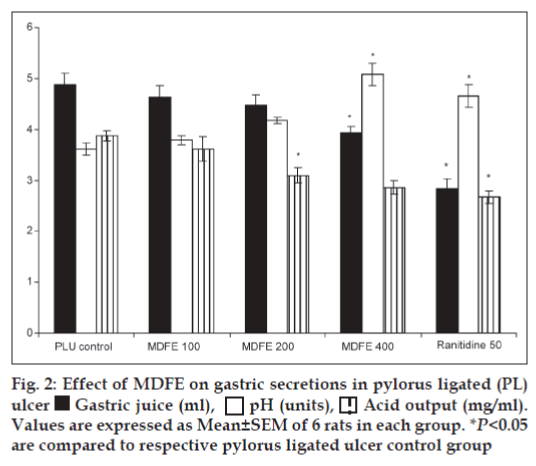
Pylorus ligation-induced ulcers are caused by enhanced acid pepsin secretion leading to autodigestion of the gastric mucosa and break down of the gastric mucosal barrier[28], the digestive effect of accumulated gastric juice and interference of gastric blood circulation are responsible for the induction of ulceration[29]. In this study pylorus ligation increases the acid secretion that is why the gastric volume was increased. The pre-treatment of MDFE 400 mg/kg dose significantly reduced the gastric juice volume (P<0.05), simultaneously the above dose of MDFE increased the pH value significantly (P<0.05), and decreased the acid output at 200, 400 mg/kg (P<0.05) respectively as compared with the pylorus-ligated ulcer group rats (fig. 2). All the results of MDFE are comparable with the standard drug ranitidine at 50 mg/kg dose.
Thus, our study shows that oral administration of M. dioica fruit extract showed antiulcer effects. The gastro protective role of M. dioica in ethanol induced ulcer might be the resultant of decreased gastric lesions, proton pump activity and increased gastric wall mucus. In cold restraint stress-induced model it reduces LPO, SOD levels. Finally M. dioica extract reduces the gastric volume; acid output and increases pH value in Pylorus ligation-induced ulcer model strengthen the claim of potential anti ulcer activity of this plant. Thus, it can be concluded that possible mechanism of antiulcer activity of Momordica dioica fruits may be due to its free radical-scavenging and antioxidant activity. However, further studies are needed to confirm involvement of specific compounds and its isolation, therapeutic evaluation for ulcer.
References
- Glavin GB, Paré WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. NeurosciBiobehav Rev 1994;18:223-49.
- Lima ZP, Severi JA, Pellizzon CH, Brito AR, Solis PN, Cáceres A, et al. Can the aqueous decoction of mango flowers be used as anantiulcer agent? J Ethnopharmacol 2006;106:29-37.
- Berenguer B, Sánchez LM, Quílez A, López-Barreiro M, de Haro O, Gálvez J, et al. Protective and antioxidant effects of Rhizophoramangle L. against NSAID-induced gastric ulcers. J Ethnopharmacol2006;103:194-200.
- Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res 2002;35:523-34.
- Joseph JK, Antony VT. Ethnobotanical investigations in the genusMomordicaL. in the Southern Western Ghats of India. Genet ResourCrop Evol 2008;55:713-21.
- Kirtikar KR, Basu BD. Indian Medicinal Plants. Vol 2. Dehradun: International Book Distributors; 1999.
- Jain A, Soni M, Deb L, Jain A, Rout SP, Gupta VB, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of MomordicadioicaRoxb. leaves. J Ethnopharmacol 2008;115:61-6.
- Jain A, Singhai AK. Nephroprotective activity of MomordicadioicaRoxb. incisplatin-induced nephrotoxicity. Nat Prod Res 2010;24:846-54.
- Jain A, Singhai AK. Effect of MomordicadioicaRoxb on gentamicin model of acute renal failure. Nat Prod Res 2010;24:1379-89.
- Shreedhara CS, Pai KS, Vaidya VP. Postcoital antifertility activity of the roots of MomordicadioicaRoxb. Indian J Pharm Sci 2001;63:528-31.
- Nowak A, Sadliński C, Górka Z, Nowakowska E, Rudzki J, Gibiński K. Ranitidine in the treatment of acute upper gastrointestinal haemorrhage-a comparative study. Preliminary report.Hepatogastroenterology 1981;28:267-9.
- Hollander D, Tarnawski A, Krause WJ, Gergely H. Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat Macroscopic, histologic, ultrastructural, and functional time sequence analysis. Gastroenterology 1985;88:366-74.
- Sanyal AK, Pandey BL, Goel RK. The effect of a traditional preparation of copper, tamrabhasma, on experimental ulcers and gastric secretion. J Ethnopharmacol 1982;5:79-89.
- Nagaya H, Satoh H, Maki Y. Actions of antisecretory agents on proton transport in hog gastric microsomes. BiochemPharmacol 1987;36: 513-9.
- Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol 1974;242:116-7.
- Gupta MB, Nath R, Gupta GP, Bhargava KP. A study of the anti-ulcer activity of diazepam and other tranquillosedatives in albino rats. ClinExpPharmacolPhysiol 1985;12:61-6.
- Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem 1993;125:115-25.
- Aebi H. Catalase. Methods of Enzymatic Analysis. 2nd ed. New York: Academic Press; 1974. p. 673-7.
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. BiochemBiophys Res Commun 1972;46:849-54.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J BiochemBiophys 1984;21:130-2.
- Jamall IS, Smith JC. Effects of cadmium on glutathione peroxidase, superoxidase dismutase and lipid peroxidation in the rat heart: A possible mechanism of cadmium cardiotoxicity. ToxicolApplPharmacol 1985;80:33-42.
- Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H. A simple method for the uniform production of gastric ulceration. Gastroenterology 1945;5:43-61.
- Zhu M, Lew TH, Luk CT. Gastric protective effect of Lentinusedodes against ethanol-induced ulceration. Fitoterapia 1997;68:537-42.
- Koo MW, Ogle CW, Cho CH. Effects of verapamil, carbenoxolone and N-acetylcysteine on gastric wall mucus and ulceration in stressed rats. Pharmacology 1986;32:326-34.
- Jain KS, Shah AK, Bariwal J, Shelke SM, Kale AP, Jagtap JR, et al. Recent advances in proton pump inhibitors and management of acid-peptic disorders. Bioorg Med Chem 2007;15:1181-205.
- Miller TA. Mechanisms of stress-related mucosal damage. Am J Med 1987;83:8-14.
- RaoChV, Verma AR, Vijayakumar M, Rastogi S. Gastroprotective effect of standardized extract of Ficusglomerata fruit on experimental gastric ulcers in rats. J Ethnopharmacol 2008;115:323-6.
- Eswaran MB, Surendran S, Vijayakumar M, Ojha SK, Rawat AK, RaoChV. Gastroprotective activity of Cinnamomumtamala leaves on experimental gastric ulcers in rats. J Ethnopharmacol 2010;128:537-40.
- Patel AV, Santani DD, Goel RK. Antiulcer activity and the mechanism of action of magaldrate in gastric ulceration models of rat. Indian J PhysiolPharmacol 2000;44:350-4.
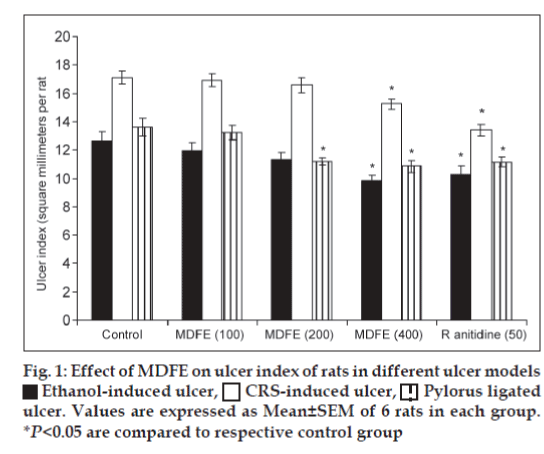
 Ethanol-induced ulcer,
Ethanol-induced ulcer,  CRS-induced ulcer,
CRS-induced ulcer,  Pylorus ligated
ulcer. Values are expressed as Mean±SEM of 6 rats in each group.
*P<0.05 are compared to respective control group
Pylorus ligated
ulcer. Values are expressed as Mean±SEM of 6 rats in each group.
*P<0.05 are compared to respective control group