- *Corresponding Author:
- M. Sokar
Department of Pharmaceutics, Faculty of Pharmacy, Alexandria University, 1st El Khartoum Square, Azarita, Elmesalla, P.O. Box 21521, Alexandria, Egypt
E‑mail: magdasokar@yahoo.com
| Date of Submission | 31 May 2014 |
| Date of Revision | 26 January 2015 |
| Date of Acceptance | 04 August 2015 |
| Indian J Pharm Sci 2015;77(4):470-477 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The aim of the present study was to design and evaluate a chronomodulated time-clock pulsatile tablets of valsartan to release it after a certain lag time, independent of the gastrointestinal pH, in its absorption window to cope with the circadian rhythm of human body for blood pressure elevation. Core tablets were prepared by direct compression of a homogenous mixture of valsartan, Avicel PH101, croscarmellose sodium, magnesium stearate and Aerosil. The core tablets were then sprayed coated with a sealing layer formed of ethyl cellulose that was subsequently coated with a release-controlling layer. Three different aqueous dispersions namely; carnauba wax or beeswax or a mixture in a ratio of 2.5:1, respectively, were used to form five time-clock tablet formulations having the release controlling layer with different thickness {B5, B10, B20, BW5 and CW5}. Quality control testing were carried out to the core tablets. Differential scanning calorimetry was also performed to detect the possible drug excipient interaction in the core tablet formulation. The release was carried out, for the prepared time-clock tablet formulations, in 0.1 N hydrochloric acid for the first 2 h, followed by phosphate buffer (pH 6.8) for 4.5 h. The effect of pH on valsartan release was studied through a release study in 0.1 N hydrochloric acid for 6.5 h. Two phase dissolution study was performed to the selected time-clock tablet formulation to predict the drug permeation through the gastrointestinal tract. Stability study of the selected formula was performed at 25°/60% RH and at 40°/75% RH for 3 months. Results showed that a release-controlling layer composed of a mixture of carnauba wax and beeswax in a ratio of 2.5:1 showed a reasonable release lag time. The release lag time of the tablets increased with the increase of the coat thickness, thus B20>B10>B5 with corresponding lag time values of 4.5, 3 and 2.5 h, respectively. Selected B5 tablet formula exhibited a reasonable lag time after which the highest, complete % drug release at pH 6.8 was obtained. In addition, a good partitioning of valsartan, between the aqueous and organic phases in a ratio of 1:7, was observed. The selected formula was stable for at least 3 months under standard long-term and accelerated storage conditions. In conclusion, in vitro studies revealed that the novel time-clock system could be used successfully to deliver valsartan in a pulsatile pH-independent manner. It provided a desirable lag time followed by a rapid and complete drug release accompanied by an expected effective permeation through the biological membranes upon release in the duodenum; the window of absorption, as indicated by the two phase release study.
Keywords
Time‑clock, pulsatile, pH‑independent, valsartan, lag time, carnauba wax, bees wax, permeation
Human body shows 24 h variation in blood pressure. There is an increase in the atrial blood pressure in the morning between 4:00 to 8:00 am and a gradual decrease at night. Valsartan is angiotensin II inhibitor used in treatment of hypertension. The pharmacokinetics of valsartan showed that valsartan peak plasma concentration is reached in 2 to 4 h after conventional drug dosage form administration. Valsartan shows an average elimination half‑life of about 6 h. Conventional drug delivery system of valsartan is inappropriate for the delivery of drug, as they cannot be administered just before the symptoms are worsened. Bedtime dosing of conventional drug dosage of valsartan will not provide a sufficient therapeutic plasma drug concentration at the early hours of morning.
The time‑clock system is a system having a lag time independent of the gastric residence time, intestinal enzymes, mechanical action of stomach or gastrointestinal pH. Pozzi et al. [1] developed the time‑clock system for delivery of salbutamol sulfate using the conventional coating technique. The coating was made up of a hydrophobic‑surfactant layer [(Carnauba wax (CW) and beeswax (BW) along with tween 80] applied as aqueous dispersion, to which a water soluble polymer (HPMC) was added to improve adhesion to the core. This coat, dried on the core during coating, retains the capacity to rehydrate and redisperse in an aqueous environment in a time proportional to the film thickness. Following redispersion, the core would be available for dissolution [1].
After performing the in vitro release studies, Pozzi et al. [1] evaluated the time‑clock tablets in vivo in healthy volunteers via gamma‑scintigraphy using samarium oxide which was added to the tablet formulation [1]. The time‑clock tablets of salbutamol [1] are targeted to the colon where they reached after 283 min. The first drug conc. appeared in the blood after Tlag of 299‑393 min with mean Tmax of 305 min.
Afterwards, Wilding et al. [2] tried to develop an enteric coated time‑clock release system for colonic targeting. However, the primary objective in case of drug delivery to the colon was that the extended gastric residence, premature mesalazine release in the small bowel can occur [2]. To overcome this problem, and to make use of the relatively consistent small intestinal transit time [3], an enteric coating was applied to the formulation to prevent dispersion of the hydrophobic layer whilst in the stomach [2].
The lag time is the time needed for the waxes’ coat to emulsify and erode by the help of surfactants such as polyoxyethylene sorbitan monooleate. The lag time is proportional to the film thickness [1,2]. The aim of the present study was to prepare valsartan time‑clock pulsatile tablets to release the drug after certain lag time in its absorption window to cope with the circadian rhythm of human body for blood pressure elevation.
Materials and Methods
Valsartan (a gift from European Egyptian Pharmaceutical Industries Company, Alexandria, Egypt), carnauba wax (CW), beeswax (BW), hydroxypropyl methylcellulose K4M (HPMC) and Tween 80 (gifts from Pharco Pharmaceuticals Co., Alexandria, Egypt), ethylcellulose (EC), Ashacel® (Asha Cellulose, India). Hydrochloric acid and sodium phosphate dodecahydrate, (E. Merck, Darmstadt, Germany). Microcrystalline cellulose (Avicel PH‑101), magnesium stearate, croscarmellose sodium (CCNa), colloidal silicon dioxide (Aerosil® 130) and ethanol 95% (El‑Nasr Pharmaceutical Chemicals Co., Cairo, Egypt), n‑octanol (Hopkin and Williams, England.
Preparation of pulsatile valsartan tablets, core tablet
Valsartan core tablets were prepared as described by Sokar et al. [4]. Valsartan (80 mg), Avicel PH‑101 (22.5 mg) and CCNa (5 mg) were mixed by geometric addition technique. Aerosil 130 (0.5 mg) and magnesium stearate (2 mg) were then added to the mixed powders. All the powders used were passed through sieve no. 45 separately. Finally, 110 mg of the blend was weighed and compressed by a single punch tablet press machine (Royal artist, India), equipped with 6 mm concave‑faced punches.
Preparation of time‑clock tablets
The time‑clock tablets were prepared as stated by Pozzi et al. [1] with some modifications. An extra sealing layer was added between the core tablet and the outer release‑controlling layer.
Core tablets were weighed accurately before coating then arranged manually in a tray and sprayed with EC ethanolic solution (3%). The number of sprayings and distance between the sprayer and tablets were fixed. The sprayed core tablets were dried using hot air oven (Human‑lap, Korea) adjusted at 60°, then allowed to cool in ambient air. Half‑sprayed core tablets were reciprocated in the tray and the other sides were sprayed as mentioned previously. After cooling, tablets were reweighed. The sprayed core tablets were coated with CW, BW and HPMC. These polymers were previously ground, sieved and a size range of (90‑180 μm) was selected. Three different aqueous dispersions were prepared, whose composition was listed in Table 1. All the dispersions were prepared using the same technique.
| Ingredients | Aqueous dispersion composition (g%) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| CW | 2.5 | 3.5 | ‑ |
| BW | 1 | ‑ | 3.5 |
| HPMC K4M | 0.25 | 0.25 | 0.25 |
| Tween 80 | 0.5 | 0.5 | 0.5 |
HPMC: Hydroxypropyl methylcellulose, CW: carnauba wax, BW: beeswax
Table 1: Compositions Of Different Release‑ Controlled Layer Aqueous Dispersions
Tween 80 was mixed with water then heated to 80° and HPMC was sprinkled slowly. Dispersion was allowed to cool and waxes were added gradually while mixing using a magnetic stirrer (Daihan Scientific Co., Korea). Core tablets, previously sprayed with the sealing layer, were arranged in the tray and sprayed using the previously mentioned technique.
After spraying the core tablets with the aqueous dispersion, they were allowed to dry in ambient air and weighed to determine the total % weight increase. The coating steps were repeated until the desired weight was gained. Five batches of the time‑clock tablets were prepared with different coat thicknesses (Table 2) using the previously prepared aqueous dispersions.
| Formulation | Aqueous dispersion used | Percentage of weight increase |
|---|---|---|
| B5 | 1 | 5 |
| B10 | 1 | 10 |
| B20 | 1 | 20 |
| CW5 | 2 | 5 |
| BW5 | 3 | 5 |
CW: Carnauba wax, BW: beeswax
Table 2: Composition Of Valsartan Time‑Clock Tablet Formulations
Differential scanning calorimetry
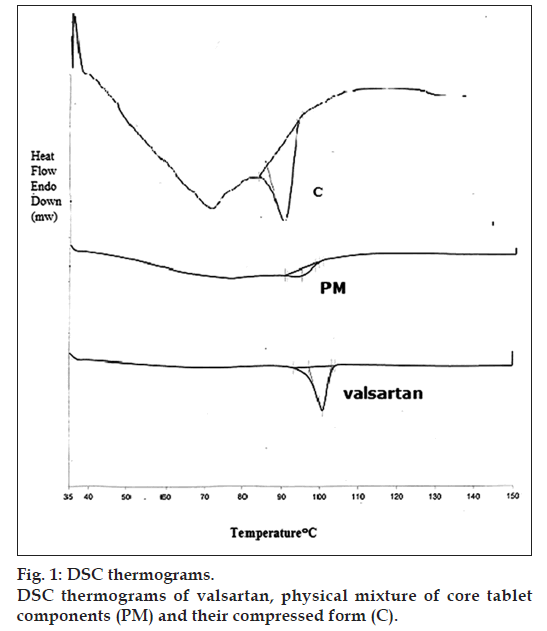
Differential scanning calorimetry (DSC) was performed using a DSC‑6 (Perkin‑Elmer, England). The samples [valsartan, components of tablet core formulation as a physical mixture (PM), and the final compressed tablet core form (C)] were investigated. The instrument was calibrated with indium standard. Valsartan powder (10 mg) and 14 mg samples of PM and ground C containing an equivalent amount of the drug were weighed and placed in a closed aluminum sample pans with pin hole under nitrogen flow (30 ml/min) at a scanning rate of 5°/min from 35 to 150°. The heat flow as a function of temperature was measured for all samples [4].
Quality control testing of the prepared core tablets
Disintegration time of the core tablets was determined using the disintegration tester (Copley, Scientific Ltd, UK) in 900 ml water, 0.1N HCl and phosphate buffer (pH 6.8), in triplicates. Tablets were also subjected to friability test using the tablet friability test apparatus (Copley, UK). Tablet breaking force of 10 randomly selected tablets was determined using the tablet hardness tester (Dr. Schleuniger Pharmatron AG, Switzerland).
Tests were performed according to the official USP method [5]. The weight variation test was carried out as stated in the BP [6]. The prepared core tablets were subjected to uniformity of thickness and diameter using a Vernier caliper (For‑bro Engineers, Mumbai, India). The tests were carried out on 20 randomly selected tablets [4].
In vitro drug release studies
The drug release testing from the time‑clock tablets was carried out using USP dissolution apparatus II (Hanson Research Corporation, USA), in 730 ml 0.1 N HCl for 2 h after which the pH of the medium was changed to 6.8 by adding 270 ml of 0.2 M Na3PO4.12H2O solution [7]. The study was performed at 37±0.5° and a stirring rate of 50 rpm. At different time intervals, 5 ml sample was withdrawn, filtered through a cellulose acetate membrane filter (0.45 μm) and analyzed using UV/Vis spectrophotometer at 250 nm (Shimadzu Corporation, Japan). Each withdrawn sample was compensated with 5 ml of fresh corresponding medium. All release experiments were carried out in triplicates. Validation parameters of the spectrophotometric assay was limited to linearity, inter‑day precision and intra‑day precision data.
Modeling of release profiles
To determine Valsartan release kinetics from the selected formulation, the release data was treated according to Higuchi, Korsmeyer‑Peppas, Hixson‑Crowell and Weibull models along with zero and first order patterns [8] using the Add‑In ‘DDSolver’ software [9].
Solubility studies
Valsartan solubility was investigated in 0.1N HCl, phosphate buffer (pH 6.8) and n‑octanol by adding a known excess amount of valsartan to a flask containing 25 ml of each medium. The flasks were shaken for 24 h in a water bath at 37° and then they were left to attain equilibrium for another 24 h. Solutions were filtered, diluted and analyzed using UV/Vis spectrophotometer (Shimadzu Corporation, Japan) at the predetermined λmax in each medium [10,11].
Two‑phase dissolution study
A two‑phase dissolution study was performed for the selected time‑clock tablet formulation in USP dissolution apparatus II using a 150 ml vessel containing 50 ml 0.1N HCl as the lower aqueous phase and 50 ml n‑octanol as the upper organic phase. The temperature was adjusted at 37±0.5° and the stirring rate was 50 rpm [12]. The study was performed in duplicates.
The dissolution vessel was filled with the aqueous phase followed by addition of one tablet. Then, n‑octanol was slowly added over the aqueous phase (being careful to prevent the contact of n‑octanol with the tablet). After 2 h, the pH of the lower phase was changed to 6.8 by adding 18.5 ml of 0.2 M Na3PO4.12H2O solution [7]. At different time intervals, 1 ml sample was withdrawn from each layer, filtered through a cellulose acetate membrane (0.45 μm) (for samples withdrawn from the aqueous phase) or a PTFE syringe filter (0.45 μm, ABIMED, Deutschland) (for samples withdrawn from the organic phase) and analyzed using UV/Vis spectrophotometer at the predetermined λmax for each layer. Each withdrawn sample was compensated with 1 ml of the fresh corresponding medium.
Stability studies
The formula of choice was tested for its physical stability according to the ICH guidelines [13]. Standard long‑term stability study was performed at 25° and 60% RH for 3 months. Accelerated stability study was carried out at 40° and 75% RH for 3 months [14]. At the specified time intervals, the tablets were examined for their physical appearance and in vitro drug release.
Statistical analysis
Results were depicted as mean values±SD. All statistical comparisons were performed using Microsoft Excel 2007. The obtained data of repeated measurements was subjected to Student’s t‑test and a P value ≤0.05 was considered as significant.
Results and Discussion
The core tablets were compressed using concave punches to minimize edges’ space so as to obtain homogenous coating [15]. EC sealing layer was applied on the core tablets during the preparation to act as an insoluble barrier protecting the core from early disintegration. It was added in a single layer thickness; so the weight increase of core tablets was negligible.
Pozzi et al. [1] prepared the coating aqueous dispersion using waxes and HPMC in concentrations higher than that used in this study. Similar high concentrations resulted in an impractically viscous dispersion and nozzle blockage. Consequently, the concentrations of the coating aqueous dispersion used mentioned in Table 1 to facilitate the spraying process. HPMC was incorporated to help the adherence and cohesiveness of waxes to the core as they exhibit poor mechanical properties because of their lack of cohesive structural integrity [16].
Five formulations were prepared (Table 2) with the following %weight increase; B5 (4.85±0.2%), B10 (10.31±0.2%), B20 (19.81±0.5%), CW5 (5.13±0.2%) and BW5 (4.92±0.3%). In DSC studies, the thermogram of valsartan showed a clear melting endothermic peak at 100.8°. Valsartan peak in PM thermogram was slightly shifted to appear at 95.31° and in C thermogram it was shifted to 96.07°. Valsartan peak intensities in both PM and C thermograms were decreased and broadened markedly; fig. 1. The decreased and broadened peaks indicate the less ordered crystallinity, rather than completely amorphous state [4]. This change could be due to the adsorption of Aerosil particles on valsartan which changed its crystalline nature to an amorphous powder [17]. Mixing valsartan with Avicel PH‑101, CCNa or Mg stearate did not seem to be responsible for this change [17,18].
The average weight the core tablets was 107.31±0.89 mg. Core tablets showed a uniform thickness (3.32±0.08 mm) and diameter (6.06±0.02 mm). The core tablet hardness was 3.87±0.4 kg/cm2. Concerning the friability, it was within the official limits as the percentage weight loss was 0.59%. The disintegration time of the core tablets was instantaneous in 900 ml water, 0.1 N HCl (pH 1.2) and phosphate buffer (pH 6.8) [4].
Validation parameters of the spectrophotometric assay was limited to linearity, inter‑day precision and intra‑day precision data. Valsartan showed a linear relationship in 0.1 N HCl and pH 6.8 over a conc. range of (1‑3 mg%) in both media with a value of coefficient of determination (R2) 0.998 and 0.9995, respectively. Inter and intra‑day precision data were reasonable and showed maximum value for % CV of 6.44%. The results are similar to those obtained by Sokar et al. [4].
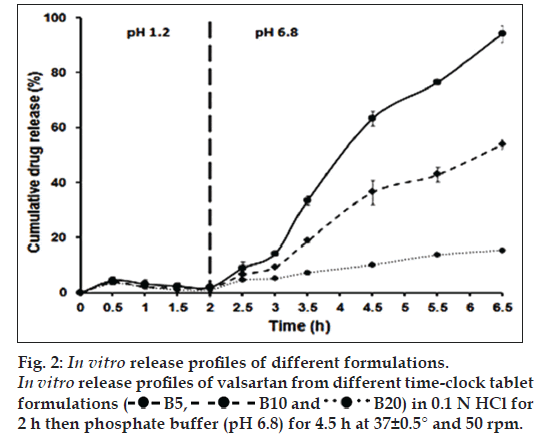
The drug release from the time‑clock system is surfactant‑based. The release‑controlling coat was formed of waxes and a surfactant (tween 80) [1]. Upon contact with water, tween 80 acts to emulsify the waxes gradually thus dissolving the coat. As the coat thickness increases, the time necessary for its complete dissolution increases. Therefore, the experiments showed that B5, B10 and B20 formulations demonstrated a lag time of 2.5, 3 and 4.5 h, respectively, depending on the weight gained by the core; fig. 2. Their ascending lag times were in accordance with their ascending coating thickness. The lag time was defined as the time until 10% of the drug has been released [19].
To investigate the effect of pH on the lag time of B5, B10 and B20 tablets, a release study was also performed in 0.1 N HCl for 6.5 h using dissolution apparatus II, at 37±0.5° and a stirring rate of 50 rpm. B5, B10 and B20 tablets demonstrated a lag time of 2.5, 3 and >6.5 h, respectively. Although the pH of the medium was not shifted from pH 1.2 to pH 6.8 as described above, B5, B10 and B20 formulations showed nearly the same lag time as in case of the previously mentioned pH change release procedure. Thus, the pH of the medium has no effect on the drug release. Therefore, the time‑clock system is pH‑independent and the main factor controlling the drug release is the coat thickness.
It is worth mentioning that in 0.1 N HCl the drug release after the lag time was slow in comparison with the release after changing the pH of the medium to 6.8. This could be due to the low intrinsic solubility of valsartan in acidic medium.
B5 tablet formula (having the smallest % weight increase among the previously mentioned time‑clock formulae) exhibited reasonable lag time (2.5 h). After 2.5 h, the tablet will be delivered to the upper part of the intestine (duodenum) at pH 6.8. At this pH the drug will be released instantaneously. Consequently, B5 formulation was selected for further stability studies.
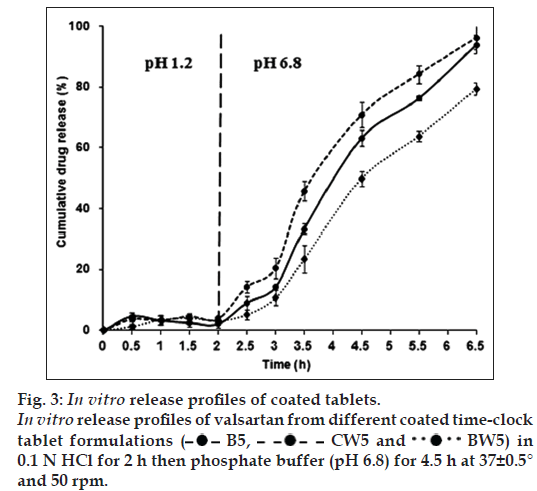
Up to our knowledge, all previous time‑clock studies, a mixture of BW and CW with different concentrations for coating was used. To investigate the effect of using of a single wax type on the drug release and lag time, two time‑clock tablet formulations were prepared involving CW or BW solely as the coating wax (CW5 and BW5, respectively). Thus, aqueous dispersions (2) and (3) were prepared with the composition mentioned previously in Table 1.
As shown in fig. 3, CW5 and BW5 showed 2 and 3 h lag time, respectively. Although the drug release from CW5 seems faster than that from B5, this difference is considered insignificant (P>0.05). BW5 showed a bit slower release than B5 but the difference in drug release was insignificant too (P>0.05).
It is preferred to use a mixture of CW and BW for tablet coating to combine the advantages of both waxes. CW is incorporated in the aqueous dispersion so as to obtain tablets of good luster without rubbing, increase the coat strength and facilitate swallowing. Whilst, BW is used to increase the elasticity of the coat but due to its low melting point (61°) it may result in softer and more elastic coats [20]. Therefore, a mixture of both waxes was used in the reported ratio to improve the properties and appearance of the coat. Therefore, B5 was chosen as the selected valsartan time‑clock tablet formulation for further studies.
After treating the release data of valsartan from B5 formulation, according to the different release patterns previously mentioned under section 2.5. It was found that B5 fits into Weibull model [21] in both release studies (0.1 N HCl for 2 h then pH 6.8 for 4.5 h) and (0.1 N HCl for 6.5 h) as indicated by the values of R2 (coefficient of determination); 0.992 and 0.934, respectively. As b, the shape parameter, is ˃1 (1.869 and 1.062, respectively), the shape of the curve gets sigmoidal with a turning point. The sigmoidal curve shape is indicative of complex release mechanisms i.e. the rate of release does not change monotonically.
In fact, the release rate initially increased nonlinearly up to the inflection point and thereafter decreased asymptotically [22].
Because of the lipid nature of biological membranes, drug lipophilicity has long been considered an important determinant of absorption. The octanol/water partition coefficient (P) at a selected pH is a key indicator of drug lipophilicity and could predict the drug permeation through the gastrointestinal membrane [23]. Valsartan log P value is 1.499, indicating that the compound has a rather hydrophilic character at physiological pH [24]. Thus, valsartan is classified, according to BCS, as Class III drug with low permeability and high solubility [25].
As maintaining ‘sink condition’ throughout the release study of poorly soluble drugs can be difficult, in vitro data obtained from the single‑phase dissolution system may not satisfactorily reflect the in vivo drug absorption characteristics [26].
The two‑phase dissolution system maintains sink condition all the time. In addition, the transfer of drug between the two phases mimics the entire process of drug release, dissolution and absorption within the GIT. Thus, it could be used to predict the formulation in vivo behavior [27].
The choice of n‑octanol as the organic phase was based on its desirable physicochemical properties. It is less dense than water (specific gravity of n‑octanol is 0.825 g/cm3 at 20°) and practically insoluble in water (0.05% w/w). It has low volatility (boiling point=195°) hence, n‑octanol will not readily evaporate at 37°. Thus, a relatively constant upper phase volume could be maintained throughout the experiment [27].
As solubility of valsartan in n‑octanol is 17 g/l, sink condition is guaranteed. Thus, the dissolved drug is continuously removed from the aqueous phase into the organic phase of the dissolution medium, mimicking the process of absorption in the systemic circulation [27].
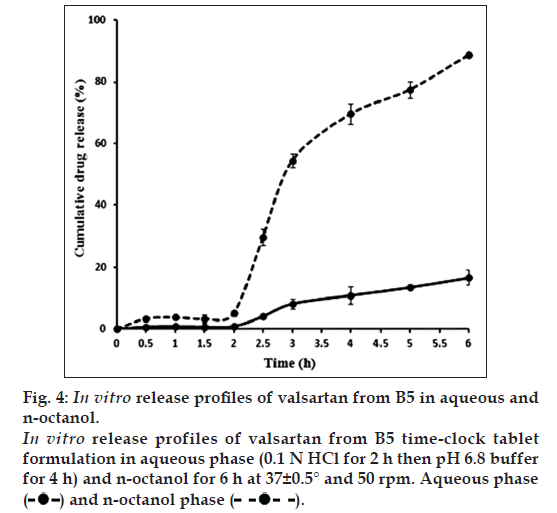
As shown in fig. 4, during the first 2 h, there was almost no drug release observed in both the aqueous and organic media. After changing the pH of the aqueous phase to 6.8; at 2.5 h from the beginning of the experiment, drug release was initiated and valsartan was partitioned between the aqueous and organic phases in a ratio of 1:7, respectively.
Thus, valsartan favors being dissolved in the organic layer rather than the aqueous layer. Consequently, it is supposed that upon drug release in the duodenum, the window of absorption, valsartan would permeate effectively through the biological membranes.
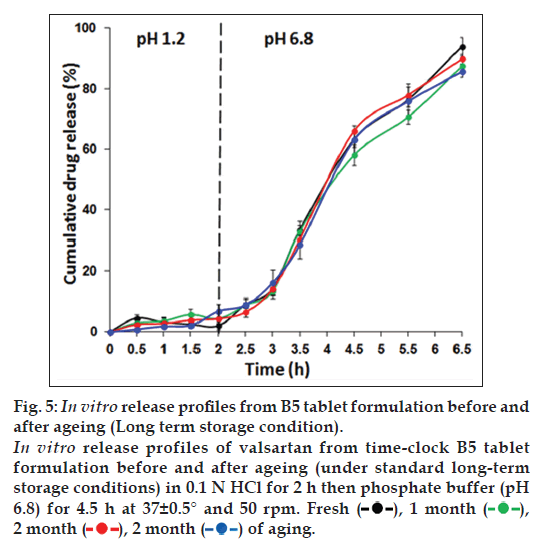
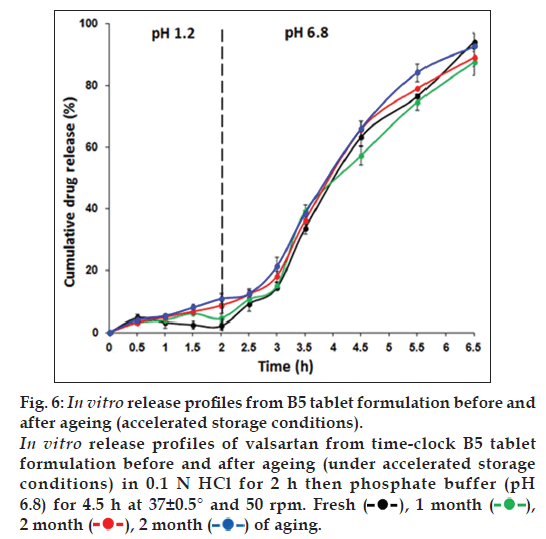
The in vitro release profiles of valsartan from freshly prepared B5 time‑clock tablet formulation and the aged tablets for 1, 2 and 3 months under standard long‑term and accelerated storage conditions, were similar with no significant difference (P>0.05), as shown in figs. 5 and 6, respectively. No cracking or color changes of the coat were observed during the study period.
In conclusion, time‑clock system could be used successfully to deliver valsartan in a pulsatile pH‑independent manner. It provided a desirable lag time, which could be controlled by the coat thickness, followed by rapid and complete drug release. The two phase dissolution study confirmed that valsartan would permeate effectively through the biological membranes upon release in the duodenum; the window of absorption.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pozzi F, Furlani P, Gazzaniga A, Davis SS, Wilding IR. The time clock system: A new oral dosage form for fast and complete release of drug after a predetermined lag time. J Control Release 1994;31:99‑108.
- Wilding IR, Davis SS, Pozzi F, Furlani P, Gazzaniga A. Enteric coated timed release systems for colonic targeting. Int J Pharm 1994;111:99‑102.
- Coupe AJ, Davis SS, Wilding IR. Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm Res 1991;8:360‑4.
- Sokar M, Hanafy A, El‑Kamel A, El‑Gamal S. Pulsatile core‑in‑cup valsartan tablet formulations: In vitro evaluation. Asian J Pharm Sci 2013;8:234‑43.
- United States Pharmacopoeial Commission. United States Pharmacopoeia 35 and National Formulary 30. 1st ed. Rockville: United States Pharmacopoeial Commission; 2012.
- Her Mayesty’s Stationary Office. British Pharmacopeia. Vol. IV. London: HMSO; 2013.
- El Gamal S, Naggar V, Sokar M. Colon targeting of mebeverine HCl from pH‑dependent tablet formulations. J Am Sci 2012;8:632‑8.
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010;67:217‑23.
- Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: An add‑in program for modeling and comparison of drug dissolution profiles. AAPS J 2010;12:263‑71.
- Mbah CJ. Physicochemical properties of valsartan and the effect of ethyl alcohol, propylene glycol and pH on its solubility. Pharmazie 2005;60:849‑50.
- Chella N, Shastri N, Tadikonda RR. Use of the liquisolid compact technique for improvement of the dissolution rate of valsartan. Acta Pharm Sin B 2012;2:502‑8.
- Shi Y, Gao P, Gong Y, Ping H. Application of a biphasic test for characterization of in vitro drug release of immediate release formulations of celecoxib and its relevance to in vivo absorption. Mol Pharm 2010;7:1458‑65.
- Branch SK. Guidelines from the International Conference on Harmonisation (ICH). J Pharm Biomed Anal 2005;38:798‑805.
- Bajaj S, Singla D, Sakhuja N. Stability testing of pharmaceutical products. J Appl Pharm Sci 2012;2:129‑38.
- Suzzi D, Toschkoff G, Radl S, Machold D, Fraser SD, Glasser BJ, et al. DEM simulation of continuous tablet coating: Effects of tablet shape and fill level on inter‑tablet coating variability. Chem Eng Sci 2011;69:107‑21.
- Gontard N, Marchesseau S, Cuq JL, Guilbert S. Water vapour permeability of edible bilayer films of wheat gluten and lipids. Int J Food Sci Technol 1995;30:49‑56.
- Shrivastava AR, Ursekar B, Kapadia CJ. Design, optimization, preparation and evaluation of dispersion granules of valsartan and formulation into tablets. Curr Drug Deliv 2009;6:28‑37.
- Cao QR, Liu Y, Xu WJ, Lee BJ, Yang M, Cui JH. Enhanced oral bioavailability of novel mucoadhesive pellets containing valsartan prepared by a dry powder‑coating technique. Int J Pharm 2012;434:325‑33.
- Nayak UY, Shavi GV, Nayak Y, Averinen RK, Mutalik S, Reddy SM, et al. Chronotherapeutic drug delivery for early morning surge in blood pressure: A programmable delivery system. J Control Release 2009;136:125‑31.
- Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients. London: Pharmaceutical Press; 2006.
- Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol 1972;24:979‑81.
- Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm 2006;309:44‑50.
- Pallicer JM, Sales J, Rosés M, Ràfols C, Bosch E. Lipophilicity assessment of basic drugs (log P(o/w) determination) by a chromatographic method. J Chromatogr A 2011;1218:6356‑68.
- Criscione L, Bradley WA, Bühlmayer P, Whitebread S, Glazer R, Lloyd P, et al. Valsartan: Preclinical and clinical profile of an antihypertensive angiotensin‑II antagonist. Cardiovasc Drug Rev 1995;13:230‑50.
- Saydam M, Takka S. Bioavailability file: Valsartan. FABAD J Pharm Sci 2007;32:185‑96.
- Mudie DM, Shi Y, Ping H, Gao P, Amidon GL, Amidon GE. Mechanistic analysis of solute transport in an in vitro physiological two‑phase dissolution apparatus. Biopharm Drug Dispos 2012;33:378‑402.
- Heigoldt U, Sommer F, Daniels R, Wagner KG. Predicting in vivo absorption behavior of oral modified release dosage forms containing pH‑dependent poorly soluble drugs using a novel pH‑adjusted biphasic in vitro dissolution test. Eur J Pharm Biopharm 2010;76:105‑11.