- *Corresponding Author:
- Mangal S. Nagarsenker
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Santacruz (East), Mumbai-400 098, India
E-mail: mangal_nag511@yahoo.co.in
| Date of Submission | 20 February 2010 |
| Date of Revision | 5 May 2010 |
| Date of Acceptance | 21 September 2010 |
| Indian J Pharm Sci 2010, 72 (5): 637-640 |
Abstract
The objective of the present investigation was to develop a parenteral microemulsion delivering artemether, a hydrophobic antimalarial drug and to evaluate antimalarial activity of the microemulsion in comparison to the marketed oily injection of artemether (Larither® ). The microemulsion was evaluated for various parameters such as globule size, ability to withstand centrifugation and freeze-thaw cycling and effect of sterilization method on the drug content and globule size. The in vivo antimalarial activity of the microemulsion was evaluated in P. berghei infected mice in comparison to the Larither; . The stability of the microemulsion was evaluated at 5º for 1 month. The microemulsion exhibited globule size of 113 nm and it could successfully withstand centrifugation and freeze-thaw cycling. The method of sterilization did not have any significant effect on the artemether content and globule size of the microemulsion. The microemulsion showed around 1.5-fold higher antimalarial activity and higher survival as compared to that of marketed artemether injection Larither® and it showed a good stability at the end of 1 month.
Keywords
Artemether, poor water solubility, microemulsion, parenteral delivery
Artemether (ARM) is a potent and rapidly acting antimalarial agent which is used as fi rst line therapy for the treatment of severe multi-drug resistant malaria [1,2]. It is effective against Plasmodium vivax as well as chloroquine-sensitive and chloroquine-resistant strains of P. falciparum and is enlisted in WHO list of Essential Medicines (WHO web site). Although ARM has good therapeutic potential in malaria, its poor aqueous solubility is a major limiting factor that hampers clinical effi cacy of ARM [3]. Currently, ARM is available as capsules for oral therapy and as an intramuscular (IM) oily injection for the treatment of severe malarial infections. Currently available oily IM injection of ARM causes considerable pain to patients on injection and it also shows slow and erratic absorption of ARM on IM administration [4]. Thus, development of a parenteral formulation of ARM to achieve effective and optimal utilization of ARM is needed for quick eradication of the malarial infection. It has been demonstrated that IV delivery of ARM results in the highest availability to body as compared to all other routes such as IM and intraperitoneal (IP) and can lead to quick eradication of the malarial infection [5]. However, the oily nature of current parenteral formulation of ARM makes it unsuitable for administration by intravenous (IV) route. Hence, the objective of this investigation was to develop a suitable IV formulation of ARM that enables its quick availability to the body with concomitant reduction in the pain on injection. Researchers have explored the potential of other novel delivery approaches such as liposomes [6] and nanostructured lipid carriers [7] to enable IV delivery of ARM and also to improve the therapeutic effi cacy of ARM. However, the utility of the microemulsion approach has not been established for improving the delivery of ARM. Microemulsions have gained a great interest as a drug delivery vehicle for hydrophobic drugs since last 2 decades. Their applications in oral [8], topical [9], nasal [10] and parenteral [11] delivery of hydrophobic drugs have been well established. In view of this, we aimed at developing aqua based microemulsion of ARM which can be administered by IV route. The microemulsion of ARM was evaluated for physical stability and also for its ability to improve anti-malarial activity of ARM as compared to the commercially available oily solution of ARM.
Artemether was obtained from IPCA Ltd., Mumbai, India, Crodamol EO (ethyl oleate) was from Croda India, Mumbai, India, Epikuron 200 was procured from Degussa, Germany, and Labrasol from Gattefosse India, Mumbai, India and all these were received as gift samples. Dextrose, ethanol, toluene, Cholesterol and chloroform (AR grade) were purchased from Qualigens, Mumbai, India. All the materials were used as received without any purifi cation. Larither®, IPCA Ltd., Mumbai, India, was procured from a local pharmacy store. Double distilled water was prepared freshly whenever required.
Based on the phase diagrams and preliminary solubility studies on ARM (data not shown), a microemulsion composition that could solubilize ARM to yield a concentration of 20 mg/ml was selected. Briefly, a suitable quantity of ARM was dissolved in a mixture of Crodamol EO, Labrasol, ethanol and Epikuron. This homogenous mixture was diluted with water or suitable parenteral vehicle (5% dextrose) to yield a microemulsion. The composition of the microemulsion is listed in Table 1. The average globule size and polydispersity index (PI) of microemulsion were determined (n=3) by the photon correlation spectroscopy (PCS; Beckman Coulter N5, Wipro, India). Microemulsion was diluted with double distilled water to ensure that the light scattering intensity (between 6e+004 to 1e+006), was within the instrument’s sensitivity range. Measurements were made at an angle of 900 for all the microemulsions. ARM microemulsion was subjected to various stress tests such as centrifugation at 5000 rpm for 20 min and freeze-thaw cycling. The globule size and physical stability of the ARM microemulsion after these stress tests was evaluated. The drug content of the microemulsion was evaluated by a previously reported validated HPTLC method [12].
| Components | Composition (% w/v) |
|---|---|
| Epikuron 200 | 4.1 % |
| Lutrol F68 | 2.5 % |
| Labrasol+Ethanol (7:3 v/v) | 16.4 % |
| Ethyl oleate | 10.3% |
| SWFI q.s.* | 100 ml |
Table 1: Composition of Artemether Microemulsion
The ARM microemulsion was sterilized by fi ltration and autoclaving and the effect of the sterilization method on the globule size and ARM content of the microemulsion was evaluated in triplicate.
The protocol for animal studies was approved by the Institutional Animal Ethics Committee of Bombay College of Pharmacy. The study design was based on papers reported by Chimanuka et al. and Joshi et al. with slight modifications [6,7]. The lethal ANKA strain of Plasmodium berghei was used for the experiments. In-house bred and mycoplasma free male Swiss mice (weighing around 25 g each) were infected by intraperitoneal inoculation of donor mouse blood diluted in acid citrate dextrose (ACD) buffer containing approximately 106 infected RBCs on day ‘0’. The mice were randomly divided into following groups (n=6 per group); Group 1 was control (no treatment), Group 2 was marketed ARM formulation (Larither®), Group 3 was blank microemulsion and Group 4 was ARM microemulsion.
On day 4 post infection when the mean parasitemia reached a level of 6.3% (±1.5%), Group 2, and 4 were administered with Larither® and ARM microemulsion (equivalent to 4 mg/kg of ARM) by intraperitoneal route. The dose of the ARM used for animal studies was same as that of Joshi et al. [7]. Group 3 received 100 μl of blank microemulsion. On day 8, blood was withdrawn from the tail vein and the blood smears were prepared. Blood smears were fixed with methanol and stained with Giemsa’s stain and the parasites were counted. Parasitemia was reported as percentage parasitemia after counting 1000 RBCs from each slide. The number of surviving animals in all the groups was recorded on the 31st day. Data was expressed as mean±SD and parasitemia of different groups were statistically assessed by the unpaired t test performed using Graphpad Instat software. Differences were considered signifi cant at P<0.05.
Chemical and physical stability of ARM micreomulsion was assessed at 5±3º for 1 month. ARM microemulsion was stored in glass vials with rubber stoppers and aluminum-crimped tops. Samples were removed at 0 and 30 days and assessed for ARM content. Physical stability of microemulsion was determined by monitoring mean globule size and polydispersity index. The data obtained about the ARM content and mean globule size of the microemulsion was subjected to statistical analysis. The statistical signifi cance of differences in the data was analyzed utilizing analysis of variance (ANOVA) followed by Bonferroni’s test (GraphPad InStat). Differences were considered statistically signifi cant at P<0.05.
Preliminary solubility studies indicated that ARM has good and nearly similar solubility in oily phases such as fixed oils, medium chain triglycerides and ethyl oleate (Crodamol EO). Hence, the selection of the oily phase for the microemulsion was done on the basis of the ease of microemulsification of the various oils. It is known that ethyl oleate can be easily microemulsifi ed as compared to the fi xed oils and medium chain triglycerides due to its low molecular volume [11]. Hence, it was selected as an oily phase of the microemulsion. Epikuron 200 (lecithin), Lutrol F68 (poloxamer 188), Labrasol and ethanol are known to have good parenteral acceptability/hemocompatibility [13]. The phase behavior of lecithin+poloxamer-labrasol+ethanol-ethyl oleate system has already been presented by us earlier [14]. The microemulsion could be spontaneously formulated with a fairly good ARM loading of 20 mg/ml. The microemulsion exhibited yellow color due to presence of Epikuron 200. The mean globule size of the microemulsion was found to be 113 nm and the polydispersity index was 0.7 (n=3). The mean globule size was not signifi cantly changed when the aqueous phase of the microemulsion was replaced with 5% dextrose solution. The various stress tests such as centrifugation and freeze thaw cycling did not affect the physical stability and the globule size of the ARM loaded microemulsion. The percent ARM content of the microemulsion was 99.2±1.5% (n=3). Various sterilization methods such as autoclaving and fi ltration did not affect the physical stability, globule size and ARM content of the microemulsion. It is well known fact that phospholipid based emulsions can withstand autoclaving whereas polyoxyethylene based surfactants can offer protection from freeze thaw cycles [15]. Since, the microemulsion described herein contains phospholipid and polyoxyethylene based surfactant (Poloxamer), it could withstand autoclaving and freeze thaw cycling.
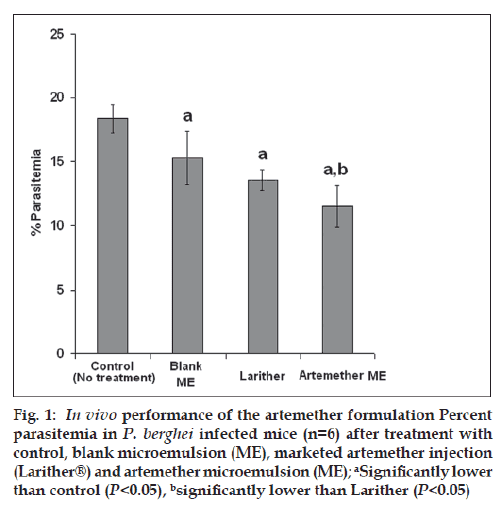
The effi cacy of the ARM microemulsion and marketed formulation (Larither®) with respect to reduction in percent parasitemia is depicted in fig. 1. As shown in fig. 1, the control group (no treatment) showed highest parasitemia, validating the animal model used for the study. As compared to the control group, all the other groups showed reduction in the parasitemia during the course of study (P<0.05). Interestingly, ARM microemulsion showed highest reduction in the parasitemia (or highest antimalarial activity) as compared to all other groups (P<0.05). The higher antimalarial activity of the microemulsion could be attributed to the quick and complete release of ARM from nanostructure of microemulsion on contact with blood enabling optimal utilization of ARM for antimalarial activity. On the contrary, oily injection results in incomplete and sustained release of ARM. Furthermore, the availability of the ARM from oily injection is erratic and can vary greatly from individual to individual. All these factors considerably limit the therapeutic efficacy of the oily formulation. The utility of the microemulsion is further substantiated by the results of the survival study. The marketed formulation showed 50% survival rate whereas ARM microemulsion showed ~66% survival.
Figure 1: In vivo performance of the artemether formulation Percent parasitemia in P. berghei infected mice (n=6) after treatment with control, blank microemulsion (ME), marketed artemether injection (Larither®) and artemether microemulsion (ME); aSignificantly lower than control (P<0.05), bsignificantly lower than Larither (P<0.05)
Thus, microemulsion reported in the present investigation is expected to offer signifi cant advantage over current oily injectable formulation. The components used in the current microemulsion are easily available and are cost effective. Additionally, the microemulsion described in the present investigation can be easily formulated by simple mixing which indicates that microemulsion approach is commercially viable. Furthermore, microemulsions are easy to fabricate and are cost effective as compared to liposomes and they would not face the regulatory issues unlike nanostructured lipid carriers. Hence, microemulsion may have an edge over other novel strategies which have been explored earlier.
It should be noted that all formulations namely, ARM microemulsion, blank microemulsion and marketed formulation were administered by intraperitoneal route instead of IV route. Since marketed formulation can not be administered by IV route, it would be difficult to compare the marketed formulation with the microemulsion. Hence, intraperitoneal route was employed for comparative evaluation. Future studies would be focused on the antimalarial effi cacy of ARM microemulsion on IV administration.
The ARM microemulsion did not show any reduction in the ARM content at the end 1 month when stored at 5±3º. There were no signs of any physical instability and the mean globule size and polydispersity index of the ARM microemulsion were unchanged at the end of the 1 month.
The utility of microemulsion approach in improving the in vivo antimalarial activity of artemether was successfully established. The ease of preparation and commercial viability of the microemulsion approach indicate that it can be a good alternative to the currently available injectable formulation of artemether.
Acknowledgements
Authors are thankful to IPCA Ltd., Degussa GmBH, Gattefosse India Ltd. for the gift samples of drug and various excipients. The authors are thankful to Dr. Abhijit Date for his help in compiling the manuscript. Nitin Tayade is thankful to Indian Council of Medical Research for providing Senior Research Fellowship.
References
- Lefèvre G, Thomsen MS. Clinical Pharmacokinetics of artemether and lumefantrine (Riamet). Clin Drug Invest 1999;18:467-80.
- deVries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 1996;52:818-36.
- Karbwang J, Na-Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur J Clin Pharmacol 1997;52:307-10.
- Hien TT, Davis TM, Chuong LV, Ilett KF, Sinh DX, Phu NH et al. Comparative pharmacokinetics of intramuscular artesunate andartemether in patients with severe falciparum malaria. Antimicrob Agents Chemother 2004;48:4234-9.
- Li G, Peggins JO, Fleckenstein LL, Masonic K, Heiffer MH, Brewer TG. Pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J Pharm Pharmacol 1998;50:173-82.
- Chimanuka B, Gabriels M, Detaevernier MR, Plaizier-Vercammen JA. Preparation of -artemether liposomes, their HPLC-UV evaluation and relevance for clearing recrudescent parasitemia in Plasmodium chabaudi malaria-infected mice. J Pharm Biomed Anal 2002;28:13-22.
- Joshi M, Pathak S, Sharma S, Patravale V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: NanojectInt J Pharm 2008;364:119-26.
- Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 2000;45:89-121.
- Date AA, Patravale VB. Microemulsions: Applications in transdermal and dermal delivery. Crit Rev Ther Drug Carrier Syst 2007;24:547-96.
- Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Intranasal mucoadhesive microemulsions of clonazepam: Preliminary studies on brain targeting. J Pharm Sci 2006;95:570-80.
- Date AA, Nagarsenker MS. Parenteral microemulsions: an overview. Int J Pharm 2008;355:19-30.
- Tayade NG, Nagarsenker MS. Validated HPTLC method of analysis for artemether and its formulations. J Pharm Biomed Anal 2007;43:839-44.
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res 2004;21:201-30.
- Tayade NG, Nagarsenker MS. Formulation and characterization of microemulsions of artemether for parenteral use. In: Proceedings, AAPS Annual Meet and Exposition, USA, Abstract No. 1210 (2004).
- Jumaa M, Mueller BW. Parenteral emulsions stabilized with a mixture of phospholipids and PEG-660-12-hydroxy-stearate: Evaluation of accelerated and long-term stability. Eur J Pharm Biopharm 2002;54:207-212.