- *Corresponding Author:
- P. B. Rathi
Department of Pharmaceutics, Shri Bhagwan College of Pharmacy, N-6, CIDCO, Auranagabad-431003, India
E-mail: pavanbrathi@gmail.com
| Date of Submission | 23 January 2010 |
| Date of Revision | 2 July 2010 |
| Date of Acceptance | 4 October 2010 |
| Indian J. Pharm. Sci., 2010, 72 (5): 671-674 |
Abstract
Satranidazole, a potent broad spectrum antiprotozoal, is a poorly water-soluble drug and has low bioavailability on oral administration. One of the important methods to improve the solubility and bioavailability of a less water-soluble drug is by the use of cosolvents. The solubility enhancement produced by binary blends with a cosolvent (dioxane) was studied against the solubility parameter of solvent blends (d1 ) to evaluate the solubility parameter of drug (d2 ). Solubility parameter of drug (d2 ) was evaluated in blends of dioxane-water system. The results obtained were compared with the d2 values obtained using Molar Volume Method and Fedor's Group Substitution Method. The binary blend water-dioxane (10:90) gave maximum solubility with an experimental d2 value of 11.34 (Cal/cm 3 ) 0.5 that was comparable to the theoretical values of 11.34 (Cal/cm 3 ) 0.5 determined by Molar Volume Method and 11.3928 (Cal/cm 3 ) 0.5 when determined by Fedor's Group Substitution Method, which is in good agreement with solubility measurement method.
Keywords
Fedor’s group substitution method, molar volume method, satranidazole, solubility parameter
Satranidazole, 1-methylsulphonyl-3-(1-methyl-5- nitro-2-imidazolyl)-2-imidazolidinone, is one of the large series of nitroimidazoles with potent antiprotozoal activity against E. hystolytica, T.vaginalis and giardia [1]. It is not official as yet in IP, USP and BP [2,3]. Though the molecule is found to be effective against these microorganisms, its therapeutic efficacy is hindered due to its poor aqueous solubility [4]. The poor aqueous solubility and wettability of satranidazole gives rise to difficulties in pharmaceutical formulations meant for oral or parenteral use, which may lead to variation in bioavailability [5]. Co-solvency is one of the methods to improve solubility especially in case of liquid formulations. The choice of the appropriate co-solvent is important to obtain maximum solubility of drug [6]. Evaluation of solubility parameter in different solvent blends of various polarities would provide important insight about the solubility of drug. Solubility parameter (δ) is an intrinsic physicochemical property that influences structure activity and transport kinetics of a drug substance [7]. Literature survey revealed that solubility parameter of satranidazole is not estimated by any method till date. So the present study attempts to determine the solubility parameter of satranidazole in different blends of dioxane-water. Experimental values obtained were compared with the theoretical values obtained by molar volume method and Fedor’s group substitution method [8]. Dioxane and water were selected based on their Hildebrand values. Water and dioxane exhibit extremities of polarity [9].
Satranidazole, obtained as gift sample from Alkem Laboratories Ltd., Baddi, India, was purified by recrystallization process. The solvent used for recrystallization of satranidazole was acetone. 1,4-Dioxane and acetone were obtained as gift sample from E. Merck, Ltd.; Mumbai, India and Qualigens Fine Chemicals, Mumbai, India respectively. Double distilled water was used for experimental purpose throughout the study. All chemicals and reagents used in the study were of analytical grade and used as such. Double beam UV/ Vis spectrophotometer, Shimadzu model 1601 with spectral bandwidth of 2 nm, wavelength accuracy ±0.5 nm and a pair of 10 mm matched quartz cells was used to measure absorbance of the resulting solutions. Citizen balance, CX-100, was used for weighing of satranidazole.
Experimental determination of solubility parameter was based upon the maximum solubility of satranidazole in cosolvent-water blends. For most of cases, 1,4-dioxane and water were chosen as miscible solvent blends, which provides the two extremes of solubility parameters (δ1) 10 and 23.4 (Cal/cm3)0.5, respectively. Binary solvent system was prepared by using dioxane and water, ranging from 0% to 100%, respectively, in screw capped vials. The final volume of binary solvent system was kept constant at 2 ml. A slight excess quantity of satranidazole was introduced into each vial and these were shaken for 3 h by keeping on rotary shaker at constant speed at 150 rpm followed by saturation equilibration for 24 h at room temperature (~ 25°). Preliminary studies showed that this time was sufficient to ensure saturation equilibrium [10]. After equilibrium was reached, solutions were filtered through Whatman filter paper No. 41 and analyzed after appropriate dilutions with double distilled water by UV Spectrophotometer at 319.80 nm (λmax). All experimental results are expressed as the average of at least three determinations. The coefficient of variation (SD/mean×100) was within 2% among replicated samples for the solubility measurements.
Solubility parameter determination of satranidazole (δ2) was achieved using the solubility measurement method (experimental method) and by theoretical methods, namely, molar volume method and by Fedor’s group substitution method proposed by Fedor [11]. In solubility measurement method, the solubility parameter of satranidazole is assumed to be similar to that of the solubility parameter of the solvent (δ1) in which the drug exhibits maximum solubility [12]. Hence, the solubility data (Table 1) obtained by the method described in preceding section was used to determine δ2.
| Solvent blend Water: Dioxane (%v/v) | δ1(Cal/cm3)0.5 | Δδ (δ1-δ2) | Solubility(g/ml) | Mole fraction solubility (X2×10-3) |
|---|---|---|---|---|
| 1 00:0 | 23.40 | +12.06 | 0.0005821 | 0.036317 |
| 90:10 | 22.06 | +10.72 | 0.0009112 | 0.078740 |
| 80:20 | 20.72 | +9.38 | 0.0012872 | 0.14198 |
| 70:30 | 19.38 | +8.04 | 0.0020104 | 0.26954 |
| 60:40 | 18.04 | +6.70 | 0.0039050 | 0.61647 |
| 50:50 | 16.70 | +5.36 | 0.0064722 | 1.1757 |
| 40:60 | 15.36 | +4.02 | 0.0109196 | 2.2456 |
| 30:70 | 14.02 | +2.68 | 0.0180788 | 4.1594 |
| 20:80 | 12.68 | +1.34 | 0.0286006 | 7.2968 |
| 10:90 | 11.34 | +0.00 | 0.0344943 | 9.6347 |
| 0:100 | 10.00 | -1.34 | 0.0262503 | 7.8876 |
δ1= Solubility parameter of solvent blend, δ2= Solubility parameter of drug in solvent blend. The binary solvent blends, δ1 and δ1-δ2 and the corresponding values of equilibrium experimental solubility and mole fraction solubility
Table 1: Mole fraction solubility of satranidazole in binary solvent blends
The solubility parameter of satranidazole was determined by molar volume method by calculating the mole fraction solubility (X2) of satranidazole in solvent blends containing water and dioxane in different ratios as shown in Table 1. The mole fraction solubility was calculated by using the equation, mole fraction solubility, X2=η2/ η1+η2 (1), where η1 and η2 are the number of moles of solvent and solute respectively. A plot of mole fraction solubility of satranidazole in the various ratios of the binary mixtures was made against Δ δ (δ1-δ2), difference between solubility parameter of solvent and solute respectively. The solubility parameter of the solvent blend (δ1) in which satranidazole showed peak mole fraction solubility represents the solubility parameter of satranidazole (δ2) [13].
Fedor’s group substitution method is based on the determination of solubility parameter of satranidazole (δ2) by using the Eqn. δ = (Δ u/ Δ V)0.5 (2), in which Δ u represents the substituent fragment constant and Δ V represents the fragmental molar volume constant. Further, the solubility parameter of Satranidazole was determined by dividing the structure of satranidazole (fig. 1) into different fragments and corresponding values of cohesive energy per mole (Δ u) and molar volume (Δ V) for each fragment was obtained from literature survey as shown in Table 2.
| Drug fragments | No. of fragments | Cohesive energy (Cal/mol) | Molar volume (Cm3/mol) |
|---|---|---|---|
| =CH | [1] | 1030 | 13.5 |
| =C< | [3] | 1690 | 6.5 |
| -CH3 | [2] | 1125 | 33.5 |
| -NO2 | [1] | 3670 | 32.0 |
| -SO2 | [1] | 3700 | 23.8 |
| >N- | [3] | 2800 | 5.0 |
| -CH2- | [2] | 1180 | 16.1 |
| =N- | [1] | 2800 | 5.0 |
| Conjugation | [2] | 400 | -2.2 |
| Ring closure | [2] | 250 | 16 |
| Total | 30580 | 235.6 | |
| δ (Cal/cm3)0.5 | =(30580/235.6)0.5 | =11.3928 |
δ2= Solubility parameter of satranidazole
Table 2: Calculation of solubility parameter of satranidazole by fedor’s group substitution method
Solubility of satranidazole was evaluated in solvent blends containing water:dioxane for the determination of δ2 as the varying blends of these provided a range of 10.00-23.40 (Cal/cm3)0.5 of δ1. The peak solubility (X2) of 0.0344943 g/ml for satranidazole was observed in a solvent blend of water:dioxane (10:90) with δ1 of 11.34 (Cal/cm3)0.5. Thus, the solubility parameter for satranidazole can be defined as 11.34 (Cal/cm3)0.5 as according to the solubility measurement method; δ2 is that value of δ1 at which the drug exhibits maximum solubility. Table 1 lists the solvent blends, the Hildebrand solubility parameter (δ1) of the solvent blends and the experimentally determined solubilities (g/ml) of satranidazole.
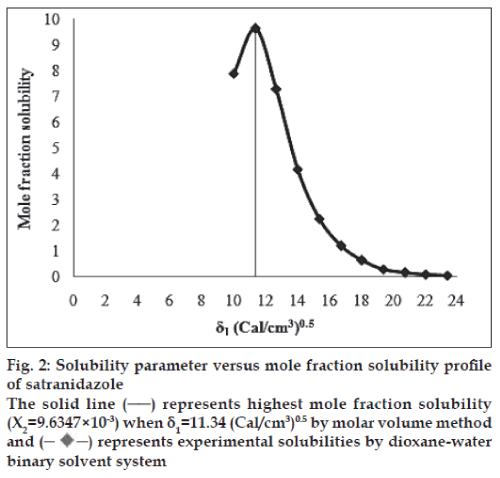
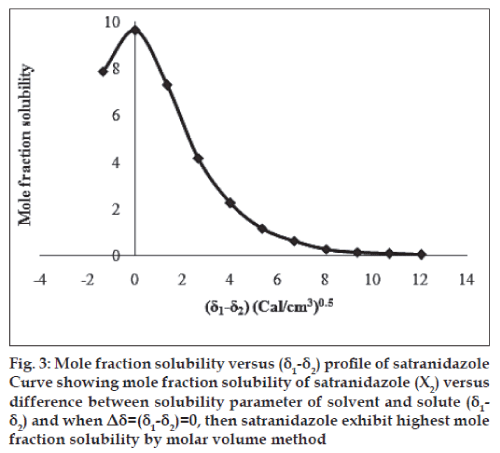
The molar volume method was used to determine the peak mole fraction solubility of satranidazole in various solvent blends and the mole fraction solubilities X2 of satranidazole and Δ δ are tabulated in Table 1. Peak mole fraction solubility was determined to be 9.6347×10-3 in solvent blend (water:dioxane, 10:90) with δ1 value 11.34 (Cal/ cm3)0.5, which is in agreement with the value obtained using solubility measurement method. A plot of δ1 and X2 (fig. 2) showed a bell shaped curve suggesting that both at lower and higher values δ1= 11.34 (Cal/ cm3)0.5 the solubility of satranidazole decreased. When Δ δ was plotted against X2 (fig. 3), the solubility parameter of satranidazole was confirmed at 11.34 (Cal/cm3)0.5 as it is that value of δ1 at which Satranidazole exhibited peak mole fraction solubility and Δ δ =0. δ2 determined by the Fedor’s group substitution method was found to be 11.3928 (Cal/cm3)0.5, which is comparable to the value obtained by solubility measurement method and molar volume method (Table 2).
Fig. 2: Solubility parameter versus mole fraction solubility profi le
of satranidazole
The solid line (─) represents highest mole fraction solubility
(X2=9.6347×10-3) when δ1=11.34 (Cal/cm/m3)0.5 by molar volume method
and (-♦-) represents experimental solubilities by dioxane-water
binary solvent system
Fig. 3: Mole fraction solubility versus (δ1-δ2) profi le of satranidazole Curve showing mole fraction solubility of satranidazole (X2) versus difference between solubility parameter of solvent and solute (δ1- δ2) and when Δδ=(δ1-δ2)=0, then satranidazole exhibit highest mole fraction solubility by molar volume method
Therefore, experimentally determined solubility parameter of satranidazole in water-dioxane binary solvent system was in good agreement with that of the theoretically determined solubility parameter by Molar volume method and by Fedor’s group substitution method.
Acknowledgements
The author is grateful to M/S Alkem Laboratories Limited, Baddi for providing gift sample of satranidazole. Author also wishes to express his gratitude to E. Merck, Ltd.; Mumbai, India and Qualigens Fine Chemicals, Mumbai, India for providing gift sample of 1,4-dioxane and acetone, respectively.
References
- Mruthyunjayaswamy BH, Patil SM, Raju SA. Spectrophotometric Methods for the Estimation of Satranidazole in Pharmaceutical Formulations. Indian J Pharm Sci 2001;63:433-6.
- Burange P, Arote S, Damle M, Godge R. A Validated RP-HPLC Method for Simultaneous Estimation of Ofloxacin and Satranidazole from Tablets. J Pharm Sci 2008;7:70-2.
- Wankhede SB, Nanda RK, Prakash A, Chitlange SS. Simultaneous Spectrophotometric Estimation of Ofloxacin and Satranidazole in Tablet Dosage Form. J Pharm Sci 2008;7:92-4.
- Josyula VR, Karanth H. In Vitro Studies on Solubility Parameter of AmoxycillinTrihydrate: Influence on Release and Antibacterial Activity. Indian J Pharm Sci 2005;67:342-5.
- Derle D, Magar M. Studies on the Preparation, Characterization and Solubility of β-Cyclodextrin- Satranidazole Inclusion Complexes. Indian J Pharm Edu Res 2006;40:232-6.
- Etman MA, Salama RO, Shamsedeen MA, El-Kamel A. Solubilization of Etodolac for Parenteral Administration. Indian J Pharm Sci 2001;63:459-67.
- Greenhalgh DJ, Williams AC, Timmins P, York P. Solubility Parameters as Predictors of Miscibility in Solid Dispersions. J Pharm Sci 1999;88:1182-90.
- Mourya VK, Yadav SK, Saini TR. Solubility Studies of Metronidazole in Binary Solvent Blends. Indian J Pharm Sci 1997;59:200-02.
- Martin A, Wu PL, Velasquez T. Extended Hildebrand Solubility Approach: Sulfonamides in Binary and Ternary Solvents. J Pharm Sci 1985;74:277-82.
- Martin A, Newburger J, Adjei A. Extended Hildebrand Solubility Approach: Solubility of Theophylline in Polar Binary Solvents. J Pharm Sci 1980;69:487-91.
- Barra J, Bustamante P, Pena MA. Partial solubility parameters of naproxen and sodium diclofenac. J Pharm Pharmacol 1998;50:975-82.
- Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm 1997;148:1-21.
- Kuksal K, Pathak K. Use of Solubility Parameter to Design Dry Suspension of Cefaclor as a Dual Pack System. Indian J Pharm Sci 2008;70:609-13.