- *Corresponding Author:
- A. Khedr
Pharmaceutical Chemistry Department, Faculty of Pharmacy, Saudi Arabia
E-mail: akhedr@kau.edu.sa
| Date of Submission | 22 June 2015 |
| Date of Revision | 18 October 2016 |
| Date of Acceptance | 25 October 2016 |
| Indian J Pharm Sci 2016; 78(5): 680-687 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The major acidic and phenolic biogenic materials in adult-camel urine were characterized using gas chromatography-mass spectrometry. The adult-camel urine sample was treated with glucuronidase/arylsulfatase enzyme followed by extraction on Sep-Pak® C8 column. The water soluble compounds, including urea and creatinine, were washed out using water containing 0.4% trifluoroacetic acid. Ethyl acetate:diethylether, 1:1 v/v, was used as extraction solvent. The extraction residue was reprivatized with N-methyl-N-(trimethylsilyl) trifluoroacetamide followed by gas chromatography-mass spectrometry analysis. The average concentrations of, phenol, p-cresol, salicylic acid, cinnamic acid, azelaic acid and benzoic acid were 4.0, 107.2, 42.7, 3.2, 68.6 and 490.6 mg/100 ml, respectively. The calibration range of each of these compounds was spanning the range of 1.0 to 50 ng/μl. The extraction recovery of all studied compounds was 100±0.8%. A standard solution mixture containing the major acidic and phenolic compounds was prepared to contain the same corresponding concentration in adult-camel urine. Both, adult-camel urine and prepared standard mixture were active against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Aspergillus flavus (fungus) and Candida albicans (fungus) after 72 h of incubation.
Keywords
Camel urine, antimicrobial, gas chromatography-mass spectrometry, azelaic acid, p-cresol, cinnamic acid
Traditionally, adult camel urine (CUR) has been claimed to exhibit anticancer, antimicrobial and antifungal effect upon administration orally or topically. To date, there is no evidence that these effects are due to the urine of these camel. The objective of this work is to find out the bioactive material in relation to any of the claimed activities. The chemical composition of camel urine has been reported to contain organic nitrogen, ammonia, urea, creatinine, creatine, hippuric acid and chloride [1]. Recently, more details about camel urine composition using liquid chromatography-mass spectrometry have been reported [2]. This information showed many metabolites in camel urine. Benzoic acid (BEN), urea, creatinine, phenylacetate, citric acid and hippuric acid have been reported as the major constituents in camel urine. The concentration of these materials was matched with the amounts found in different camels, elephant and rat urine. The amount of benzoate salt was greatest in camel urine. To date, the fully characterized chemical composition of camel urine, not yet reported. Al-Abdalall [3], proved that camel urine at low concentrations had no significant inhibitory effect on fungal growth, while inhibition can be obviously recorded after using high concentrations. Moreover, camel urine has been proved to have a potent antiplatelet activity against adenosine diphosphateinduced and arachidonic acid induced platelet aggregation; neither human nor bovine urine exhibited such properties [4].
Our research investigated the chemical nature and composition of camel urine to know which substance(s) gave antimicrobial and antifungal activities. This leads us to characterize the most suspicious materials in CUR that could have antimicrobial activity. Herein, described a sequential procedures including, enzymatic hydrolysis of glucuronides/sulfate conjugates, solidphase extraction, followed by derivatization. The derivatized sample extract was analyzed by gas chromatography-mass spectrometry (GC-MS) to characterize and to measure the major bioactive antimicrobial materials.
Materials and Methods
Adult camel urine (1-5 y old) samples were collected, freely voided, at the morning, just before sunrise, in clean containers. Adult human urine was collected from healthy volunteers, n=6. Sample volume and pH were measured and immediately treated for the analysis or maintained at -80° in labeled separate containers.
Sodium benzoate (BEN), purity >99%, p-cresol (CRE), phenol (PHE), azelaic acid (AZE), cinnamic acid (CIN) and salicylic acid (SAL), were purchased from Sigma-Aldrich (Fluka, Steinheim, Germany). β-glucuronidase/arylsulfatase (Helix pomatia, type HP-2 ≥500,000 units/ml, Sigma units β-glucuronidase and ≤37.5 units sulfatase activity) and N-methyl-Ntrimethylsilyl- trifluoroacetamide (MSTFA) 99.8% w/v, were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-hydroxymethyl-propyphenazone (3-OH-MP, m. wt. 246) was prepared in our laboratory by boiling, 1 g of 3-bromomethyl-propyphenazone in 50 ml water for 30 min [5]. The obtained crystals were washed with 0.1% sodium carbonate followed by water, filtered and dried over sodium hydroxide pellets in desiccators. The purity of 3-OHMP was verified by GC-MS, melting point and LC-MS. A concentration of 40 ng/μl of 3-OHMP was prepared in acetone and used as an internal standard. The tested micro-organisms, bacteria and yeast strains were obtained from the Micro-Analytical Center, Faculty of Science, Cairo University, Egypt. Gram-positive bacterial strains: Staphylococcus aureus (ATCC 12600) and Bacillus subtilis (ATCC 6051) and Gram-negative bacterial strains: Escherichia coli (ATCC 11775), Pseudomonas aeruginosa (ATCC 10145), yeast: Candida albicans (ATCC26555) and fungi: Aspergillus flavus (ATCC 204304) were used for antibacterial and antifungal screening. Solid-phase extraction (SPE) columns, Sep- Pak® Vac 1cc (100 mg) C8 cartridge were purchased from Waters, Ireland. Extraction manifold, 16×75 mm tubes, with Buchi vacuum pump V-700 and the screwcapped (PTFE/silicon) 1 ml total recovery autosampler vials (12×32 mm) were purchased from Waters (Waters, Milford, MA, USA). Screw-capped borosilicon minireaction vials (v-shaped, with TFE liners, Alltech, GmbH, Unterhaching, Germany) were used for derivatization at elevated temperature. Solvents and all other materials were of analytical grade.
Instrument and conditions:
GC-MS: Clarus 500 GC-MS (Perkin Elmer,Shelton, CT, USA) was utilized throughout the experiments. The software controller/integrator was TurboMass version 5.4.2.1617. An Elite-1 GC capillary column, Crossbond® 100% dimethyl polysiloxane (30 m×0.25 mm ID×0.25 μm df, Perkin Elmer) was used. The carrier gas was helium (purity 99.9999%) and flow rate was 0.9 ml/min. Source (EI+): source temperature was 270°. GC line temperature was 210°. Electron energy was 70 eV, and trap-emission was 100 V. The oven was programmed as follows: initial temperature was 70° (hold 2 min) to 150° (rate 10°/min, hold 5.0 min), followed by an increase to 220° (rate 10.0° /min, hold 5 min), then increased to 280° (rate 20°/min, hold 2.0 min). The injector temperature was 260°. The injection volume was 1.0 μl and the split ratio was 40:1. The run time was 32 min. Samples were acquired by applying a total MS scan from 40 to 350 m/z (500 scan/s).
Enzymatic hydrolysis:
β-Glucuronidase arylsulphatase enzyme was used to hydrolyze the glucuronides or suphate conjugated compounds in the urine. In 250 ml round flask, a volume of 30 ml camel urine was mixed with 1 ml of 2 M sodium acetate and adjusted to pH 5.5 with acetic acid or 1 M sodium hydroxide solution. This solution was mixed with 200 μl β-glucuronidase/ arylsulphatase. The reaction mixture was left for 24 h at 42° in thermostatically controlled water bath. The reaction was stopped by addition of 100 μl of CH2Cl2 or extracted immediately using SPE columns.
Extraction of camel urine:
The Sep-Pak® Vac 1cc (100 mg) C8, 100 μl, extraction cartridge was fitted to a vacuum manifold and condition with 2 ml methanol followed by 2 ml water containing 0.4% trifluoroacetic acid (v/v). The column end was closed and a volume of 100 μl 0.4% TFA and 100 μl CUR sample solution was added. The sample was allowed to flow through the column at a flow rate of 10 drops/min. The sample was cleaned-up with 1 ml 0.4% TFA solution, followed by 1 ml water and left under vacuum for 5 min to expel the adsorbed water. The column was fitted to another port with a clean test tube containing 100 μl of 0.6% v/v, triethylamine in acetone and 100 μl of 3-OHMP, 40 ng/μl. Two milliliter of the extraction solvent (ethylacetate:diethylether; 1:1, v/v) was added and allowed to flow through the column at slow flow rate. The eluent was then dried under a gentle stream of nitrogen gas at room temperature. The residue was reconstituted in 100 μl ethylacetate: diethylether; 1:1 v/v, transferred to total recovery vial and dried with nitrogen gas. Trimethylamine solution was used to suppress the volatilization of phenolic compounds during the step of drying with nitrogen gas.
Reaction with MSTFA:
The hydroxyl, carboxylic, and amino compounds were trimethylsilylated using MSTFA. The vial containing the extract residue was mixed with 50 μl of MSTFA, closed cap and heated at 80° for 10 min, using block heater (designed for half insertion of vials). The reaction mixture was cooled to room temperature, and a volume of 1 μl was injected for GC-MS analysis.
Preparation of calibration mixture:
The calibration mixture was prepared by dissolving 100 mg of each; phenol (PHE), p-cresol (CRE), benzoic acid (BEN), salicylic acid (SAL), cinnamic acid (CIN) and azelaic acid (AZE), with the aid of sonication, in 100 ml water containing 100 mg of KOH, 100 mg glycine and 100 mg glycerol. A volume of 0.5 ml from this solution was diluted to 100 ml with water to obtain a concentration of 50 ng/μl, of each compounds. A serial dilution was prepared spanning the range of 1 to 50 ng/μl of each compound. A volume of 100 μl, from each concentration level, was extracted using Sep-Pak® Vac 1cc (100 mg) C8, derivatized with MSTFA, and analyzed by GC-MS. The calibration curves were constructed by plotting the peak area ratio of corresponding compound to 3-OHMP versus concentration as ng/μl. Potassium hydroxide was used to form less volatile and soluble salts. Glycine and glycerol were also added as solvent matrix.
Preparation of antimicrobial mix:
The antimicrobial mix was prepared to contain same average amount of characterized compounds that measured in CUR after enzymatic treatment. A weight of 40, 1072, 4906, 427, 32, 686 mg of phenol, p-cresol, benzoic acid, salicylic acid, cinnamic acid and azelaic acid, respectively, was prepared in 100 ml volumetric flask. This powder mixture was mixed with 17 mg KOH (equimolar amount), 80 ml water and sonicated for about 10 min. The pH was adjusted to 7.9 using 0.5 M KOH or 1 M phosphoric acid and the final volume was adjusted to 100 ml with water. A volume of 10 ml of this solution was diluted with water, to 100 ml, filtered through a sterile 0.22 μ nylon membrane and tested for antimicrobial activity.
Testing of antimicrobial activity:
Three types of samples were tested for antimicrobial activity. Samples were include, fresh camel urine, enzymatic hydrolyzed CUR sample and prepared standard solution mixture containing same concentrations of the claimed bioactive materials found in CUR. Mueller-Hinton agar was used as the culture medium for bacteria, while Czapek’s Dox agar (sucrose-nitrate agar) was used for yeasts and fungi. Blank paper disks (Schleicher and Schuell) with a diameter of 8.0 mm, were impregnated with the tested compounds. Standard discs of ampicillin and amphotericin B served as positive controls for the antimicrobial activity and filter discs impregnated with dimethyl sulfoxide (DMSO) were used as a negative control. The modified Knirby-Bauer disc diffusion method was used in antimicrobial testing [6]. Briefly, 100 μl of the test bacteria/fungi were grown in 10 ml of fresh media until they reached a count of, approximately, 108 cell/ml for bacteria or 105 cell/ml for fungi. About 100 μl of microbial suspension was spread onto agar plates corresponding to the broth in which they were maintained. Paper disks impregnated with 10 μl of the tested compounds were added. Samples were incubated at 35-37° for 72 h and yeast incubated at 30° for 72 h and the diameters of the inhibition zones were measured in millimeters.
Results and Discussion
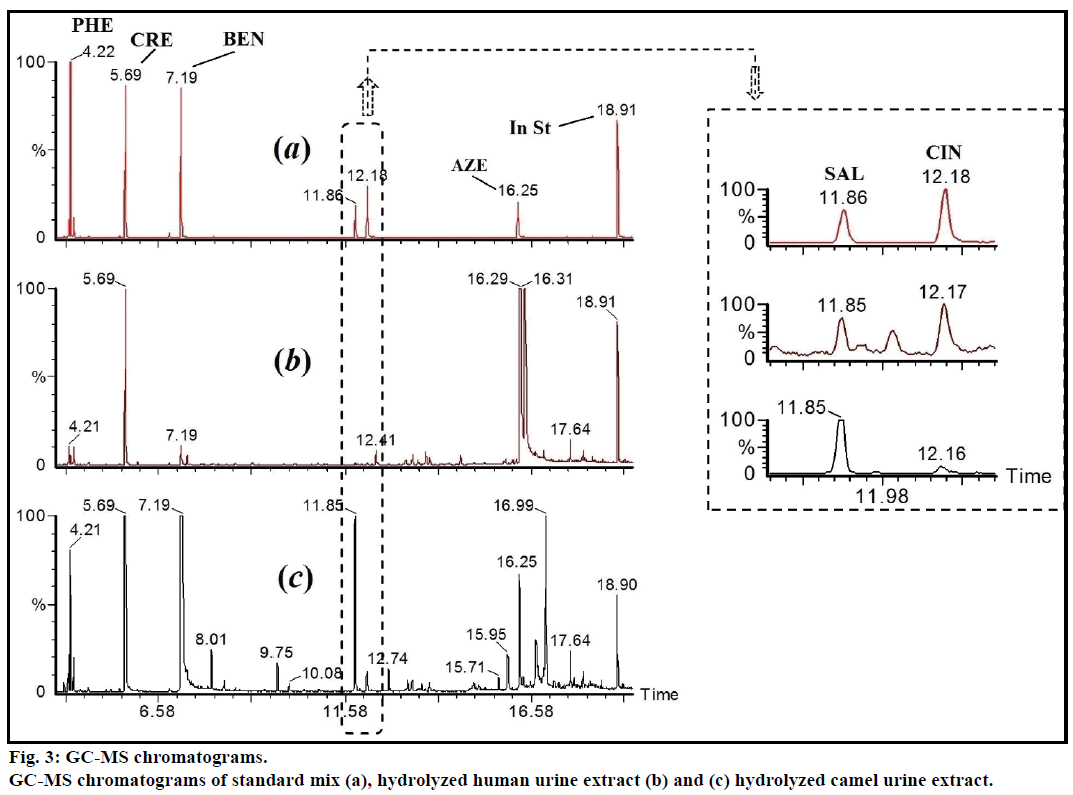
The pH of fresh camel urine was ranged from 8.2 to 9.2. This basic pH might be due to high concentration of potassium salts [7]. Amazingly, it was very difficult to filter the fresh camel urine through 0.22 or 0.45 μ nylon filters. In addition, camel urine was found not miscible with acetonitrile, even after shaking or sonication. The liquid-liquid extraction gave low and fluctuated percentage recoveries of targeted materials. The glucuronides metabolites were enzymatically hydrolyzed before the extraction. The reported major polar compounds were identified by liquid chromatography-mass spectrometry (LC-MS) and GC-MS [2]. The confirmed major constituents in nonhydrolyzed samples were include: hippuric acid, creatinine, urea, phenaceturic acid and benzoic acid. These substances needs large amount of MSTFA and showed intense GC-MS peaks. The use of 2 ml 0.4% trifluoroacetic acid in water, as clean-up solvent, was essential to remove this major polar unwanted compounds and to ovoid co-elution/overlap with the targeted substances.
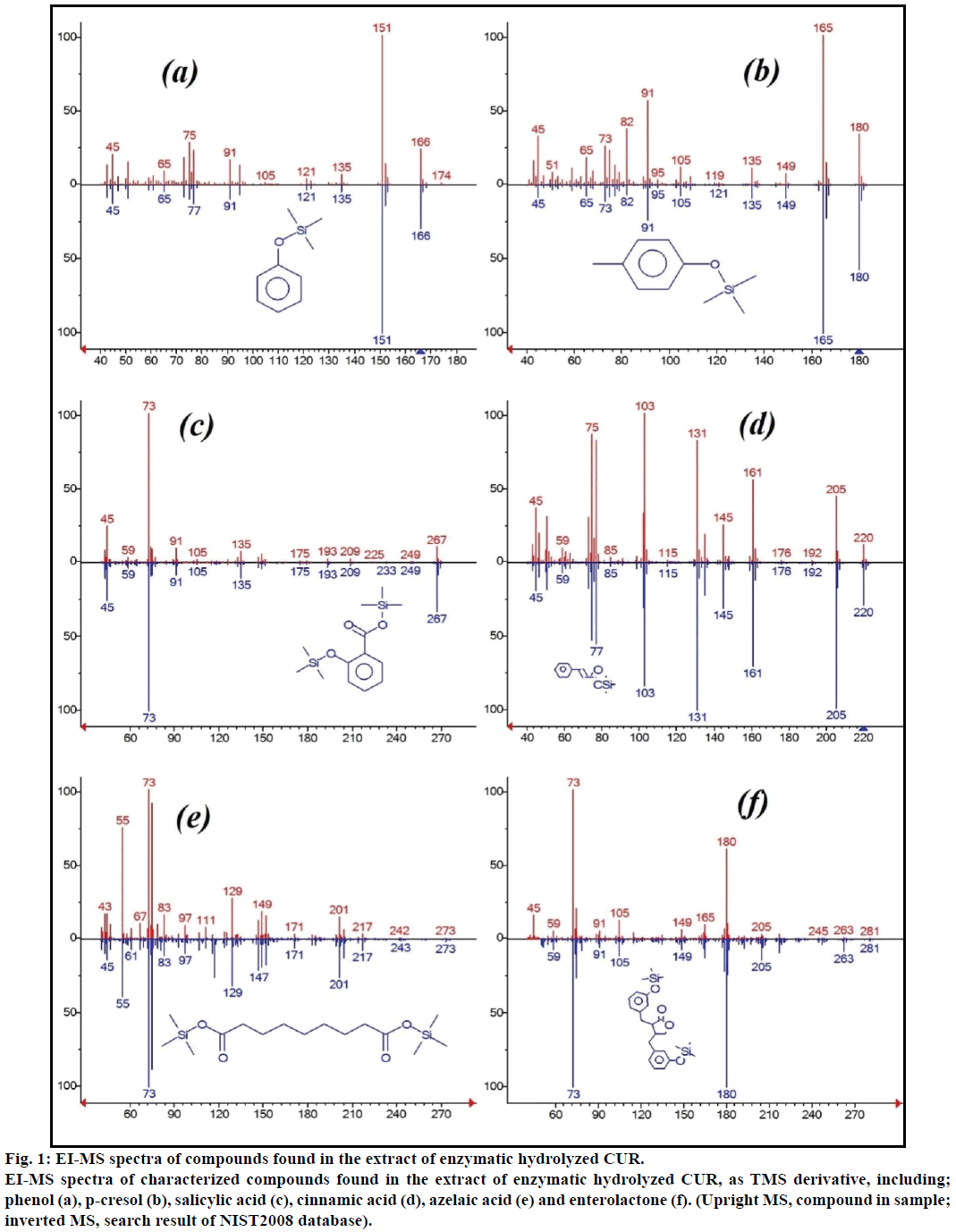
The enzymatic hydrolyzed CUR was extracted on Sep-Pak C8 column, washed with 0.4% TFA in water, followed by water and the eluent was derivatized with MSTFA. Figure 1 showed the GC-MS of MSTFA derivatized CUR extract. The EI-MS spectra of investigated peaks showed most reported compounds.
Figure 1: EI-MS spectra of compounds found in the extract of enzymatic hydrolyzed CUR.
EI-MS spectra of characterized compounds found in the extract of enzymatic hydrolyzed CUR, as TMS derivative, including; phenol (a), p-cresol (b), salicylic acid (c), cinnamic acid (d), azelaic acid (e) and enterolactone (f). (Upright MS, compound in sample; inverted MS, search result of NIST2008 database).
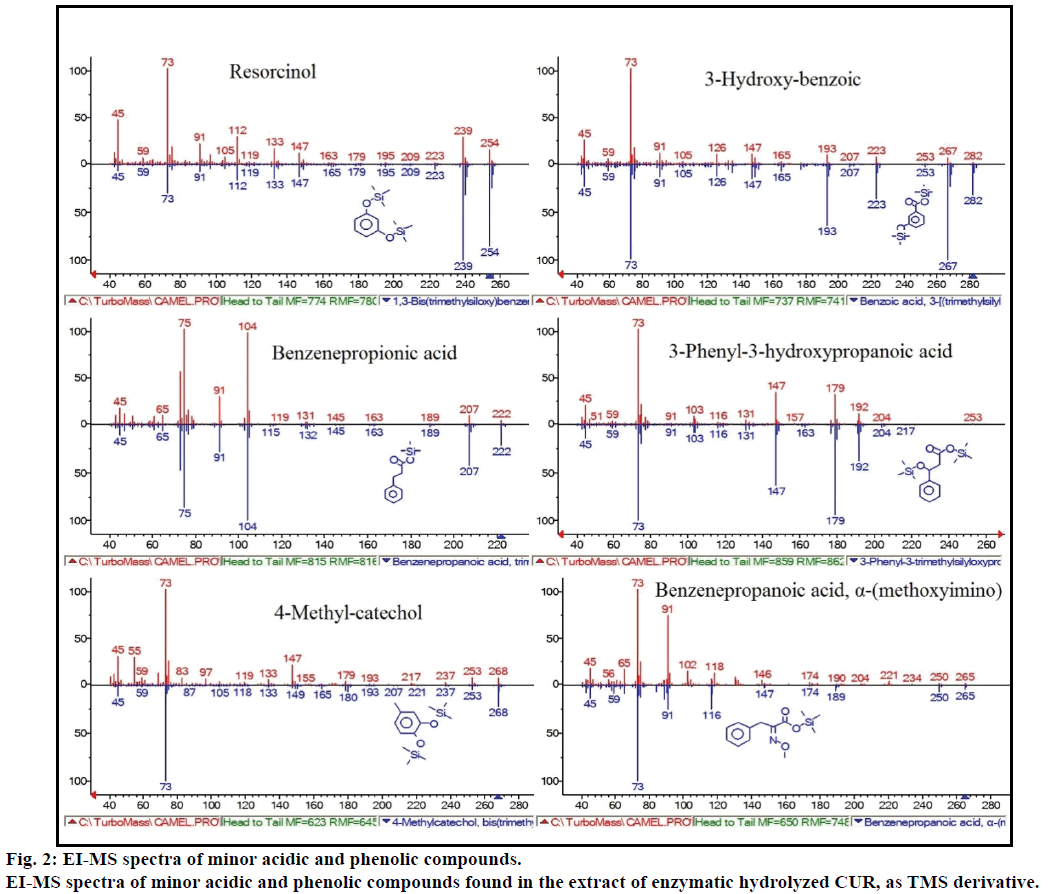
Moreover, the GC-MS analysis showed additional bioactive compounds which are not mentioned in any previous literature. These compounds were include, phenol, p-cresol, salicylic acid, cinnamic acid, azelaic acid and enterolactone. P-cresol was the predominant phenolic compound, as per GC-MS. Other minor GCMS peaks were characterized by NIST2008, after enzymatic hydrolysis, and ignored from the assay. Those compounds were identified as TMS-derivative of; resorcinol, 3-hydroxy-benzoic acid, benzene propionic acid, 3-phenyl-3-hydroxypropanoic acid, 4-methylcatechol and α-(methoxyimino) benzenepropanoic acid (Figure 2).
The simulated camel urine was prepared to contain the most interesting compounds that known to exhibit therapeutic activity and marketed as drugs. A topical formulation contain 6% benzoic acid and 3% salicylic acid has been described an effective treatment of Tinea Unguium [8]. Azelaic acid was one of the major new constituents found in camel urine. Skinoren® cream is Italian pharmaceutical topical formulation found in the market. This formulation contains 20% w/w, of azelaic acid and prescribed for the treatment of acne and hyperpigmentation in skin. Azelaic acid has been proved as an effective treatment of Papulopustular rosacea and hyperpigmentation in skin [9].
The GC-MS analytical method was optimized using standard substances. Many extraction solvents were tried including, methanol, dichloromethane, chloroform, ether and n-hexane. Methanol extract showed many interfering extracted substances including the residual amounts of polar compounds. Chloroform and dichloromethane showed a very low % recover of the targeted compounds (<72%). The optimal extraction solvent was a mixture of ethylacetate and diethylether (1:1 v/v). This solvent mix showed optimal extraction of targeted compounds with minimal co-eluted interfering polar components. The average percentage recovery, of all determined compounds, was 100±0.8%, using spiked water solution. The determined compounds include phenol (PHE), p-cresol (CRE), benzoic acid (BEN), salicylic acid (SAL), cinnamic acid (CIN) and azelaic acid (AZE) using 3-OH-MP as internal standard. The Sep-Pak® C8 column, 100 mg, showed a good results (100% recovery) on loading 100-200 μl sample volume. After washing the SPE column, nitrogen gas was purged forcedly throughout the column to expel the residual amount of adsorbed water, because MSTFA was rapidly deactivated in moisture containing solvent. The eluted extract was mixed with 100 μl 0.6% triethylamine (in acetone) to ovoid the loss of volatile phenolic compounds during the process of drying. These precautions showed a precise extraction recovery value, reached to 100±0.8%. The hydrolyzed CUR samples were immediately extracted, or left inside the refrigerator (at 4°), because phenol and p-cresol were turned red if stand for 2 h (day light). The SPE C8 and C18 columns showed the same recovery results. However, more volume of the extraction solvent (4 ml) was required in case of using C18/1 ml columns. The calibration parameters of the determined compounds are listed in Table 1.
| Name | Slope | Intercept | r* | LOD,g/ml | LOQ,μg/ml | Range, μg/ml |
|---|---|---|---|---|---|---|
| Phenol | 0.00689937 | -0.05158 | 0.9991 | 0.05 | 0.10 | 2.0–500 |
| p-cresol | 0.00571764 | -0.01628 | 0.9981 | 0.04 | 0.10 | 2.0–500 |
| Benzoic acid | 0.0057987 | 0.01096 | 0.9995 | 0.05 | 0.20 | 4.0–500 |
| Salicylic acid | 0.00145584 | -0.01006 | 0.9987 | 0.05 | 0.20 | 4.0–500 |
| Cinnamic acid | 0.00243503 | -0.02321 | 0.9985 | 0.05 | 0.20 | 4.0–500 |
| Azelaic acid | 0.00371740 | 0.00596 | 0.9981 | 0.05 | 0.20 | 4.0–500 |
Table 1: Calibration Parameters, Lod and Loq of Assayed Compounds
The regression coefficient was close to unity, for all compounds, as shown in Table 1. The average EI-MS scan, of each peak at definite retention times, was defined to the Turbo Mass software to integrate the GCMS peaks selectively. This method showed an inter-day and intraday precision values of not more than 1.62 and 0.81% (relative standard deviation, RSD), respectively. The percentage error of spiked sample, over three levels, includes 75, 100 and 125% of claimed content, were not more than 0.07%. The method deemed to be accurate, precise and selective.
The free and conjugated major acidic compounds were determined in CUR and matched with the measured concentrations in urine of healthy human, n=6. The quantitation data showed that the average concentrations of p-cresol and azelaic acid in camel urine were 107 and 69 mg/100 ml, respectively (Table 2). The total concentration of benzoic acid (free and conjugated) in CUR were about 410 mg/100 ml, while, the concentration of the free (unconjugated) form was 40.0 mg/100 ml (Figure 3). The reported amount of benzoic acid in non-hydrolyzed CUR was 1484 μmol/ mmol creatinine (equivalent to 18 mg/100 ml) [2]. This variation could be due to the geographical location of used camels. Moreover, the measured total amount of benzoic acid was equal to ten folds as free form. This clarify that benzoic acid is existed majorly in form of hippuric acid, i.e. glycine conjugate. These results confirmed that benzoic acid, phenol, p-cresol and cinnamic acid are existed majorly in conjugated form. The concentrations of targeted compounds were very low in human urine, relative to the camel urine (Table 2). Azelaic acid was not detected in human urine.
| Substance | Camel urine@ | Human urine@@ | ||
|---|---|---|---|---|
| Free | Total | Free | Total | |
| Phenol | 0.2 (8.8) | 4.0 (7.5) | 0.0 | 0.14 (8.1) |
| p-cresol | 33.8 (5.1) | 107.2 (8.9) | 0.2 (7.9) | 2.84 (15.8) |
| Benzoic acid | 40.0 (3.7) | 490.6 (12.5) | 0.0 | 0.37 (11.2) |
| Salicylic acid | 4.5 (1.2) | 42.7 (2.2) | 0.0 | 0.18 (1.9) |
| Cinnamic acid | 0.3 (6.6) | 3.2 (4.5) | 0.0 | 0.23 (0.7) |
| Azelaic acid | 55.9 (4.4) | 68.6 (6.7) | 0.0 | 0.0 |
Table 2: Concentration of Targeted Components in Fresh and Enzymatically Hydrolyzed, Camel Urine and Human Urine
The estimated compounds were prepared in water and mixed with an equimolar amount of KOH (Table 2). This solution mixture prepared to contain the same corresponding total concentrations in camel urine samples. The prepared solution showed approximately the same results showed by the enzymatically hydrolyzed CUR sample. However, the fresh CUR sample showed, relatively, less antimicrobial and antifungal activities. In Table 3 it is shown that, the prepared standard solution mixture and CUR (hydrolyzed) are effective against Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus flavus (fungus) and Candida albicans (fungus).
| Sample | Inhibition zone diameter (mm/mg sample) | |||||
|---|---|---|---|---|---|---|
| B.subtilis | E. coli | P.aeruginosa | S.aureus | A.flavus (fungus) | C.albicans (fungus) | |
| Control: DMSO | 0 | 0 | 0 | 0 | 0 | 0 |
| Ampicillin | 20 | 22 | 17 | 18 | N/A | N/A |
| Amphotericin B | N/A | N/A | N/A | N/A | 17 | 19 |
| Camel urine (fresh) | 9 | 11 | 8 | 12 | 13 | 12 |
| Camel urine (hydrolyzed) |
15 | 20 | 16 | 17 | 18 | 20 |
| Simulated solution | 13 | 18 | 18 | 18 | 18 | 20 |
Table 3: The Antimicrobial and Antifungal Test Results of Investigated Samples
In conclusion, a relatively, high concentrations of bioactive materials were found in camel urine, including; phenol, p-cresol, cinnamic acid, salicylic acid and azelaic acid. These compounds, separately reported to exhibit antiseptic, antiinflammatory, antiacne, antiscabies and anticancer effects. The high concentration of p-cresol and azelaic acid, relative to known doses, confirm the antibacterial activities. These compounds execrated majorly as glucuronide conjugate. Moreover, the adapted extraction and analysis procedure were suitable for detection and quantification of phenolic and acidic bioactive constituents in camel urine.
Acknowledgements
The Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah (grant no. 1434/166/125) funded this study. The author therefore acknowledges and thanks the DSR for technical and financial support.
Financial support and sponsorship:
The financial support from the Deanship of Scientific Research (DSR) is gratefully acknowledged.
Conflicts of interest:
The author declares no competing interests.
References
- Read BE. Chemical constituents of camel's urine. J BiolChem 1925;64:615-7.
- Antakly T. Bioactive compounds in camel urine and milk. WO Patents 2012; WO2012019295A1.
- Al-Abdalall AHA. The inhibitory effect of camel's urine on mycotoxins and fungal growth. Afr J Agric Res 2010;5:1331-7.
- Alhaidar A, Abdel Gader AG, Mousa SA. The antiplatelet activity of camel urine. J Altern Complement Med 2011;17:803-8.
- Neugebauer M, Khedr A, el-Rabbat N, el-Kommos M, Saleh G. Stereoselective metabolic study of famprofazone. Biomed Chromatogr 1997;11:356-61.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J ClinPathol 1966;45:493-6.
- Yagil R, Berlyne GM. Sodium and potassium metabolism in the dehydrated and rehydrated bedouin camel. J ApplPhysiol 1976;41:457-61.
- Taplin D, Meinking TL. Scabies, lice and fungal infections. Prim Care 1989;16:551-76.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol 2013;12:434-7.