- *Corresponding Author:
- B. Yilmaz
Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, 25240, Erzurum, Turkey
E-mail: yilmazb@atauni.edu.tr
| Date of Submission | March 08, 2012 |
| Date of Revision | June 27, 2012 |
| Date of Acceptance | June 29, 2012 |
| Indian J Pharm Sci, 2012, 74 (3): 248-253 |
Abstract
This article describes gas chromatography-flame ionization detection method for determination of 17 β-estradiol in rabbit plasma. 17 β-estradiol and internal standard progesterone were extracted from plasma using liquid-liquid extraction method. Linearity was found between 0.25 and 20 μg/ml (r 2 =0.994) for plasma samples. Intra-day and inter-day precision, expressed as the relative standard deviation were less than 5.5%, and accuracy (relative error) was less than 3.5%. The mean recovery of 17 β-estradiol samples was 94.4%. The limits of detection and quantification of method for plasma samples were 0.10 μg/ml and 0.15 μg/ml, respectively. Also, clinically used other 10 drugs were investigated to check for potential interferences and the method was successfully applied to the determination of 17 β-estradiol in New Zealand white rabbits.
Keywords
Flame ionization detection, gas chromatography, liquid–liquid extraction, pharmacokinetics, validation, 17 β-estradiol
17 β-estradiol (fig. 1) is the most potent of the natural human estrogens [1]. It is the most potent estrogen of a group of endogenous estrogen steroids which includes estrone and estriol. It is responsible for the growth of breast and reproductive epithelia, maturation of long bones, and development of secondary sexual characteristics. 17 β-estradiol and its semi‑synthetic esters are primarily used as menopausal hormones. It may also be used as replacement therapy for female hypogonadism or primary ovarian failure. The decrease of 17 β-estradiol at menopause is often accompanied by vascular instability and an increasing risk of osteoporosis [2].
Several methods have been reported for the determination of 17 β-estradiol including voltammetry [3], high performance liquid chromatography (HPLC) [1,4‑8], liquid chromatography‑tandem mass spectrometry (LC‑MS‑MS) [9] and gas chromatography‑mass spectrophotometry (GC‑MS) [10‑15].
In addition, no method is reported till date for determination of 17 β-estradiol by gas chromatography‑flame ionization detection (GC‑FID) in rabbit plasma. Therefore, the present work describes the determination of 17 β-estradiol in rabbit plasma. The developed method was validated by sensitivity, linearity, precision, accuracy and stability parameters according to the Center for Drug Evaluation and Research (CDER) guidance for bio‑analytical method validation [16].
The advantages of the present method include simple and single step extraction procedure using inexpensive chemicals and short run time. Also, this method was used to assay the 17 β-estradiol in plasma samples obtained from three rabbits which had been given as an oral tablet of Estrofem tablet (4 mg/kg, 17 β-estradiol).
Materials and Methods
17 β-estradiol and progesterone (internal standard [IS]) were purchased from Sigma (St. Louis, Mo, USA). Ethylacetate, hexane, methanol, ethanol and acetic acid were purchased from Sigma‑Aldrich (St. Louis, MO, USA). All chemicals were of analytical grade. Distilled water was prepared as required using aquaMAX™ ultra, Young instrument (Korea) ultrawater purification system. Estrofem tablet containing 17 β-estradiol was obtained from the local market (Erzurum, Turkey). All gases were supplied by Havas (Erzurum, Turkey).
Chromatographic analyses were carried out on a Agilent 6890N Network gas chromatography system equipped with a flame ionization detector. HP‑5 column with 0.25 μm film thickness (30 m×0.320 mm I.D., USA) was used for separation.
Injection and detector temperature is 250 and 300°, respectively. Splitless injection was used. The carrier gas (N2) flow‑rate was kept constant during the run at 2 ml/min. Nitrogen (30 ml/min), hydrogen (44 ml/min) and dry air (400 ml/min) were used as auxiliary gases for the flame ionization detector.
Preparation of the standard and quality control solutions
Calibration standards of 17 β-estradiol at concentrations of 0.25, 0.50, 0.75, 1.25, 2.5, 5, 7.5, 10, 12.5, 15 and 20 μg/ml were prepared by spiking appropriate amount of the standard solutions in blank plasma. Standard solutions were stored at –20°. IS stock solution was made at an initial concentration of 100 μg/ml. The IS working solution (30 μg/ml) was made from the stock solution using methanol dilution.
The concentrations of 17 β-estradiol were 1, 9 and 17.5 μg/ml rabbit plasma to represent low, middle and high quality controls, respectively. Appropriate volumes from stock solution of 17 β-estradiol were added to normal rabbit plasma to get low, middle and high quality control samples, respectively, and stored at –20°. The quality control samples were taken out from storage for analysis to determine intra‑ and inter‑day precision and accuracy.
Plasma sample preparation procedure
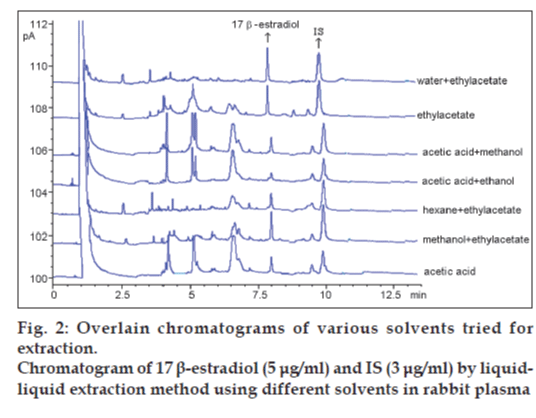
Various solvents were tested for the extraction of 17 β-estradiol in our laboratory. They were water+ethylacetate, ethylacetate, acetic acid+methanol, acetic acid+ethanol, hexane+ethylacetate, methanol+ ethylacetate and acetic acid (fig. 2). Water and ethylacetate solvent mixture was selected for liquid– liquid extraction method as shown in Table 1.
| Solvent | % Recovery |
|---|---|
| Water+ethylacetate | 97.4 |
| Ethylacetate | 92.8 |
| Acetic acid+methanol | 72.4 |
| Acetic acid+ethanol | 84.5 |
| Hexane+ethylacetate | 84.3 |
| Methanol+ethylacetate | 86.9 |
| Acetic acid | 69.8 |
Table 1: Recoveries of 17 β‑estradiol byliquid‑liquid extraction method using different solvents in rabbit plasma
Ethylacetate is an extensively used extracting solvent because of its high polarity and volatility [14]. The solvent ethylacetate gave good recovery and the absolute recoveries of 17 β-estradiol and IS from rabbit plasma. No interfering peak and ghost peak were detected in the blank rabbit plasma in analysis (fig. 3c).
The use of IS is very important. When, IS is not available commercially, an alternative approach has been used. IS chosen should match the chromatographic properties, recovery and chemical properties of the analyte. Progesteron was found to match these criteria, and therefore was chosen as an IS. Other IS’s such as growth hormone, estriol, diazepam, atenolol, carbamazepine, a‑tocopherol, medazepam and prilocaine were also tried, but were rejected because of their low recovery and inefficient extraction. Progesteron was selected because of its high recovery as IS. Good chromatographic separation was obtained.
Blood samples were collected into the tubes containing disodium ethylenediaminetetraacetic acid (EDTA) and centrifuged at 4500×g for 10 min. A 0.2 ml of the resultant plasma samples was spiked with 0.1 ml of 17 β-estradiol and 0.1 ml of IS. After vortex mixing for 5 s, 1.9 ml water and then 5 ml ethyl acetate was added. The mixture was vortexed for 10 s and then centrifuged at 3000×g for 3 min. The organic phase was transferred into another tube and evaporated to dryness at room temperature under nitrogen gas. The dry residue was dissolved in 1 ml methanol. Two microlitre volumes were injected into the GC‑FID system.
Method development
The method development for the assay of 17 β-estradiol was based on its chemical properties. The column and acquisition parameters were chosen to be a starting point for the method development. 17 β-estradiol is a polar molecule. Therefore, the capillary column coated with 5% phenyl and 95% dimethylpolysiloxane is an adequate choice for separation of 17 β-estradiol.
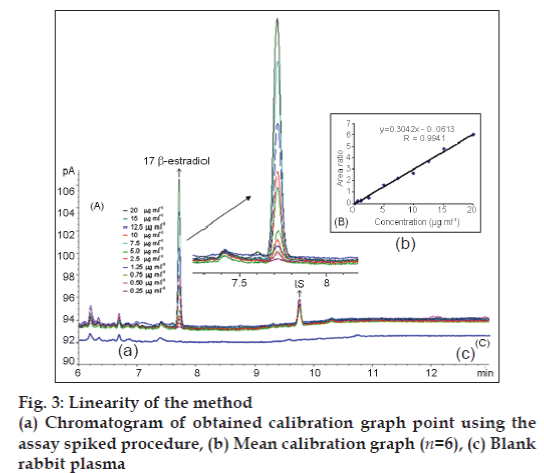
The GC‑FID parameters used in the method development were based on the boiling point. The injection port and detector temperature was set to 250 and 300°, respectively [14]. Different temperature programs were investigated for method. At the end of this investigation, the temperature program of the GC‑FID was as follows: Initial temperature was 150°, held for 1.5 min, increased to 260° at a rate of 50°/ min held for 5 min, and finally to 270°/min at a rate of 10°/min and held for 3.3 min. The head pressure was set to ensure a hydrogen flow of 44 ml/min. The splitless mode was chosen. The solvent, column and acquisition parameters were chosen to be a starting point for the method development. In addition, preliminary precision and linearity studies performed during the development of the method showed that the 2 μl injection volume was reproducible and the peak response was significant at the analytical concentration chosen. Typical 17 β-estradiol and IS chromatogram obtained in rabbit plasma is presented in fig. 3.
Specifity
It was determined that liquid‑phase extraction process was necessary at the sample preparation procedure. Several solvents (ethylacetate, hexane, dichloromethane, acetonitrile, butanol and chloroform) were tested for the extraction. Finally, ethylacetate and water mixture proved to be the most efficient in extracting 17 β-estradiol from rabbit plasma. There was no interference in the chromatogram of blank rabbit plasma. The retention times of 17 β-estradiol and IS in plasma were approximately 7.8 and 9.8 min, respectively (fig. 3a, c).
Linearity
Eleven 17 β-estradiol standard samples at different concentration levels ranging from 0.25 to 20 μg/ml with constant concentration of IS (3 μg/ml) in spiked rabbit plasma samples were prepared and injected onto the GC‑FID system. The calibration curve (fig. 3b) was established by plotting the ratio of the peak areas of 17 β-estradiol and IS obtained after extraction of the spiked rabbit plasma sample. The regression equations were calculated from the calibration graphs, along with the standard deviations of the slope and intercept on the ordinate [Table 2].
| Linearity (µg/ml) | 0.25–20 |
| Regression equationa | y=0.3042x–0.0613 |
| Standard deviation of slope | 8.99×10–3 |
| Standard deviation of intercept | 3.14×10–2 |
| Correlation coefficient | 0.9941 |
| Standard deviation of correlation coefficient | 1.50×10–3 |
| Limit of detection (µg/ml) | 0.10 |
| Limit of quantification (µg/ml) | 0.15 |
aBased on six calibration curves, y: peak‑area ratio, x: 17 b‑estradiol concentration (µg/ml)
Table 2: Linearity of 17 β‑estradiol in rabbitplasma
Precision and accuracy
The precision of the method was determined by repeatability (intra‑day) and intermediate precision (inter‑day). Repeatability was evaluated by analysing spiked blank rabbit plasma six times per day, at three different concentrations (1, 9, 17.5 μg/ml). The intermediate precision was evaluated by analysing the same plasma samples once daily for 3 days. The relative standard deviation (RSD) of the predicted concentrations from the regression equation was taken as precision. The accuracy of this method was assessed as the percentage relative error (% relative error= [(found-added concentration)/added concentration]×100). For all the concentrations studied, intra‑ and inter‑day RSD values were ≤5.15% and for all concentrations of 17 β-estradiol the relative errors were ≤3.43%. These results are given in Table 3.
| Added (µg/ml) | Intra‑day | Inter‑day | ||||
|---|---|---|---|---|---|---|
| Found (Mean±SDa) | Precision% RSDb | Accuracyc | Found (Mean±SDa) | Precision % RSDb | Accuracyc | |
| 1 | 0.98±0.046 | 4.69 | -2.00 | 0.97±0.050 | 5.15 | -3.00 |
| 9 | 9.20±0.285 | 3.09 | 2.22 | 9.30±0.446 | 4.79 | 3.33 |
| 17.5 | 16.90±0.439 | 2.59 | -3.43 | 17.10±0.633 | 3.70 | -2.34 |
SDa = Standard deviation of six replicate determinations; RSD = Relative standard deviation; bAverage of six replicate determinations; Accuracyc = (% relative error) (found‑added)/added ×100
Table 3: Precision And Accuracy Of 17β-Estradiol In Rabbit Plasma (N=6)
Limits of detection and quantification
The limit of detection (LOD) is a parameter that provides the lowest concentration in a sample that can be detected from background noise, but not quantitated. The limit of quantification (LOQ) is defined as the lowest concentration of analyte that can be determined with acceptable precision and accuracy. The LOD and LOQ were studied to test the sensitivity of the method. The LOD was defined as a signal/noise ratio of 3:1 and the LOQ was with a S/N ratio of 10 [17]. The LOD and LOQ values for 17 β-estradiol were found to be 0.10 and 0.15 μg/ml, respectively (Table 2).
Recovery
The analytical recovery of 17 β-estradiol from rabbit plasma was assessed by direct comparison of concentrations of 17 β-estradiol obtained after the whole extraction procedure using six replicate at 11 concentrations levels (0.25, 0.50, 0.75, 1.25, 2.5, 5, 10, 12.5, 15 and 20 μg/ml) in the calibration graph versus standard 17 β-estradiol solutions. The extraction recoveries of 17 β-estradiol from rabbit plasma were between 88.0 and 101.6% as shown in Table 4.
| Added (µg/ml) | Found (Mean±SDa) | % Recovery | % RSDb |
|---|---|---|---|
| 0.25 | 0.22±0.014 | 88.0 | 6.36 |
| 0.50 | 0.46±0.026 | 92.0 | 5.65 |
| 0.75 | 0.72±0.037 | 96.0 | 5.14 |
| 1.25 | 1.16±0.049 | 92.8 | 4.22 |
| 2.5 | 2.36±0.108 | 94.4 | 4.58 |
| 5 | 4.78±0.185 | 95.6 | 3.87 |
| 10 | 10.16±0.369 | 101.6 | 3.63 |
| 12.5 | 11.85±0.472 | 94.8 | 3.98 |
SDa: Standard deviation of six replicate determinations RSD: Relative standard deviation bAverage of six replicate determinations
Table 4: Recovery Of 17 Β‑Estradiol In Rabbitplasma (N=6)
Stability
For the determination of the stability of 17 β-estradiol in plasma at room temperature, 4° and –20° refrigeration temperature, low (2.5 μg/ml) and high (15 μg/ml). 17 β-estradiol concentrations were kept for 24 h and 3 days. Then the stability measurements were carried out. Rabbit plasma samples were found to be stable after 3 days with no significant change in concentration when stored at 4 and –20°. The results of these stability studies are given in Table 5, where the percent recovery ratios are within the acceptance range of 90-110%.
| Concentration (mg/ml) | Room temperature 24 h | Room temperature 72 h | Refrigaratory 4°,24 h | Refrigaratory 4°,72 h | Frozen -20°, 24 h | Frozen -20°, 72 h |
|---|---|---|---|---|---|---|
| 2.5 | 2.44±5.12 | 2.43±7.18 | 2.46±3.16 | 2.44±5.23 | 2.47±2.54 | 2.47±4.97 |
| 15 | 14.9±4.36 | 14.8±6.29 | 15.2±3.46 | 15.45±4.62 | 15.17±2.89 | 15.34±3.26 |
Table 5: Stability of 17 β‑estradiol in rabbit plasma
Interference study.
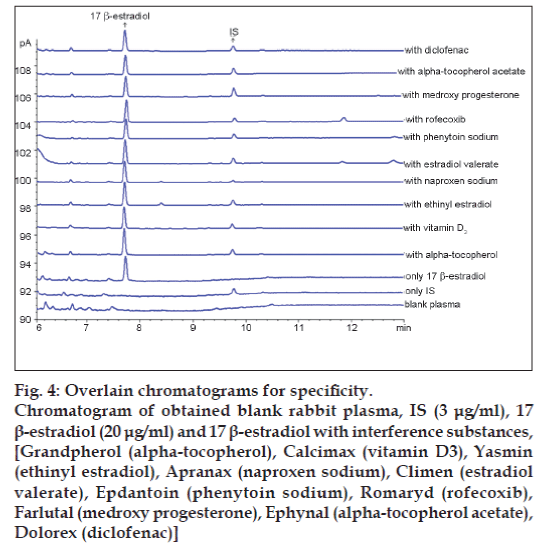
In order to assess the possible analytical applications of the proposed method, the effect of common drugs used in patients was studied by analysing spiked plasma samples. Commonly prescribed drugs Grandpherol (alpha‑tocopherol), Calcimax (vitamin D3), Yasmin (ethinyl estradiol), Apranax (naproxen sodium), Climen (estradiol valerate), Epdantoin (phenytoin sodium), Romaryd (rofecoxib), Farlutal (medroxy progesterone), Ephynal (alpha‑tocopherol acetate), Dolorex (diclofenac) were analysed for possible interference. No interference was observed under the chromatographic conditions. These results are given in fig. 4.
Figure 4: Overlain chromatograms for specificity.
Chromatogram of obtained blank rabbit plasma, IS (3 μg/ml), 17β-estradiol (20 μg/ml) and 17 β-estradiol with interference substances, [Grandpherol (alpha-tocopherol), Calcimax (vitamin D3), Yasmin
(ethinyl estradiol), Apranax (naproxen sodium), Climen (estradiol valerate), Epdantoin (phenytoin sodium), Romaryd (rofecoxib), Farlutal (medroxy progesterone), Ephynal (alpha-tocopherol acetate), Dolorex (diclofenac)]
Application of the method
Prior to the study, the ethical protocol was approved by the Ethical Committee for Medical Experimental Research and Application Centre, Ataturk University.
Three rabbits were housed with free access to food and water, except for the final 2 h before the experiment. After a single oral administration of 4 mg/kg of Estrofem tablet, 1.0 ml of blood samples were collected from the marginal ear vein at 0, 15, 30, 60, 120, 180, 240, 360, 420 and 480 min time‑points into EDTA collection tubes. The blood was immediately centrifuged 6000 rpm for 10 min at ambient temperature. The supernatant plasma layer was separated and analysed for 17 β-estradiol concentrations as described above.
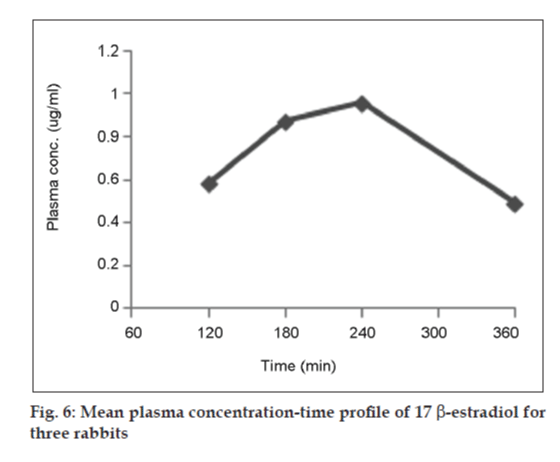
The maximum plasma concentration (Cmax) and the time to reach maximum concentration (Tmax) were directly determined from the plasma concentration versus time curves. The area under the curve from 0 to t (AUC0‑t) was calculated by the linear trapezoidal rule. The area under the curve from 0 h to infinity (AUC0‑∞) was estimated by summing the area from 0 to t (AUC0‑t) and t to infinity (AUCt‑∞), where AUCt‑∞= Ct/Kel, with Ct defined as the last measured plasma concentration at time t, and kel the slope of the terminal portion of the ln (plasma concentration) versus time curve. The elimination half‑life (t1/2) was calculated using the pharmacokinetic relationship t1/2 = ln (2)/kel [18].
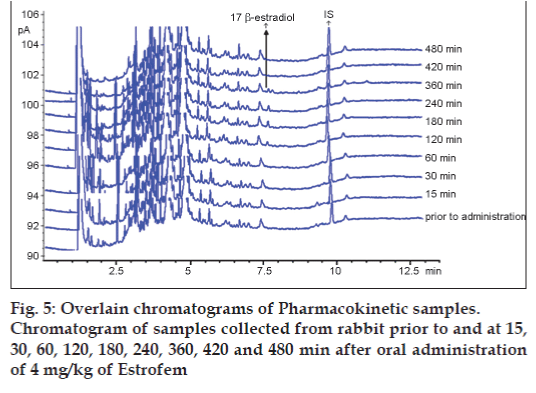
GC‑FID chromatogram of rabbit plasma after oral administration of Estrofem tablet is shown in fig. 5. Representative mean plasma concentrations versus time profiles following oral administration of 17 β-estradiol to three rabbits are presented in fig. 6.
Results and Discussion
Ingrand et al. [9] has reported LC method with tandem mass detection for the analysis of 17‑β estradiol in in effluents of wastewater treatment plants. 17‑β estradiol is extracted via solid‑phase extraction using C18 cartridge. The calibration curve of LC‑MS‑MS method was linear for 17‑β estradiol in the range 10-50 ng/l. Intra‑ and inter‑day precision values were lower than 15%. The maximum recovery of 17‑β estradiol was 84%. The LOQ of method was found 10 ng/l. Detection using LC‑MS‑MS would be a more sensitive approach, but is costly and not yet available for every laboratory.
GC‑FID method (proposed method) is a little simpler and faster with respect to the deproteinization step and analysis time. In this study, the recovery percentage of 17‑β estradiol is higher than other reported method [9,10]. There is not a derivatization process. Also, the extraction processes do not take much time [4,10‑13]; additionally, the retention time is short which is an advantage [4,10,12,13].
17‑β estradiol was extracted with a solid phase extraction procedure in other techniques [4,10‑13]. These methods are also the most comprehensive method which can extract 17‑β estradiol in a single extraction procedure.
In conclusion, a new rapid, simple GC‑FID method has been developed for the determination of 17 β-estradiol in rabbit plasma. Also, the method was completely validated by sensitivity, stability, specificity, linearity, accuracy and precision parameters for determination of 17 β-estradiol in rabbit plasma. The method was found to be linear over an analytical range of 0.25-20 μg/ml. Additional advantages of this method include small sample volume (0.2 ml), good extraction recovery from plasma and a readily available IS. To our knowledge, this is the first description of 17 β-estradiol pharmacokinetics in rabbit plasma by GC‑FID method in the literature. It can be very useful and an alternate to performing pharmacokinetic studies in determination of 17 β-estradiol for clinical use.
Acknowledgements
The authors are grateful to the University of Ataturk for the financial support of this work.
References
- Russell JA, Malcolm RK, Campbell K, Woolfson AD. High‑performance liquid chromatographic determination of 17β-estradiol and 17β-estradiol‑3‑acetate solubilities and diffusion coefficents in silicone elastromericintravaginal rings. J Chromatogr B Biomed SciAppl 2000;744:157‑63.
- Havlíková L, Nováková L, Matysová L, Sícha J, Solich P. Determination of estradiol and its degradation products by liquid chromatography. J Chromatogr A 2006;1119:216‑23.
- Salci B, Biryol I. Voltammetric investigation of beta‑estradiol. J Pharm Biomed Anal 2002;28:753‑9.
- López de Alda MJ, Barceló D. Determination of steroid sex hormones and related synthetic compounds considered as endocrine disrupters in water by fully automated on‑line solid‑phase extraction‑liquid chromatography‑diode array detection. J Chromatogr A 2001;911:203‑10.
- Kimura A, Taguchi M, Arai H, Hiratsuka H, Namba H, Kojima T. Radiation‑induced decomposition of trace amounts of β-estradiol in water. RadiatPhysChem 2004;69:295‑301.
- Terada H, Yamamoto K, Miyabe M. Determination of corticosteroids and anabolic agents in health food by high performance liquid chromatography. Japan J Toxicol and Environ Health 1992;38:537‑44.
- Mao L, Sun C, Zhang H, Wang X, Li Y, Wu D. Determination of 17α‑estradiol and 17β‑estradiol in urine by high performance liquid chromatography with pre‑column derivatization. FenxiHuaxue 2003;31:1446‑9.
- Nygaard L, DrøhseKilde H, Andersen SG, Henriksen L, Overby V. Development and validation of a reversed‑phase liquid chromatographic method for analysis of degradation products of estradiol in Vagifem tablets. J Pharm Biomed Anal 2004;34:265‑76.
- Ingrand V, Herry G, Beausse J, de Roubin MR. Analysis of steroid hormones in effluents of wastewater treatment plants by liquid chromatography‑tandem mass spectrometry. J Chromatogr A 2003;1020:99‑104.
- Zacharia LC, Dubey RK, Jackson EK. A gas chromatography/mass spectrometry assay to measure estradiol, catecholestradiols, and methoxyestradiols in plasma. Steroids 2004;69:255‑61.
- Fotsis T, Adlercreutz H. The multicomponent analysis of estrogens in urine by ion exchange chromatography and GC‑MS‑I. Quantitation of estrogens after initial hydrolysis of conjugates. J Steroid Biochem 1987;28:203‑13.
- Quintana JB, Carpinteiro J, Rodríguez I, Lorenzo RA, Carro AM, Cela R. Determination of natural and synthetic estrogens in water by gas chromatography with mass spectrometric detection. J Chromatogr A 2004;1024:177‑85.
- Kawaguchi M, Ito R, Sakui N, Okanouchi N, Saito K, Nakazawa H. Dual derivatization‑stir bar sorptive extraction–thermal desorption–gas chromatography‑mass spectrometry for determination of 17 β-estradiol in water sample. J Chromatogr A 2006;1105:140‑7.
- Fedeniuk RW, Boison JO, MacNeil JD. Validation of a gas chromatography‑mass spectrometry method for the determination of pg/ml levels of 17beta‑estradiol and 17beta‑trenbolone in bovine serum. J Chromatogr B AnalytTechnol Biomed Life Sci 2004;802:307‑15.
- Watabe Y, Kubo T, Nishikawa T, Fujita T, Kaya K, Hosoya K. Fully automated liquid chromatography‑mass spectrometry determination of 17beta‑estradiol in river water. J Chromatogr A 2006;1120:252‑9.
- Center for Drug Evaluation, and Research (CDER). Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2001.
- Proceedings of the International Conference on Harmonization (ICH), Commission of the European Communities; 1996.
- Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker; 1990.