- *Corresponding Author:
- Alaa M. Hayallah
Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Assiut University, Assiut-71526, Egypt
E-mail: alaa_hayalah@yahoo.com
| Date of Submission | 10 September 2013 |
| Date of Revision | 1 July 2014 |
| Date of Acceptance | 14 July 2014 |
| Indian J Pharm Sci 2014;76(5):387-400 |
Abstract
Cytohesins are small guanine nucleotide exchange factors that stimulate ADP ribosylation factors, Ras-like GTPases, which control various cellular regulatory networks ranging from vesicle trafficking to integrin activation. A small molecule SecinH3 (1,2,4-triazole derivative) in an aptamer displacement assay as a pan-cytohesin Sec7-domain inhibitor was previously identified. Here a series of different SecinH3-analogues was designed and synthesised as potential cytohesin Sec7-domain inhibitors. All final synthesized compounds 6-8, 43-58 and their intermediates were confirmed by 1 H NMR, 13 C NMR and high resolution Mass. Preliminary biological screening of target compounds indictaed that some of the new synthesized secinH3 derivatives showed higher potency and promising activity more than secinH3 itself (unpublished results). Compund 9 and 10 were approximate equal to secinH3 activity as cytohesin antagonist activity. Furthermore compound 52 showed twice inhibition potency if compared to secinH3
Keywords
SecinH3, synthesis, 1,2,4-triazole, pipronyloyl moiety, cytohesin inhibitors
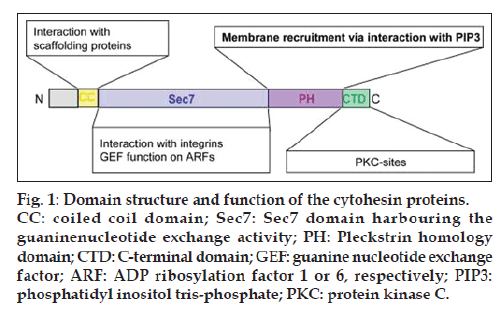
Cytohesins are small (ca. 45 kD) guanine nucleotide exchange factors (GEFs) that stimulate ADP ribosylation factors, or ARFs, which represent ubiquitously expressed Ras-like GTPases [1-3]. The most widely studied cytohesin-dependent ARF members are ARF1 and ARF6. These ARFs, and thereby also the cytohesins as their activators, control various cellular regulatory networks ranging from vesicle trafficking, remodeling of the actin cytoskeleton, or cell adhesion to integrin activation [4]. Four highly related cytohesin homologs are known in mammals: cytohesin 1, cytohesin 2, cytohesin 3, and cytohesin 4. Cytohesins are multi-domain proteins, and all of them consist of an N-terminal coiled coil domain, a Sec7 domain that harbours the GEF activity, a Pleckstrin homology (PH) domain, and a C-terminal polybasic domain fig.1.
Figure 1: Domain structure and function of the cytohesin proteins. CC: coiled coil domain; Sec7: Sec7 domain harbouring the guaninenucleotide exchange activity; PH: Pleckstrin homology domain; CTD: C-terminal domain; GEF: guanine nucleotide exchange factor; ARF: ADP ribosylation factor 1 or 6, respectively; PIP3: phosphatidyl inositol tris-phosphate; PKC: protein kinase C.
A related class of ARF-GEFs that also contain a Sec7-domain are the members of the large (i.e. ca. 200 kD) GEF-family, such as the human BIG1 and BIG2, or the yeast Gea2 [5-7]. The fungal metabolite brefeldin A (BFA), which selectively inhibits guanine nucleotide exchange of the large Sec7 GEFs on ARFs, but not of the small cytohesin GEF members, has established itself as a tremendously useful chemical biology tool for elucidating the biological function of the large GEFs.
To obtain selective inhibitors for the small GEFs of the cytohesin family, we have previously selected an RNA aptamer (M69) that binds to the Sec7 domain of cytohesins. M69 discriminates between large and small GEFs, but it binds with similar affinity to cytohesins 1, 2, and 3. When expressed as an intramer in the cytoplasm of Jurkat cells, M69 allowed implicating cytohesin GEF activity in actin cytoskeletal reorganization [8]. However, while the use of intramers in cell culture is straightforward, their application in whole organisms is hampered by their instability, bioavailability, and transmembrane delivery, at least when targeting intracellular proteins [9-11]. Thus, the development of therapeutic aptamers that target intracellular target is a process that faces complications usually associated with the nucleic acid-based drugs [12].
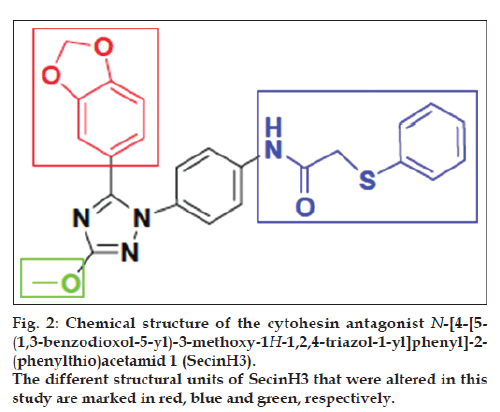
To cope with these limitations, we have established aptamer displacement assays [13-15]. And aptamer regulated allosteric ribozymes [16-19] to screen for small drug-like molecules with similar inhibitory profile as the parent aptamers. For identifying a small molecule cytohesin antagonist, we have used a 5’-fluorescein-labeled version of the aptamer M69 [20]. The screening assay was based on aptamer displacement, followed by fluorescence polarization. We identified the 1,2,4-triazole derivative 1, called SecinH3 (Scheme 1), which bound to the Sec7-domain of cytohesins and inhibited guanine nucleotide exchange on ARF1 and ARF6, respectively [21]. The binding to cytohesins is highly specific [22] and its IC50 value for the GEF activity of the cytohesin-1 Sec7 domain on Δ17-ARF1 was determined to be 10-11 μM [23]. When applied in human liver cells, flies, and mice, SecinH3 allowed implicating insulin receptor complexassociated cytohesins as essential for proper insulin signaling [21,24]. SecinH3 is now widely used in biological assays as either a cytohesin antagonist or as an indirect Arf6 inhibitor [25-33].
Herein we report the rational design, and synthesis of a novel series of derivatives that are derived from the SecinH3 scaffold, in which different core elements of SecinH3 were systematically altered (fig. 2).
Materials and Methods
All the chemicals used were of analytical grade and purified by standard methods prior to use. The Melting points were measured using a Stuart Scientific melting point apparatus SMP3 (UK) and are uncorrected. The NMR spectra were taken using Bruker DPX 300 MHz instrument at Organic Chemistry institute, Bonn, Germany. DMSO-d6 was used as solvent and the chemical shifts are given in δ (ppm) values. The chemical shifts of the remaining protons of the deuterated solvent served as internal standard: δ 1H: 2.49 ppm, 13C: 39.7 ppm. The EI-MS was obtained using EI- Finnigan MAT 95XL (Thermo Finnigan, Bremen) and FAB-MS was obtained using Concept 1H (Kratos, Hofheim), with m-Nitrobenzyl alcohol as matrix. Silica gel column chromatography was carried out using kieselgel 60 (Merck). TLC analysis was performed on kieselgel 60 F254 (Merck) aluminum plates.
2-(4-Hydroxyphenylthio)acetic acid, 2-(3,4-dihydro- 2,4-dioxopyrimdin-1(H)-yl)acetic acid and 2-(3,4-dihydro-5-methyl-2,4-dioxopyrimdin-1-(H)-yl) acetic acid were prepared as described according to the reported procedure [34,35]. These derivatives were synthesized as illustrated in Schemes 1 and 2.
Synthesis of O-methyl-N-acyl-thiocarbamate and O-ethyl-N-acyl-thiocarbamate derivatives (3, 19-26)
To a solution of acid chloride derivative (1, 17a-e) (37,84 mmol) in acetone (60 ml), potassium thiocyanate (3.68 g, 37.84 mmol, 1.0 eq.) under efficient stirring. The reaction mixture was refluxed at 60° for 60-120 min and monitored using TLC until the start is completely disappeared. Ethanol or methanol (94.60 mmol, 2.5 eq.) was added drop wise and the reaction mixture was refluxed for 4-7 h and then cooled, filtered, washed with acetone (15 ml). The filtrate was evaporated and the residue was purified either by silica gel chromatography if it is oil or by crystallization if it is solid to afford the desired product. Yield (74-94%).
The molecular characteristics of O-methyl-N-(benzoyl) thiocarbamate 19 as representative example for this series are as follow, (300 MHz, CDCl3): δ 9.16 (br, 1H), 7.75 (m, 2H), 7.51 (m, 1H), 7.42 (m, 2H), 4.13 (s, 3H). 13C-NMR (75 MHz, CDCl3): δ 190.38, 162.51, 133.27, 132.83, 129.06, 127.73, 59.62. EIHRMS (m/z): calcd. for C9H9NO2S 195.0354, found 195.0359 [M]+. Yield: 68%, mp 102-104°.
Synthesis of 3-ethoxy(methoxy)-5-(3-(un) substitiutedphenyl)-1-(4-nitrophenyl)-1H-1,2,4- triazol derivatives (4, 27-34)
O-Methyl-N-acyl-thiocarbamate or O-ethyl- N-acyl-thiocarbamate derivative 3, 19-26 (28.01 mmol, 1 eq.) and p-nitrophenyl-hydrazine (4.29 g; 28.01 mmol, 1 eq.) were suspended in ethanol (30.0 ml) in a round-bottom flask equipped with a reflux condenser and a bubbler. The reaction mixture was refluxed for 12-20 h. During the course of the reaction H2S evolution was observed. The reaction mixture was cooled and the precipitated product was filtered off followed by thorough washing cycles with cold ethanol. The residue was purified using silica gel column or crystallized from ethanol to afford the nitro-triazole derivatives 4, 26-32 as yellow solid.
The molecular characteristics of 3-methoxy- 1-(4-nitrophenyl)-5-phenyl-1H-1,2,4-triazole 27 as representitve example for this series are as follow, 1H-NMR (400 MHz, DMSO): δ 8.31 (d, J=9.19 Hz, 2H), 7.61 (d, J=9.19 Hz, 2H), 7.47 (m, 5H), 3.98 (s, 3H). 13C-NMR (100 MHz, DMSO): δ 168.63, 154.21, 146.96, 142.86, 131.09, 129.32, 129.29, 127.64, 126.25, 125.30, 57.23. EI-HRMS (m/z): calcd. for C15H12N4O3 296.0909, found 296.0908 [M]+. Yield: 80%, mp 142-44°.
Synthesis of 4-(5-(un)substitutedphenyl)-3- methoxy(ethoxy)-1H-1,2,4-triazol-1-yl)phenyl amine derivatives (5, 35-42)
As per method A, nitrotriazole derivative 4, 27-34 (0.63 g, 1.85 mmol, 1 eq.), ammonium chloride (0.30 g, 5.61 mmol, 3.0 eq.) and Fe powder (0.52 g, 9.31 mmol, 5 eq.) were suspended in 1:1 solution of methanol and water (20 ml). Concentrated HCl (0.25 ml) was added to the above reaction mixture and was heated at 65° for 3 h. The reaction mixture was filtered when hot and was washed several times with hot methanol (8×15 ml). The filtrate was evaporated to remove methanol and the precipitated product was filtered and washed with cold water. The crude product was purified by silica gel chromatography to afford the product as pale white solid. Yield obtained was 35-43%.
In the method B, to a suspension of the nitro derivative 4, 27-34 (7.61 mmol) in ethanol (70 ml), (Pd/C10% 2.87 g) was added portion wise after washing with argon gas three times. The reaction mixture was transferred into autoclave under H2 atmosphere and 20 bar pressure and stirred overnight. The reaction mixture was filtered through celite pad and was washed with ethanol (120 ml) and then hot methanol (5×120 ml). The solvent was concentrated and the rest was cooled in the refrigerator to afford the amine product (5, 35-42) as white crystals. Yield obtained was 65-82%.
The molecular characteristics of 4-(3-Methoxy-5- phenyl-1H-1,2,4-triazol-1-yl)phenyl amine 35 as representative example of this series are as follows, 1H-NMR (300 MHz, DMSO): 7.33-7.45 (m, 5H), δ 6.99 (d, J=8.5 Hz, 2H), 6.57 (d, J=8.5 Hz, 2H), 5.49 (br s, 2H), 3.91 (s, 3H). 13C-NMR (75 MHz, DMSO): δ 167.80, 152.73, 149.97, 130.20, 128.88, 128.69, 128.17, 127.41, 126.56, 114.04, 56.77. EI-HRMS (m/z): calcd. for C19H22N4O 322.1794, found 322.1790 [M]+. Yield obtained was 76% with an mp 198-200°.
N-(4-(5-(benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)2-(phenylthio)-acetamide 6 was synthesized by adding to a solution of 5 (1.277 g, 4.12 mmol, 1 eq.) and triethyl amine (1.14 ml, 8.22 mmol, 2 eq.) in dichloroethane (20 ml) and phenylthioacetyl chloride (0.61 ml, 4.11 mmol, 1 eq.). The reaction mixture was stirred overnight at room temperature (RT) and was diluted with dichloromethane (100 ml). The reaction mixture was washed with 5% aqueous Na2CO3 solution (25 ml) and water (2×50 ml), respectively. The organic phase was dried over MgSO4, filtered and evaporated to dryness. The crude product was purified by silica gel chromatography using ethylactate/cyclohexane (1:1) as eluent system to give the product as pale white foam. Yield obtained was 67%, mp 202-204°.
1H-NMR (400 MHz, CDCl3): δ 8.60 (br, 1H), 7.48 (d, J=8.84 Hz, 2H), 7.30-7.18 (m, 7H), 6.90 (dd, J=8.08 Hz, J=1.64 Hz, 1H), 6.87 (d, J=1.64 Hz, 1H), 6.66 (d, J=8.08 Hz, 1H), 5.90 (s, 2H), 3.97 (s, 3H), 3.71 (s, 2H); 13C-NMR (100 MHz, d6-DMSO): 167.48, 167.16, 152.45, 148.67, 147.23, 139.19, 135.66, 132.90, 128.99, 128.15, 126.44, 126.05, 123.18, 120.99, 119.52, 108.40, 108.36, 101.63, 56.41, 37.52; FAB (m/z): 461.1 [M + 1]+.
2-(4-Hydroxyphenylthio)-N-(4-(5-(benzo [d] [1,3] dioxol-5-yl)-3-methoxy-1H-1,2,4-triazol-1-yl)phenyl) acetamide 7 was synthesised by adding under argon atmosphere, to a solution of 4-hydroxyphenylthioacetic acid (0.42 g, 2.26 mmol), EDC (0.44 g, 2.26 mmol) and DMAP (0.09 g, 0.74 mmol) in DCM (10 ml) and DMF (6 ml), the triazole amine derivative 5 (0.7 g, 2.26 mmol). After the reaction mixture was stirred at RT overnight, the solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography using (cyclohexane/ ethylacetate 1:1) as eluent system to give the product as white powder. Yield obtained was 0.32 g, (31%) and the mp was 212-214°.
1H-NMR (400 MHz, d6-DMSO): δ 10.27 (br s, 1H), 9.59 (br s, 1H), 7.63 (d, J=8.5 Hz, 2H), 7.28-7.32 (m, 4H), 6.88-6.91 (m, 3H), 6.72 (d, J=8.5 Hz, 2H), 6.05 (s, 2H), 3.92 (s, 3H), 3.65 (s, 2H). 13C-NMR (100 MHz, d6-DMSO): 168.03, 157.65, 152.92, 149.93, 149.16, 147.71, 139.76, 133.69, 126.91, 123.65, 123.38, 121.46, 119.97, 116.52, 108.89, 108.85, 102.13, 56.89, 40.68. EI-HRMS (m/z): calcd. for C24H20N4O5S 476.1154, found 476.1156 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H- 1,2,4-triazol-1-yl)phenyl-2-(phenyl-sulfonyl)acetamide 8 was prepared by adding to a stirred solution of 6 (117 mg, 0.254 mmol) in DCM (5 ml), a solution of peracetic acid (39% in AcOH, 175 mg, 1.07 mmol). The reaction mixture was stirred for 7 h before being extracted with DCM (30 ml) and water (30 ml). The organic phase was washed with water (2×25 ml) and the combined aqueous phases were extracted with DCM (50 ml). The combined organic phases were dried over MgSO4 and evaporated in vacuo to yield the product as white powder, which purified by column chromatography using ethylactate/ cyclohexane (1:1) as eluent system. Yield obtained was 0.105 mg (84%) with an mp of 217-219°.
1H-NMR (300 MHz, CDCl3): δ 8.65 (br s, 1H), 7.85 (d, J=8.85 Hz, 2H), 7.62-7.68 (m, 1H), 7.49-7.55 (m, 4H), 7.25 (d, J=8.85 Hz, 2H), 6.87-6.93 (m, 2H) 6.67 (d, J=8.05 Hz, 1H), 5.92 (s, 2H), 4.11 (s, 2H), 3.98 (s, 3H). 13C-NMR (75 MHz, CDCl3): 168.32, 158.57, 153.09, 149.27, 147.80, 137.87, 137.23, 134.87, 134.80, 129.74, 128.12, 126.28, 123.67, 121.28, 120.65, 109.09, 108.53, 101.60, 62.84, 56.89. EI-HRMS (m/z): calcd. for C24H20N4O6S 492.1104, found 492.1105 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy- 1H-1,2,4-triazol-1-yl)phenyl-2-(pyridin-2-ylthio) acetamide 9 was sythesised as follows. To a solution of (2-pyridylthio)acetic acid (0.55 g, 3.2 mmol), EDC (0.617 g, 3.2 mmol) and DMAP (0.12 g, 0.99 mmol) in DCM (10 ml) and DMF (5 ml) under argon atmosphere, the amine 5 (1.0 g, 3.2 mmol) was added. The reaction mixture was stirred at RT overnight and the solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography using 3-7% MeOH/DCM to give the desired product with an yield of 51 % (0.76 g) and mp 176-178°.
1H-NMR (300 MHz, CDCl3): δ 10.40 (br s, 1H), 8.46-8.49 (m, 1H), 7.47-7.50 (m, 3H), 7.18-7.28 (m, 3H), 7.07-7.11 (m, 1H), 6.86-6.90 (m, 2H), 6.64 (dd, J=8.40 Hz, J=1.00 Hz, 1H), 5.90 (s, 2H), 3.96 (s, 3H), 3.81(s, 2H). 13C-NMR (75 MHz, CDCl3): 168.50, 158.12, 152.93, 148.91, 147.70, 138.85, 137.10, 133.64, 126.19, 123.59, 122.99, 121.47, 120.86, 119.83, 109.09, 108.43, 101.52, 56.83, 35.37. EI-HRMS (m/z): calcd. for C23H19N5O2S 461.1158, found 461.1160 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H- 1,2,4-triazol-1-yl)phenyl-2-(pyridin-4-ylthio)acetamide 10 was prepared by the same procedure of 9 using (4-pyridylthio)acetic acid. Yield (0.77 g, 52 %), mp 198-200°.
1H-NMR (300 MHz, CDCl3): δ 8.67 (br s, 1H), 8.39 (d, J=5.85 Hz, 2H), 7.47 (d, J=8.88 Hz, 2H), 7.21 (d, J=8.88 Hz, 2H), 7.11 (d, J=5.85 Hz, 2H), 7.30- 7.18 (m, 7H), 6.90 (dd, J=8.08 Hz, J=1.64 Hz, 1H), 6.87 (d, J=1.64 Hz, 1H), 6.65 (d, J=7.93 Hz, 1H), 5.90 (s, 2H), 3.96 (s, 3H), 3.78 (s, 2H). 13C-NMR (75 MHz, CDCl3): 168.27, 165.35, 153.04, 149.84, 149.26, 147.77, 146.26, 137.41, 134.54, 126.15, 123.64, 121.23, 120.47, 109.02, 108.52, 101.61, 56.90, 35.62. EI-HRMS (m/z): calcd. for C23H19N5O2S 461.1158, found 461.1152 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H- 1,2,4-triazol-1-yl)phenyl)-2-(3,4-dihdro-5-methyl-2,4- dioxopyrimidin-1(2H)-yl acetamide 11 was prepared using the following procedure. Thymine-1-acetic acid (0.30 g, 1.61 mmol, EDC (0.31 g, 1.61mmol) and DMAP (0.037 g, 0.31 mmol) were dissolved in 10 ml DMF under argon atmosphere. To the reaction mixture the amine 5 (0.5 g, 1.61 mmol) in DCM (10 ml) was added and the reaction was further stirred overnight at RT The solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography using DCM/MeOH (9:1) as eluent system to give the product as white powder. Yield obtained was (25 g) 32.5 %, with a mp of 197-199°.
1H-NMR (300 MHz, DMSO): 11.34 (br s, 1H), 10.53 (br s, 1H), 7.68 (d, J=8.6 Hz, 2H), 7.51 (s, 1H), 7.34 (d, J=8.6 Hz, 2H), 6.87-6.94 (m, 3H), 6.05 (s, 2H), 4.52 (s, 2H), 3.92 (s, 3H), 1.77 (s, 3 H). 13C-NMR (75 MHz, DMSO): δ 167.96, 166.58, 164.85, 152.94, 151.54, 149.15, 147.71, 142.77, 139.48, 133.36, 127.07, 123.63, 121.43, 119.90, 108.88, 188.83, 108.51, 102.13, 56.90, 50.49, 12.33. EI-HRMS (m/z): calcd. for C23H20N6O6 476.1444, found 476.1448 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H- 1,2,4-triazol-1-yl)phenyl-2-(thiophen-3-yl)acetamide 12 was prepared. To a solution of 3-thiopheneacetic acid (0.23 g, 1.6 mmol, EDC (0.309 g, 1.6 mmol) and DMAP (0.06 g, 0.50 mmol) in DCM (10 ml) and DMF (3 ml) under argon atmosphere, the amine 5 (0.5 g, 1.6 mmol) was added. The reaction mixture was stirred at RT overnight and then the solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography using cyclohexane/ethylacetate (1:2) as eluent to give the product as white crystals. Yield was 0.22 g (31.4%) and mp was 213-215°.
1H-NMR (300 MHz, CDCl3): δ 10.05 (br s, 1H), 7.78 (d, J=8.80 Hz, 2H), 7.38 (dd, J=2.83 Hz, J=2.08 Hz, 1H), 7.29-7.32 (m, 3H), 7.18 (dd, J=3.78 Hz, J=1.13 Hz, 1H), 7.01 (dd, J=6.42 Hz, J=1.69 Hz, 1H), 6.96 (d, J=1.51 Hz, 1H), 6.80 (d, J=8.12 Hz, 1H), 6.05 (s, 2H), 4.06 (s, 3H), 3.78 (s, 2H). 13C-NMR (75 MHz, CDCl3): 174.18, 172.79, 157.50, 153.86, 152.44, 144.45, 139.99, 137.93, 133.38, 130.72, 128.20, 127.27, 126.10, 124.78, 113.61, 113.16, 106.39, 61.46, 43.49. EI-HRMS (m/z): calcd. for C22H18N4O4S 434.1049, found 434.1042 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H- 1,2,4-triazol-1-yl)phenyl-2-(pyridin-2-yl)acetamide 13. To a solution of 2-pyridylacetic acid hydrochloride (0.39 g, 2.26 mmol, EDC (0.44 g, 2.26 mmol), TEA (0.6 ml, 4.54 mmol) and DMAP (0.09 g, 0.74 mmol) in DCM (20 ml) under argon atmosphere, the amine 5 (7.0 g, 2.26 mmol) was added. The reaction mixture was stirred at RT overnight and then the solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography using DCM/MeOH (3-6%) as eluent to give the product as white crystals. Yield was 0.61 g (62%) and the mp was 180-182°.
1H-NMR (400 MHz, CDCl3): δ 10.20 (br s, 1H), 8.56 (d, J=4.90 Hz, 1H), 7.63-7.67 (m, 1H), 7.57 (d, J=8.31 Hz, 2H), 7.19-7.24 (m, 4H), 6.86-6.90 (m, 2H), 6.64 (d, J=8.18 Hz, 1H), 5.89 (s, 2H), 3.96 (s, 3H), 3.82 (s, 2H). 13C-NMR (100 MHz, CDCl3): 168.27, 167.23, 155.12, 152.97, 148.94, 147.70, 138.59, 137.69, 133.97, 126.19, 124.45, 123.61, 122.48, 121.48, 120.18, 109.10, 108.42, 101.53, 56.84, 45.52. EI-HRMS (m/z): calcd. for C23H19N5O4 429.1437, found 429.1432 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy-1H- 1,2,4-triazol-1-yl)phenyl-2-(pyridin-4-yl)acetamide 14 was prepared using the same procedure of 13 using instead 4-pyridylacetic acid hydrochloride to afford 14 as a white powder. Yield was 0.76 g (78.4%) and the mp was 214-216°.
1H-NMR (300 MHz, d6-DMSO): δ 10.48 (br s, 1H), 8.51 (d, J=5.30 Hz, 2H), 7.69 (d, J=8.85 Hz, 2H), 7.31-7.36 (m, 4H), 6.87-6.91 (m, 3H), 6.86-6.90 (m, 2H), 6.05 (s, 2H), 3.92 (s, 3H), 3.74 (s, 2H). 13C-NMR (75 MHz, d6-DMSO): 168.61, 167.94, 152.91, 149.93, 149.15, 147.70, 144.83, 139.83, 133.27, 126.93, 125.13, 123.66, 121.46, 119.99, 108.88, 108.83, 102.12, 56.89, 42.78. EI-HRMS (m/z): calcd. for C23H19N5O4 429.1437, found 429.1446 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy- 1H-1,2,4-triazol-1-yl)phenyl-2-(1H-imidazol-4-yl) acetamide 15 was prepared in the following manner. To a solution of imidazole-4-acetic acid hydrochloride (0.185 g, 1.13 mmol, EDC (0.22 g, 1.13 mmol) and DMAP (0.045g, 0.37 mmol) in DCM (5 ml) and DMF (10 ml) under argon atmosphere, the amine 5 (0.35 g, 1.13 mmol) was added. The reaction mixture was stirred overnight at RT and the solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography using MeOH/ DCM (5-8%) to give the desired product. Yield obtained was 0.15 g (28%) and the mp was 196-198°.
1H-NMR (400 MHz, d6-DMSO): δ 11.91 (br s, 1H), 10.34 (br s, 1H), 7.70 (d, J=8.5 Hz, 2H), 7.56 (s, 1H), 7.31 (d, J=8.5 Hz, 2H), 6.94 (s, 1H), 6.87-6.94 (m, 3H), 6.05 (s, 2H), 3.91 (s, 3H), 3.33 (s, 2H). 13C-NMR (100 MHz, d6-DMSO): 167.92, 152.90, 149.13, 147.69, 140.15, 135.32, 133.02, 126.91, 123.61, 123.38, 121.47, 119.82, 108.87, 108.81, 102.11, 56.88, 49.01. EI-HRMS (m/z): calcd. for C21H18N6O4 418.1390, found 418.1391 [M]+.
N-(4-(5-(Benzo [d] [1,3]dioxol-5-yl)-3-methoxy- 1H-1,2,4-triazol-1-yl)phenyl)-2-(3,4-dihdro-5-2,4- dioxopyrimidin-1(2H)-yl acetamide 16. Uracil-1-acetic acid (0.274 g, 1.61 mmol, EDC (0.31 g, 1.61 mmol) and DMAP (0.037 g, 0.31 mmol) were dissolved in 10 ml DMF under argon atmosphere. To the reaction mixture the amine 5 (0.5 g, 1.61 mmol) in DCM (10 ml) was added and the reaction was further stirred overnight at RT. The solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography DCM/MeOH (9:1) as eluent system to give the product as white crystals with an yield of 0.24 g (32%) and mp of 252-254°.
1H-NMR (300 MHz, DMSO): 11.35 (br s, 1H), 10.53 (br s, 1H), 7.61-7.69 (m, 3H), 7.35 (d, J=8.31 Hz, 2H), 6.87-6.94 (m, 3H), 6.05 (s, 2H), 5.60 (d, J=8.12 Hz, 1H), 4.57 (s, 2H), 3.92 (s, 3H). 13C-NMR (75 MHz, DMSO): δ 167.96, 166.48, 164.28, 152.94, 151.56, 149.15, 147.71, 147.02, 139.45, 133.39, 127.09, 123.63, 121.42, 119.90, 108.88, 102.13, 101.07, 56.90, 50.66. EI-HRMS (m/z): calcd. for C22H18N6O6 462.1288, found 462.1289 [M]+.
N-(4-(3-Methoxy-5-phenyl-1H-1,2,4-triazol-1-yl) phenyl)-2-(phenylthio)-acetamide 43. To a solution of amine 35 (0.546 g, 2.05 mmol, 1 eq.) and triethyl amine (0.57 ml, 4.10 mmol, 2 eq.) in DCM (15 ml) was added phenylthioacetyl chloride (0.3 ml, 2.06 mmol, 1 eq.). The reaction mixture was stirred overnight at RT. Phenylthioacetyl chloride (0.1 ml, 0.67 mmol) was added and then reaction was refluxed for 3 h. The reaction mixture was diluted with dichloromethane (50 ml) and the reaction mixture was washed with 5% aq. Na2CO3 solution (25 ml) and water (2×50 ml), respectively. The organic phase was dried over MgSO4 and then filtered and evaporated to dryness. The crude product was purified by silica gel chromatography using cyclohexane: EtOAc (3:2) as eluent to give the product as white crystals. Yield, (0.38 g, 44 %), mp 120-122°.
1H-NMR (300 MHz, CDCl3): 8.65 (br s, 1H), 7.46 (d, J=8.5 Hz, 2H), 7.38 (d, J=8.5 Hz, 2H), 7.11-7.30 (m, 10H), 3.98 (s, 3H), 3.70 (s, 2H). 13C-NMR (75 MHz, CDCl3): δ 168.50, 166.29, 153.29, 137.59, 134.39, 133.96, 130.14, 129.60, 128.87, 128.61, 128.59, 127.69, 127.27, 126.14, 120.28, 56.91, 38.55. EI-HRMS (m/z): calcd. for C23H20N4O2S 416.1307, found 416.1307 [M]+.
2-(3,4-Dihydro-5-methyl-2-4-dioxopyrimidin-1(2H)- yl)-N-(4-(3-methoxy-5-phenyl-1H-1,2,4-triazol-1-yl) phenyl) acetamide 44. Thymine-1-acetic acid (0.28 g, 1.5 mmol, EDC (0.29 g, 1.5 mmol) and DMAP (0.030 g, 0.25 mmol) were dissolved in 10 ml DMF under argon atmosphere. To the reaction mixture the amine 35 (0.4 g, 1.5 mmol) in DCM (10 ml) was added and the reaction was further stirred overnight at RT. The solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography DCM/MeOH (9:1) to give the product as white crystals of an yield of 0.2 g (30.77%) and mp of 199-201°.
1H-NMR (300 MHz, DMSO): 11.34 (br s, 1H), 10.53 (br s, 1H), 7.67 (d, J=8.5 Hz, 2H), 7.51 (s, 1H), 7.39-7.41 (m, 5H), 7.34 (d, J=8.5 Hz, 2H), 4.52 (s, 2H), 3.94 (s, 3H), 1.77 (s, 3 H). 13C-NMR (75 MHz, DMSO): δ 168.16, 166.58, 164.85, 153.17, 151.54, 142.78, 139.53, 133.31, 130.52, 129.04, 127.84, 127.02, 119.88, 114.04, 108.52, 56.95, 50.50, 12.33. EI-HRMS (m/z): calcd. for C22H20N8O4 432.1546, found 432.1546 [M]+.
N-(4-(5-(2-Fluorophenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(phenylthio)-acetamide 45. To a solution of amine 36 (0.285 g, 1.01 mmol, 1 eq.) and triethyl amine (0.28 ml, 2.01 mmol, 2 eq.) in DCM (10 ml) was added phenylthioacetyl chloride (0.15 ml, 1.01 mmol, 1 eq.). The reaction mixture was stirred overnight at room temperature, 0.15 ml of phenylthioacetyl chloride was added and then the reaction was stirred at 55° for 3 h. The reaction mixture was diluted with dichloromethane (70 ml). The reaction mixture was washed with 5% aquous Na2CO3/ solution (15 ml) and water (2×25 ml), respectively. The organic phase was dried over MgSO4, filtered and evaporated to dryness. The crude product was purified by silica gel chromatography to give the product as pale white foam like crystals with a yield of 0.2 g (46%) and mp of 122-124°.
1H-NMR (400 MHz, CDCl3): δ 8.60 (br s, 1H), 7.45-7.49 (m, 1H), 7.40 (d, J=8.5 Hz, 2H), 7.33-7.36 (m, 1H), 7.23-7.28 (m, 3H) 7.11-7.19 (m, 5H), 6.91- 6.96 (m, 1H), 3.99 (s, 3H), 3.68 (s, 2H). 13C-NMR (100 MHz, CDCl3): 168.74, 166.19, 160.76, 158.25, 148.68, 137.22, 134.26, 133.96, 132.57, 132.48, 131.52, 131.50, 129.58, 128.55, 127.25, 124.43, 120.04, 116.87, 116.46, 116.25, 56.98, 38.52. EIHRMS (m/z): calcd. for C23H19FN4O2S 434.1213, found 434.1213 [M]+.
N-(4-(5-(2-Fluorophenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(3,4-dihydro-5-methyl-2-4- dioxopyrimidin-1(2H)-yl))acetamide 46. Thymine- 1-acetic acid (0.32 g, 1.76 mmol, EDC (0.33 g, 1.76 mmol) and DMAP (0.050 g, 0.42 mmol) were dissolved in 10 ml DMF under argon atmosphere. To the reaction mixture the amine 36 (0.5 g, 1.76 mmol) in DCM (10 ml) was added and the reaction was further stirred overnight at RT. The solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography with DCM/ MeOH (9:1) as eluent system and then crystallized from ethanol to give the product as white crystals. Yield was 0.42 g (53%) and mp was 200-202°.
1H-NMR (300 MHz, DMSO): 11.33 (br s, 1H), 10.45 (br s, 1H), 7.56-7.60 (m, 4H), 7.50 (s, 1H), 7.23-7.35 (m, 4H), 4.49 (s, 2H), 3.96 (s, 3H), 1.76 (s, 3 H). 13C-NMR (75 MHz, DMSO): δ 168.49, 166.51, 164.85, 160.91, 157.60, 151.52, 148.65, 142.76, 139.07, 133.38, 132.98, 132.16, 132.13, 125.14, 119.74, 116.71, 116.45, 116.43, 108.49, 57.09, 50.44, 12.33. EI-HRMS (m/z): calcd. for C22H19FN6O4 450.1452, found 450.1452 [M]+.
N-(4-(5-(2-Fluorophenyl)-3-methoxy-1H-1,2,4-triazol- 1-yl)phenyl)-2-(3,4-dihydro-2-4-dioxo-pyrimidin- 1(2H)-yl))acetamide 47 was synthesized by the same procedure of 46 using uracil-1-acetic acid instead of thymine-1-acetic acid to give the product as white crystals. Yield was 0.27 g (35.1%) and mp was 238-240°.
1H-NMR (300 MHz, DMSO): 11.34 (br s, 1H), 10.47 (br s, 1H), 7.52-7.62 (m, 5H), 7.29-7.35 (m, 1H), 7.23-6.27 (m, 3H), 6.05 (s, 2H), 5.59 (dd, J=6.12 Hz, J=2.26 Hz, 1H), 4.53 (s, 2H), 3.96 (s, 3H). 13C-NMR (75 MHz, DMSO): δ 168.49, 166.40, 164.27, 160.91, 157.60, 151.54, 148.64, 147.01, 139.06, 133.48, 133.27, 132.99, 132.16, 125.16, 119.74, 116.70, 116.45, 116.43, 101.05, 57.09, 50.62. EI-HRMS (m/z): calcd. for C21H17FN6O4 436.1295, found 436.1292 [M]+.
N-(4-(5-(2,5-Difluorophenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(phenylthio)-acetamide 48 was prepared by the same method of 45 using amine 37. Yield, (0.48 g, 53%), mp 126-128°. 1H-NMR (300 MHz, CDCl3): δ 8.60 (br s, 1H), 7.44 (d, J=8.5 Hz, 2H), 7.15-7.27 (m, 8H), 7.01-7.09 (m, 1H), 6.86-6.94 (m, 1H) 3.99 (s, 3H), 3.69 (s, 2H). 13C-NMR (75 MHz, CDCl3): 168.76, 166.21, 160.10, 160.05, 157.11, 156.79, 153.85, 153.79, 147.51, 137.46, 133.91, 129.59, 128.56, 127.27, 124.46, 120.09, 119.36, 118.93, 117.71, 57.04, 38.53. EI-HRMS (m/z): calcd. for C23H18F2N4O2S 452.1119, found 452.1118 [M]+.
N-(4-(3-Ethoxy-5-(2-fluorophenyl)-1H-1,2,4-triazol- 1-yl)phenyl)-2-(phenylthio)-acetamide 49. It was prepared by the same method of 45 using amine 38. Yield, (0.97 g, 54%), mp 150-152°. 1H-NMR (300 MHz, CDCl3): δ 8.59 (br s, 1H), 7.45-7.49 (m, 1H), 7.32-7.41 (m, 3H), 7.23-7.27 (m, 3H) 7.11-7.19 (m, 4H), 6.91-6.95 (m, 1H), 4.35 (q, J=7.00 Hz, 2H), 3.68 (s, 2H), 1,38 (t, J=7.10 Hz, 3H). 13C-NMR (75 MHz, CDCl3): 168.15, 166.17, 160.75, 158.24, 148.48, 137.14, 133.97, 132.51, 132.42, 131.52, 131.51, 129.58, 128.53, 127.23, 124.38, 120.04, 116.87, 116.46, 116.25, 65.70, 38.51, 14.78. EIHRMS (m/z): calcd. for C24H21FN4O2S 448.1369, found 448.1367 [M]+.
N-(4-(3-Ethoxy-5-(2-fluorophenyl)-1H-1,2,4-triazol-1- yl)phenyl)-2-(3,4-dihydro-5-methyl-2-4-dioxopyrimidin- 1(2H)-yl))acetamide 50. Thymine-1-acetic acid (0.31 g, 1.68 mmol, EDC (0.32 g, 1.68 mmol) and DMAP (0.050 g, 0.42 mmol) were dissolved in 10 ml DMF under argon atmosphere. To the reaction mixture the amine 39 (0.5 g, 1.68 mmol) in DCM (10 ml) was added and the reaction was further stirred overnight at room temperature. The solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography (DCM/MeOH, 9:1) as eluent system and then crystallized from ethanol to give the product as white powder with a yield of 0.41 g (52%) and mp of 241-243°.
1H-NMR (300 MHz, DMSO): 11.33 (br s, 1H), 10.44 (br s, 1H), 7.53-7.60 (m, 4H), 7.49 (s, 1H), 7.21-7.35 (m, 4H), 4.49 (s, 2H), 4.33 (q, J=7.4 Hz, 2H), 1.76 (s, 3 H), 1.37 (t, J=7.4 Hz, 3H). 13C-NMR (75 MHz, DMSO): δ 167.84, 166.50, 164.85, 160.91, 157.60, 151.52, 148.43, 142.76, 139.03, 133.44, 132.99, 132.16, 132.13, 125.09, 119.74, 116.70, 108.49, 65.54, 50.44, 15.01, 12.33. EI-HRMS (m/z): calcd. for C23H21FN6O4 464.1608, found 464.1614 [M]+.
N-(4-(3-Ethoxy-5-(2,5-difluorophenyl)-1H-1,2,4- triazol-1-yl)phenyl)-2-(phenylthio)-acetamide 51 was prepared by the same method of 45 using amine 40. Yield: (0.39 g, 42.4%), mp 156-158°. 1H-NMR (400 MHz, CDCl3): δ 8.60 (br s, 1H), 7.40 (d, J=8.5 Hz, 2H), 7.17-7.26 (m, 6H), 7.15 (d, J=8.5 Hz, 2H), 7.02- 7.07 (m, 1H), 6.87-6.93 (m, 1H), 4.34 (q, J=7.20 Hz, 2H), 3.69 (s, 2H), 4.34 (t, J=7.20 Hz, 3H). 13C-NMR (100 MHz, CDCl3): 168.18, 166.20, 159.70, 157.25, 157.20, 156.70, 154.21, 147.31, 137.38, 133.92, 129.60, 128.54, 127.27, 124.41, 120.09, 119.02, 118.05, 118.02, 117.85, 117.81, 117.77, 65.81, 38.52, 14.75. EI-HRMS (m/z): calcd. for C24H20F2N4O2S 466.1275, found 466.1269 [M]+.
N-(4-(5-(4-tert-Butylphenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(phenylthio)-acetamide 52 was prepared by the same method of 45 using amine 41. Yield: (0.41 g, 42.3%), mp 167-169°. 1H-NMR (300 MHz, CDCl3): 8.68 (br s, 1H), 7.48 (d, J=8.5 Hz, 2H), 7.19-7.33 (m, 11H), 3.97 (s, 3H), 3.70 (s, 2H), 1.21 (s, 9 H). 13C-NMR (75 MHz, CDCl3): δ 168.43, 166.32, 153.48, 153.37, 137.57, 134.59, 134.02, 129.58, 128.49, 128.59, 127.24, 126.26, 125.57, 124.69, 120.28, 56.85, 38.57, 34.86, 31.14. EI-HRMS (m/z): calcd. for C27H28N4O2S 472.1933, found 472.1926 [M]+
N-(4-(5-(4-tert-Butylphenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(3,4-dihydro-5-methyl-2-4- dioxopyrimidin-1(2H)-yl))acetamide 53. Thymine- 1-acetic acid (0.29 g, 1.55 mmol, EDC (0.30 g, 1.55 mmol) and DMAP (0.037 g, 0.31 mmol) were dissolved in 10 ml DMF under argon atmosphere. To the reaction mixture the amine 41 (0.5 g, 1.55 mmol) in DCM (10 ml) was added and the reaction was further stirred overnight at RT. The solvent was evaporated under reduced pressure. The crude product was purified by silica gel chromatography (DCM/MeOH, 9:1) as eluent system to give the product as white powder. Yield, (0.24 g, 32%), mp 225-227°.
1H-NMR (300 MHz, DMSO): 11.35 (br s, 1H), 10.53 (br s, 1H), 7.66 (d, J=8.6 Hz, 2H), 7.51 (s, 1H), 7.31 -7.42 (m, 6H), 4.53 (s, 2H), 3.93 (s, 3H), 1.77 (s, 3 H), 1.24 (s, 9H). 13C-NMR (75 MHz, DMSO): δ 168.11, 166.60, 164.85, 153.28, 151.55, 142.77, 139.57, 133.51, 128.53, 127.23, 125.88, 124.98, 119.92, 108.52, 56.90, 50.52, 34.99, 31.27, 12.34. EIHRMS (m/z): calcd. for C26H28N6O4 488.2172, found 488.2169 [M]+.
N-(4-(3-Ethoxy-5-(2-fluorophenyl)-1H-1,2,4-triazol- 1-yl)-phenyl)-2-(phenylsulfonyl)-acetamide 54 was oxidized by the same procedure of compound 8. Yield (71 mg, 86.6%), mp 204-206°. 1H-NMR (400 MHz, CDCl3): δ 8.68 (br s, 1H), 7.61-7.65 (m, 2H), 7.47-7.52 (m, 3H), 7.35-7.41 (m, 3H) 7.13-7.19 (m, 3H), 6.93- 6.97 (m, 1H), 4.36 (q, J=7.00 Hz, 2H), 4.11 (s, 2H), 1,39 (t, J=7.10 Hz, 3H). 13C-NMR (100 MHz, CDCl3): 168.15, 166.74, 158.63, 148.54, 137.97, 136.90, 134.79, 132.60, 132.52, 131.53, 131.52, 129.67, 128.16, 127.72, 124.68, 124.44, 120.45, 116.48, 116.27, 65.77, 62.87, 14.77. EI-HRMS (m/z): calcd. for C24H21FN4O4S 480.1268, found 408.1061 [M]+.
N-(4-(5-(3-Fluorophenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(thiophen-2-yl)-acetamide 55 was prepared as follows. To a solution of amine 42 (0.265 g, 0.84 mmol, 1 eq.) and triethyl amine (0.24 ml, 1.68 mmol, 2 eq.) in DCM (15 ml) was added thiophene-2-acetylchloride (0.135 g, 0.105 ml, 0.84 mmol, 1 eq.). The reaction mixture was stirred overnight at RT. The reaction mixture was diluted with dichloromethane (40 ml), washed with 5% aq. Na2CO3 solution (15 ml) and water (220 ml), respectively. The organic phase was dried over MgSO4, filtered and evaporated to dryness. The crude product was purified by silica gel chromatography using cyclohexane: EtOAc (1:1) and then repurified using another eluent system of cyclohexane: EtOAc (2:3) to afford the pure target product with a yield of 0.1 g (23%) and mp 153-155°.
1H-NMR (300 MHz, CDCl3): δ 7.57 (br s, 1H), 7.46 (d, J=8.6 Hz, 2H), 7.11-7.25 (m, 6H), 6.97-7.01 (m, 3H), 3.98 (s, 3H), 3.89 (s, 2H). 13C-NMR (75 MHz, CDCl3): 168.37, 168.07, 163.68, 161.22, 151.94, 151.91, 138.20, 135.23, 133.78, 129.46, 127.71, 126.19, 124.57, 124.53, 120.33, 117.36, 117.15, 116.08, 115.84, 57.01, 38.55. EI-HRMS (m/z): calcd. for C21H17FN4O2S 408.1056, found 408.1061 [M]+.
N-(4-(3-Ethoxy-5-(3-fluorophenyl)-1H-1,2,4-triazol- 1-yl)phenyl)-2-(thiophen-2-yl)-acetamide 56 was afforded by the same procdure of 55 using amine 39. Yield (0.250 g, 35.7%), mp 214-216°. 1H-NMR (300 MHz, CDCl3): δ 7.59 (br s, 1H), 7.45 (d, J=8.6 Hz, 2H), 7.10-7.25 (m, 6H), 6.96-7.03 (m, 3H), 4.35 (q, J=7.00 Hz, 2H), 3.88 (s, 2H), 1.38 (t, J=7.10 Hz, 3H). 13C-NMR (75 MHz, CDCl3): 168.06, 167.86, 164.07, 160.79, 151.79, 151.75, 138.12, 135.24, 133.87, 130.33, 130.22, 127.69, 126.16, 124.52, 124.47, 120.32, 117.01, 116.08, 115.76, 65.69, 38.53, 14.75. EI-HRMS (m/z): calcd. for C22H19FN4O2S 422.1213, found 422.1216 [M]+.
N-(4-(5-(2,5-Difluorophenyl)-3-methoxy-1H-1,2,4- triazol-1-yl)phenyl)-2-(thiophen-2-yl)-acetamide 57. To a solution of amine 37 (0.61 g, 2.01 mmol, 1 eq.) and triethyl amine (0.56 ml, 4.02 mmol, 2 eq.) in dichloromethane (15 ml) was added thiophene-2-acetylchloride (0.323 g, 0.25 ml, 2.01 mmol, 1 eq.). The reaction mixture was stirred overnight at RT. The reaction mixture was diluted with dichloromethane (40 ml), washed with 5% aq. Na2CO3 solution (15 ml) and water (2×20 ml), respectively. The organic phase was dried over MgSO4, filtered and evaporated to dryness. The crude product was purified by silica gel chromatography (cyclohexane: EtOAc, 1:1) two times to give the pure product. Yield: (0.26 g, 30%), mp 172-174°.
1H-NMR (300 MHz, CDCl3): δ 7.47 (br s, 1H), 7.39 (d, J=8.5 Hz, 2H), 7.17-7.24 (m, 2H), 7.14 (d, J=8.5 Hz, 2H), 6.97-7.08 (m, 1H), 6.85-6.95 (m, 3H), 3.99 (s, 3H), 3.86 (s, 2H). 13C-NMR (75 MHz, CDCl3): 168.75, 167.96, 160.09, 160.03, 156.77, 153.78, 147.53, 137.70, 135.23, 133.81, 127.93, 127.70, 126.24, 124.42, 120.08, 119.36, 118.92, 117.90, 57.04, 38.52. EI-HRMS (m/z): calcd. for C21H16F2N4O2S 426.0962, found 426.0964 [M]+.
N-(4-(3-Ethoxy-5-(2,5-difluorophenyl)-1H-1,2,4- triazol-1-yl)phenyl)-2-(thiophen-2-yl)-acetamide 58 was prepared by same procedure for compound 57 using amine derivative 40. Yield (0.27 g, 30%), mp 208-210°. 1H-NMR (300 MHz, CDCl3): δ 7.43 (br s, 1H), 7.39 (d, J=8.5 Hz, 2H), 7.19-7.25 (m, 2H), 7.13 (d, J=8.5 Hz, 2H), 7.01-7.07 (m, 1H), 6.95-6.98 (m, 2H), 6.85-6.93 (m, 1H), 4.34 (q, J=7.2 Hz, 2H), 3.87 (s, 2H), 1.39 (t, J=7.2 Hz, 3H). 13C-NMR (75 MHz, CDCl3): 167.93, 160.09, 160.03, 156.82, 153.83, 147.31, 137.62, 135.23, 133.88, 127.95, 127.71, 126.26, 124.38, 120.06, 118.87, 118.08, 117.71, 65.82, 38.53, 14.74. EI-HRMS (m/z): calcd. for C22H18F2N4O2S 440.1119, found 440.1113 [M]+.
Exchange assay of hCyt1-Sec7
[Δ17]ARF1 and ARNO-Sec-7 were subcloneds into pET15 vectors (Novagen) as described previously [13,21-23,36]. N-Terminal truncated [Δ17]ARF1 (amino acids 18-181), lacking the first 17 amino acids and cytohesin-1-Sec-7 were expressed in Escherichia coli and purified by Ni-NTA chromatography (Ni- NTA agarose, Quiagen). GDP/GTP exchange was measured on [Δ17]ARF1 by tryptophan fluorescence because a large increase in intrinsic fluorescence of ARF occurs upon exchange of GDP for GTP [34,37]. All measurements were performed in PBS pH 7.4, 3 mM MgCl2 at 37°. [Δ17]ARF1 (1 μM) in PBS without MgCl2 was preincubated with GDP (80 μM) in the presence of EDTA (2 mM) for 15 min. The bound GDP was stabilized by addition of MgCl2 (final concentration 3 mM) and incubation for 5 min. For each exchange reaction 250 nM [Δ17]ARF1 was mixed with 10 nM ARNO-Sec-7 (total volume 200 μl) in the absence or presence of inhibitor. The reaction was started by injection of GTP (50 μM). The tryptophan fluorescence was measured at an excitation and emission wavelength of 280 and 340 nm, respectively. All fluorescent measurements were performed with a Varioskan microplate reader (Thermo Scientific), in 96-well plates. For analysis all data were fitted by linear regression. IC50 values were determined in 5-fold repeated titration assays.
IGFBP1 gene expression
HepG2 cells (ECACC) 105 were seeded in 12 well plates and cultured for 24 h in EMEM (Cambrex) containing 10% FCS. Cells were then serum starved in EMEM for 24 h and stimulated for 12 h with 10 nM insulin in the presence of the inhibitor SecinH3 or control, with 0.8% final concentration of DMSO. Total mRNA was prepared using the Absolutely RNA Kit (Stratagene) and cDNA for qPCR was generated from 1 μg RNA with the High Capacity cDNA Archive Kit (Applied Biosystems). qPCR was performed in a 10 μl scale on an iQ5- cycler (BioRad) using a TaqMan Gene Expression Assay for IGFBP1 (Applied Biosystems). Data were normalized to b2-microglobulin expression.
Results and Discussion
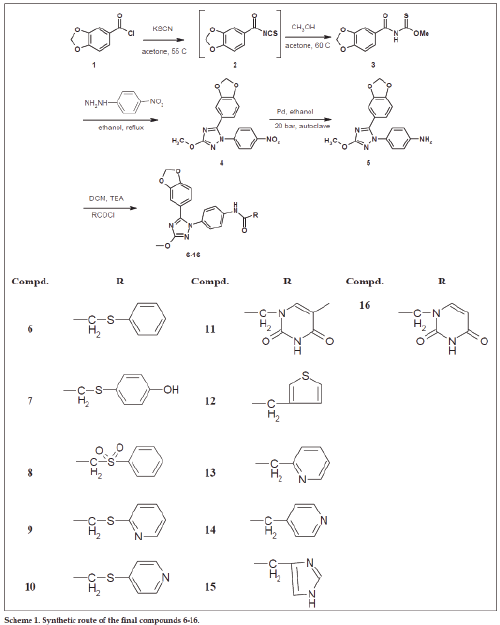
The design and synthesis of the new SecinH3 derivatives 6-15 and 43-58 is based on the change of the substitutions at three points as illustrated in fig. 2. These derivatives were synthesized as illustrated in Schemes 1 and 2. In this group of compounds in scheme 1 the blue part of SecinH3 was changed (fig. 2) taking in mind the importance of sulfur and the aryl moiety. Firstly p-OH was introduced to the phenyl moiety in compound 8 and 54 to increase the polarity and consequently the water solubility and for the same reason sulfur was oxidized the to sulfonyl group in compound 8 and 54. Trials to investigate if the phenyl group could be changed by other heterocyclic rings such as imidazole, pyridine, thiophene, uracil and thymine as aromatic bioisoester groups with retained activity of SecinH3. Finally, the sulfur bridge was also changed to investigate if it must be exocyclic or could be included in heterocyclic rings or not essential at all. From these changes the structure activity relationship (SAR) of SecinH3 could be studied.
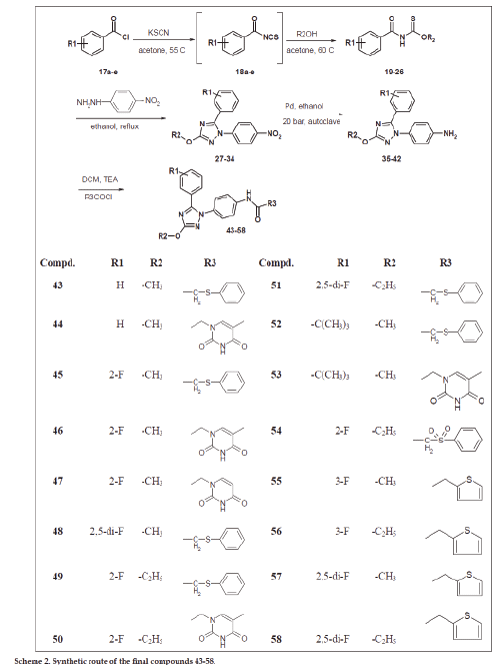
The second group of compounds Scheme 2 represents the change and study of structure activity relationship (SAR) of SecinH3 by changing both red part and green parts. The green part was modified within 2-3 carbons according our pervious study that small substituents such as methyl, ethyl and propyl could be tolerated at this position. The third point of design is the change of pipronyloyl moiety (red part) by phenyl group carrying electron attracting groups at different positions of the ring to investigate the effect of these groups on the biologicall activity of the SecinH3.
The intermediates, 2-(4-Hydroxyphenylthio)acetic acid, 2-(3,4-dihydro-2,4-dioxopyrimdin-1(H)-yl)acetic acid and 2-(3,4-dihydro-5-methyl-2,4-dioxopyrimdin-1-(H)- yl)acetic acid were prepared as described according to the reported procedure [36,37].
The compounds 3 and 19-26 were prepared by reaction of acid chloride derivatives 1 and 17a-e with KSCN and the respective alcohols as illustrated in schemes 1-2. These derivatives were confirmed using 1H-NMR, 13C-NMR, FAB-MS, EI-MS and HRMS (see exp. Part). Their 1H-NMR spectra were characterized by appearance of amidic NH around δ 9-9.6 ppm and the introduced methyl, ethyl or isopropyl moities and 13C-NMR spetra showed C=O group ranged from δ 180 to 189 ppm and C=S group ranged from δ 160 to 164 ppm. HRMS of compund 19 for example is: calcd. For C9H9NO2S 195.0354, found 195.0359 [M]+. These derivatives 3 and 19-26 were reacted with p-nitrophenylhydrazine in ethanol to afford the 3-Alkyl-5-(3-(un)substitiutedphenyl)-1- (4-nitrophenyl)-1H-1,2,4-triazol derivatives 4, 27-34. The products were characterized by disappearance of amidic NH in 1H-NMR and appearance of introduced p-phenyl moiety. Their 13C-NMR spectra showed disappearance of both signals corresponding to C=O and C=S groups and this evidence for cyclization and formation of triazole nucleus. Also, their structures were further confirmed by HRMS, for example compound 32 shwoed m/z: 346.0877 [M]+ for calcd. C16H12F2N4O3 346.0877.
4-(5-(un)substituted phenyl)-3-alkyl-1H-1,2,4- triazol-1-yl)phenyl amine derivatives 5, 35-42 were prepared by reduction of nitro derivatives 4, 27-34, respectively. The reduction process was achieved using two methods of reduction: i. Chemical reduction using ammonium chloride and iron in methanol and water, followed by purification using silica gel chromatography to afford the product as pale white solid. The problems of this method are the low yield (40-50%) and its awful efforts in purification. ii. Catalytic reduction method using palladium as catalyst under hydrogen atmosphere and 20 bar pressure using autoclave. The advantages of the second method are higher yield (70-80%), easier purification and more convenient procedure. The latter method enables us to make multigram scaling synthesis of final derivatives especially compound 6 for further biological investigations. These amine derivatives 5, 35-42 were proved by different spectral data 1H-NMR, 13C-NMR and HRMS. Compound 5 for example was characterized by appearance of broad singlet (2H) at δ 5.39 ppm corresponding to NH2 group and further confirmed by HRMS (m/z): calcd. For C16H14N4O3 310.1066, found 310.1069 [M]+.
The final compounds 6-15 and 43-58 were synthesized either by condensation of amine derivatives with the respected acid chloride in dichloromethane and triethylamine as catalyst under argon atmosphere or with the respected carboxylic acid derivative in presence of condensing agent such as EDC and DMAP in dimethylformamide under argon atmosphere. 1H-NMR spectra of these derivatives were characterized by disappearance of the signal corresponding to NH2 and appearance of downfield signal (1H) corresponding to amidic NH and signals corresponding to introduced moiety. In addition, 13C-NMR spectra showed amidic C=O signal around 167-168 ppm and new signals corresponding to introduced moiety if compared to parent amine. All of final derivatives were further confirmed using HRMS. The compound 6 was required for further biological animal experiments so it was synthesized in multi gram amounts. It was synthesized many times after optimization of synthetic method including the reduction step and the final coupling in different conditions and in each time was confirmed by spectral data in addition to HRMS (m/z): calcd. For C24H20N4O4S 460.1205, found 460.1215 [M]+ (first batch), found 460.1203 [M]+ (2nd batch) found and 460.1203 [M]+ (3rd batch). Sulfonyl compounds 8 and 54 were prepared by oxidation of compounds 6 and 49, respectively using peracetic acid (39% in AcOH) [36] and then purified by column chromatography. The 1H-NMR spetra of these derivatives showed downfieldshift of CH2-SO2- if compared to CH2-Sand further conformed by HRMS for example compound 8 (m/z): calcd. for C24H20N4O6S 492.1104, found 492.1105 [M]+.
Preliminary biological screening of the target compounds indictaed that some of the new synthesized secinH3 derivatives showed higher potency and promising activity more than secinH3 itself (detalied study is still in progress). For example compund 9 and 10 were approximate equal to secinH3 activity as cytohesin antagonist activity. Furthermore compound 52 showed twice inhibition potency if compared to secinH3. Detailed biological screening and study of the structure activity relationship (SAR) of SecinH3 new derivatives is in progress.
In conclusion, rational design, synthesis of novel SecinH3 derivatives as potential cytohesin inhibitors is reported here. Final compounds were synthesized as shown in Schemes 1-2 starting with benzo [1,3]dioxole-5-carbonyl-chloride or different benzoyl chloride derivatives and potassium isothiocyanate (KNCS). The 1,2,4-triazol ring was readily assembled from benzo [1,3]dioxole-5-carbony lisothiocyanate in two steps, using (4-nithrophenyl)- hydrazine as previously described and then further elaborated after reduction using the appropriate substituted acetic acid derivative to yield the desired compounds in the presence of HBTU in DMF. Detailed biological screening of these new derivatives is in progress.
Acknowledgements
I am grateful to Prof. Dr. M. Famulok, Director of LIMES Institute, Chemical Biology and Medicinal Chemistry Unit, University of Bonn, Germany for providing NMR and Mass spectral data and his kind collaboration in this project.
References
- Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol 2000;12:475-82.
- Kolanus W. Guanine nucleotide exchange factors of the cytohesin family and their roles in signal transduction. Immunol Rev 2007;218:102-13.
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical elements in the control of small GTPases. Cell 2007;129:865-77.
- Casanova JE. Regulation of Arf activation: The Sec7 family of guanine nucleotide exchange factors. Traffic 2007;8:1476-85.
- Donaldson JG, Honda A. Localization and function of Arf family GTPases. BiochemSoc Trans 2005;33:639-42.
- Zeghouf M, Guibert B, Zeeh JC, Cherfils J. Arf, Sec7 and Brefeldin A: A model towards the therapeutic inhibition of guanine nucleotide-exchange factors. BiochemSoc Trans 2005;33:1265-8.
- Bui QT, Golinelli-Cohen MP, Jackson CL. The Capping Domain in RalF Regulates Effector Functions. Mol Genet Genomics 2009;282:329-50.
- Mayer G, Blind M, Nagel W, Bohm T, Knorr T, Jackson CL, et al. Controlling small guanine–nucleotide-exchange factor function through cytoplasmic RNA intramers. Proc Natl Acad Sci USA 2001;98:4961-5.
- Famulok M, Mayer G. Intramers and aptamers: Applications in protein-function analyses and potential for drug screening. Chembiochem 2005;6:19-26.
- Famulok M. Exploring Chemical space with aptamers. J Med Chem 2009;52:6951-7.
- Jeong S, Lee HK, Kim MY. Use of RNA aptamers for the modulation of cancer cell signaling. Methods Mol Biol 2009;542:363-77.
- Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009;457:426-33.
- Hafner M, Vianini E, Albertoni B, Marchetti L, Grune I, Gloeckner C, et al. Displacement of protein-bound aptamers with small molecules screened by fluorescence polarization. Nat Protoc 2008;3:579-87.
- Mayer G, Faulhammer D, Grattinger M, Fessele S, Blind MA. RNA-based approach towards small-molecule inhibitors. Chembiochem 2009;10:1993-6.
- Niebel B, Lentz C, Pofahl M, Mayer G, Hoerauf A, Pfarr KM, et al. ADLOC: An aptamer-displacement assay based on luminescent oxygen channeling. Chemistry 2010;16:11100-7.
- Hartig JS, Famulok M. Reporter ribozymes for real-time analysis of domain-specific interactions in biomolecules: HIV-1 reverse transcriptase and the primer-template complex. Angew Chem Int Ed Engl 2002;41:4263-6.
- Hartig JS, Najafi-Shoushtari SH, Grüne I, Yan A, Ellington AD, Famulok M. Protein-dependent ribozymes report molecular interactions in real time. Nat Biotechnol 2002;20:717-22.
- Jenne A, Hartig JS, Piganeau N, Tauer A, Samarsky DA, Green MR, et al. Rapid identification and characterization of hammerhead-ribozyme inhibitors using fluorescence-based technology. Nat Biotechnol 2001;19:56-61.
- Yamazaki S, Tan L, Mayer M, Hartig JS, Song JN, Reuter S, et al. Aptamer displacement identifies alternative small-molecule target sites that escape viral resistance. ChemBiol 2007;14:804-12.
- Sengle G, Jenne A, Arora PS, Seelig B, Nowick JS, Jaschke A, et al. Synthesis, incorporation efficiency, and stability of disulfide bridged functional groups at RNA 5’-ends. Bioorg Med Chem 2000;8:1317-29.
- Hafner M, Schmitz A, Grune I, Srivatsan SG, Paul B, Kolanus W, et al. Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature 2006;444:941-4.
- Bi X, Schmitz A, Hayallah AM, Song JN, Famulok M. Affinity-based labeling of cytohesins with a bifunctional SecinH3 photoaffinity probe. Angew ChemInt Ed Engl 2008;47:9565-8.
- Stumpfe D, Bill A, Novak N, Loch G, Blockus H, Geppert HC, et al.Targeting multifunctional proteins by virtual screening: Structurally diverse cytohesin inhibitors with differentiated biological functions. ACS Chem Biol 2010;5:839-49.
- Fuss B, Becker T, Zinke I, Hoch M. The cytohesinSteppke is essential for insulin signalling in Drosophila. Nature 2006;444:945-8.
- Ikenouchi J, Umeda M. FRMD4A regulates epithelial polarity by connecting Arf6 activation with the PAR complex. Proc Natl Acad Sci USA 2010;107:748-53.
- Oh SJ, Santy LC. Differential effects of cytohesins 2 and 3 on beta1 integrin recycling. J Biol Chem 2010;285:14610-6.
- Lim J, Zhou M, Veenstra TD, Morrison DK. The CNK1 scaffold binds cytohesins and promotes insulin pathway signaling. Genes Dev 2010;24:1496-506.
- Torii T, Miyamoto Y, Sanbe A, Nishimura K, Yamauchi J, Tanoue A. Cytohesin-2/ARNO, through its interaction with focal adhesion adaptor protein paxillin, regulates preadipocyte migration via the downstream activation of Arf6. J Biol Chem 2010;285:24270-81.
- El Azreq MA, Garceau V, Harbour D, Pivot-Pajot C, Bourgoin SG. Cytohesin-1 regulates the Arf6-phospholipase D signaling axis in human neutrophils: Impact on superoxide anion production and secretion. J Immunol 2010;184:637-49.
- Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol 2009;11:1325-31.
- Yamauchi J, Miyamoto Y, Torii T, Mizutani R, Nakamura K, Sanbe A, et al.Valproic acid-inducible Arl4D and cytohesin-2/ARNO, acting through the downstream Arf6, regulate neurite outgrowth in N1E-115 cells. Exp. Cell Res 2009;315:2043-52.
- Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun 2009;1:194-201.
- Bill A, Schmitz A, Albertoni B, Song JN, Heukamp L, Walrafen D, et al.Cytohesins are cytoplasmic ErbB receptor activators. Cell 2010;143:201-11.
- Lebreton S, Newcombe N, Bradley M. Antibacterial single-bead screening. Tetrahedron 2003;59:10213-22.
- Dueholm KL, Egholm M, Behrens C, Christensen L, Hansen HF, Vulpius T, et al. Synthesis of peptide nucleic acid monomers containing the four natural nucleobases: Thymine, cytosine, adenine, and guanine and their oligomerization. J Org Chem 1994;59:5767-73.
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 1997;36:4675-84.
- Northup JK, Smigel MD, Gilman AG. The guanine nucleotide activating site of the regulatory component of adenylatecyclase. Identification by ligand binding. J Biol Chem 1982;257:11416-23.