- *Corresponding Author:
- Meenakshi S. Akhade
Medicinal Natural Products Research Laboratory, Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Matunga (E), Mumbai-400 019, India
E-mail: meenakshiakhade@gmail.com
| Date of Submission | 28 January 2013 |
| Date of Revision | 04 June 2013 |
| Date of Acceptance | 13 June 2013 |
| Indian J Pharm Sci 2013;75(4):476-482 |
Abstract
The aim of the present work was to develop and validate a reversed-phase high-performance liquid chromatography method for the simultaneous estimation of picroside I, plumbagin, and Z-guggulsterone in a polyherbal formulation containing Picrorhiza kurroa, Plumbago zeylanica, and Commiphora wightii extracts. The analysis was performed on a C18 column using the mobile phase consisting of solvent A (acetonitrile) and solvent B (0.1% orthophosphoric acid in water) with the following gradient: 0-12 min, 25% A; 12-17 min, 25-80% A; 17-32 min, 80% A; and 32-37 min, 80-25% A at a flow rate of 1 ml/min. Ultraviolet detection was at 255 nm. The method was validated for accuracy, precision, linearity, specificity, and sensitivity as per the norms of the International Conference on Harmonization. From the validation study, it was found that the method is specific, accurate, precise, reliable, and reproducible. Good linear correlation coefficients (r 2 >0.900) were obtained for calibration plots in the ranges tested. Limits of detection were 2.700, 0.090 and 0.099 μg/ml and limits of quantification were 9.003, 0.310, and 0.330 μg/ml for picroside I, plumbagin, and Z-guggulsterone, respectively. Intra and interday relative standard deviation (RSD) of retention times and peak areas was less than 3.0%. Recovery was found to be 100.21% for picroside I, 102.5% for plumbagin, and 103.84% for Z-guggulsterone. The established method was appropriate and the three markers were well resolved, enabling efficient quantitative analysis of picroside I, plumbagin and Z-guggulsterone. The method is a rapid and cost-effective quality control tool for routine quantitative analysis of picroside I, plumbagin, and Z-guggulsterone in tablet dosage form.
Keywords
Picroside I, plumbagin, Z-guggulsterone, Picrorhiza kurroa, Plumbago zeylanica, Commiphora wightii, high-performance liquid chromatography
For the past few decades, compounds from natural sources have been gaining importance because of the vast chemical diversity that they offer. This has led to a phenomenal increase in the demand for herbal medicines in the last two decades, and a need has been felt for ensuring quality, safety, and efficacy of herbal drugs. Phytochemical evaluation is one of the tools for quality assessment, which includes preliminary phytochemical screening, chemo‑profiling, and marker compound analysis using modern analytical techniques. High‑performance liquid chromatography (HPLC) has emerged as a simple, reliable, and efficient method for simultaneous analysis of two or more components.
Natural products have been utilized as an important resource for the maintenance of life for ages. Picrorhiza kurroa Royles ex Benth (family: Scrophulariaceae) [1], locally known as Kutki, is an important medicinal plant used in traditional and modern medicine for liver disorders as it is reputed for its hepatoprotective activity [2]. It is known for its immunomodulatory effect for cell‑mediated as well as humoral immunity [3] and used in the treatment of asthma [3] and jaundice [4]. It is reported to possess antiperiodic and cholagogue properties [5]. Other activities reported are appetite inducing, purgative, and bile flow enhancing properties, and it is effective in malarial fever [6,7]. Important chemical constituents of the plant are grouped into four categories, namely, iridoid glycosides, phenolics, phenylethanoids, and cucurbitacin glycosides [2]. The iridoids reported to be present in P. kurroa are picroside I (fig. 1) [3], picroside II [8], picroside III [9], picroside IV [10], kutkoside [3], veronicosides [11], and so on. Other identified constituents are apocynin, androsin, and nine cucurbitacin glycosides [9,12].
The whole plant of Plumbago zeylanica (family: Plumbaginaceae) and its roots have been used as folk medicine for the treatment of rheumatic pain, dysmenorrhea, carbuncles, contusion of the extremities, ulcers, and elimination of intestinal parasites [13]. In Africa, it is used in southwestern Nigerian folk medicine for parasitic diseases, scabies, and ulcers [14]. According to the Indian system of medicine, the plant is used in sprue, malabsorption syndrome, piles, and inflammatory diseases of the anorectum [15]. The principle constituents of this plant include naphthoquinones like plumbagin (fig. 1) [16], plumbagic acid and its two glucosides (3’‑O‑β‑glucopyranosyl plumbagic acid and 3’‑O‑β‑glucopyranosyl plumbagic acid methyl ester) [17,18], chitranone [19], maritinone [20], elliptinone and isoshinanolone [21], zeylanone, and isozeylanone [22].
Commiphora wightii (Arnott.) Bhandari, commonly known as guggul, is an important medicinal plant of the herbal heritage of India belonging to the family Burseraceae [23]. The oleo gum resin is traditionally used in the management of hypercholesterolemia and obesity [24‑26]. It is known for analgesic, antiinflammatory, and antiarthritic activities [27,28]. It is reported to possess anticancer properties [29] and is also known for its cardioprotective properties [30].
Other activities reported in guggul are antioxidant and antiplatelet and is reported to be used for angina, skin disorders, urinary disorders, and so on [31‑34]. The main constituent present is a steroid, namely, guggulsterone (C21H28O2) that includes E‑guggulsterone [4,17 (20)‑(cis)‑pregnadiene‑3, 16-dione] and Z‑guggulsterone [4,17 (20)-(trans)- pregnadiene‑3,16‑dione (fig. 1)] [35]. It also shows the presence of guggulsterols [36]. It contains essential oils (0.4%) consisting chiefly of myrcene. The gum resin also showed the presence of long chain aliphatic 1,2,3,4‑tetrols esterified with ferulic acid at the primary hydroxyl function [37,38].
As per the literature survey, no chromatographic or spectrometric method has been reported for the simultaneous estimation of picroside I, plumbagin, and Z‑guggulsterone in combined dosage form; hence, it is essential to develop a chromatographic method for the simultaneous estimation of the three important phytoconstituents in formulation. Many formulations are marketed individually or in combination with other drugs for the above mentioned drugs. As HPLC methods are widely used for routine analysis of drugs because of their sensitivity and accuracy, in the present work, a new, simple, and specific reversed‑phase (RP)‑HPLC method was developed for the simultaneous estimation of these marker compounds in tablet dosage form.
Materials and Methods
Picroside I (purity 98% by HPLC), plumbagin (purity 98% by HPLC), and Z‑guggulsterone (purity 97% by HPLC) were purchased from Sigma‑Aldrich, Bangalore, India. HPLC‑grade methanol and acetonitrile were obtained from Merck India. The water used was double‑distilled. The solvents were filtered through a 0.45 μm filter (Millipore Bedford, MA, USA) and degassed in an ultrasonic bath (Remi Instruments, Mumbai, India) before use. A polyherbal formulation (tablets), Arogyavardhini Gutika (average weight: 550 mg) manufactured by Zandu Emami Ltd., containing P. kurroa (225 mg), P. zeylanica (51.5 mg), and C. wightii (40.9 mg) was procured from a local market.
Chromatographic system and conditions
HPLC analysis was performed with a Jasco (Hachioji, Tokyo, Japan) system consisting of an intelligent pump (PU‑1580, PU‑2080), a high‑pressure mixer (MX‑2080‑31), a manual sample injection valve (Rheodyne 7725i) equipped with a 20 μl loop, and an UV/Vis detector (UV‑1575). The compounds were separated on a Purospher® STAR RP-18 encapped Hibar® column 250×4.6 mm, 5 μm (Merck, Darmstadt, Germany) with the mobile phase consisting of solvent A (acetonitrile) and solvent B (0.1% orthophosphoric acid in water). A constant flow gradient composition of 0-12 min, 25% A; 12-17 min, 25-80% A; 17-32 min, 80% A; and 32-37 min, 80-25% A, was used at a flow rate of 1 ml/min. The injection volume was 20 μl and the detection wavelength was 255 nm. HPLC was performed at ambient temperature and data were analyzed on a computer equipped with Borwin software. Before analysis, both the mobile phase and sample solutions were degassed by the use of a sonicator and filtered through 0.2 mm filter paper. The identities of three compounds were established by comparing retention time of the sample solution with those of standard solutions.
Preparation of standard solution and construction of calibration plots
The standard stock solutions (1 mg/ml) of picroside I, plumbagin, and Z‑guggulsterone were freshly prepared in methanol. From the stock solutions, further dilutions were prepared by diluting the required volume of solution with methanol, and their area was noted by injecting 20 μl into the system. Calibration standard solutions of various concentrations (picroside I: 200, 400, 600, 800, and 1000 μg/ml; plumbagin: 1, 2, 4, 6, and 8 μg/ml; Z‑guggulsterone: 1, 2, 4, 6, 8, and 10 μg/ml) were obtained by appropriate dilution.
Assay of tablet formulation
For analysis of tablet dosage form, 20 tablets were weighed and the average weight was determined. They were crushed to a fine powder, and a powder equivalent to 10 tablets (5.565 g) was weighed and extracted with methanol using Soxhlet apparatus. The extract was concentrated and transferred to 25 ml volumetric flask. Then, the volume was made up to the mark with methanol (stock solution). From the above stock solution, 1 ml was diluted to 10 ml with methanol (sample solution). The amounts of picroside I, plumbagin, and Z‑guggulsterone per tablet were calculated by extrapolating the value of area from the calibration curve. The analysis procedure was repeated three times with the tablet formulation.
Validation
The method was validated as per the guidelines of the International Conference on Harmonization (ICH) [39‑41] for the parameters like linearity, accuracy, precision, limit of detection (LOD), and limit of quantification (LOQ).
Linearity
The linearity of an analytical procedure is its ability to obtain test results within a given range, which are directly proportional to the concentration of the analyte in the sample. The linearity study was done by serially diluting standard stock solutions (1 mg/ml) to a given concentration range as given above. Calibration plots were constructed for all three compounds, after triplicate analysis of each calibration solution, by plotting peak area against concentration (μg/ml) of the corresponding standard solution.
LOD and LOQ
LOD is the lowest amount of analyte in a sample which can be detected but not necessarily quantified. LOQ of an individual analytical procedure is the lowest amount of analyte in a sample which can be quantified. LOD and LOQ were experimentally verified by diluting known concentrations of picroside I, plumbagin, and Z‑guggulsterone until the average response was approximately 3 to 10 times the standard deviation (SD) of response (peak area) for the three replicate determinations.
Precision and accuracy
Precision is the closeness of values between a series of measurements obtained from multiple sampling of the same sample under the prescribed conditions. Precision was determined as the intraday and interday variation of results from the analysis of five different standard solutions. Intraday precision was determined by triplicate analysis of each solution on a single day. Interday precision was determined by triplicate analysis of the solution on two successive days. The relative SD (RSD) of retention time and peak area of all three analytes were calculated as measures of precision and repeatability.
The accuracy of an analytical procedure is the closeness between the conventional true value or an accepted reference value and the value found. The accuracy of the method was determined by application of the standard addition method. Accurately known amounts of the standards (150 and 200 μg/ml for picroside I, 3 and 5 μg/ml for plumbagin, and 2 and 4 μg/ml for Z‑guggulsterone) were added to known concentrations of the formulation (180, 4, and 3 μg/ml for picroside I, plumbagin, and Z‑guggulsterone, respectively). The total amount of each compound was calculated from the corresponding calibration plot and the recovery of each compound was calculated by the use of the equation: Recovery (%)=(amount found−amount contained)/amount added×100.
Results and Discussion
Column chemistry, solvent type, solvent strength (volume fraction of organic solvent (s) in the mobile phase), detection wavelength, and flow rate were varied to determine the chromatographic conditions giving the best separation. The mobile phase conditions were optimized so that the solvent and excipients did not interfere with the components. Other criteria such as the time required for analysis, appropriate range for eluted peaks, assay sensitivity, solvent noise, and use of the same solvent system for extraction of the drug from the formulation matrices during drug analysis were also considered.
The main objective in developing this method is to achieve simultaneous determination of picroside I, plumbagin, and Z‑guggulsterone in tablet formulation under common conditions that will be applicable for routine quality control of the product in laboratories. Trials were carried out using mobile phase with isocratic and gradient pumping system. A series of mobile phases containing different volume fractions of water and acetonitrile as modifiers were also tested. In the isocratic system, the mobile phase used was acetonitrile:water (50:50, 40:60), but the peaks were eluted at high retention time. Hence, the method was not appropriate and therefore, the gradient system was chosen for method development using acetonitrile (solvent A) and water (solvent B) using gradient composition as 0-10 min, 30% A; 10-20 min, 30-80% A; and 20-25 min, 80-90% A. The peak of picroside I was eluted at five minutes; however, the retention time of Z‑guggulsterone and plumbagin were not found to be stable. The final mobile phase consisting of solvent A (acetonitrile) and solvent B (0.1% orthophosphoric acid in water) with gradient 0-12 min, 25% A; 12-17 min, 25-80% A; 17-32 min, 80% A; and 32-37 min, 80-25% A flow was tried. The peaks of picroside I, plumbagin, and Z‑guggulsterone were eluted at 9.53, 24.33, and 27.7 min, respectively with symmetry and well‑retained peaks. The flow rate was determined by testing the effect of different flow rates on the peak area and resolution; a flow rate of 1 ml/min was found to be optimum. All the experiments were carried out at ambient temperature.
To determine the appropriate wavelength for the simultaneous determination of picroside I, plumbagin, and Z‑guggulsterone, solutions of these compounds in the mobile phase were scanned by a UV/Vis spectrophotometer (Jasco V‑530) in the range 200-400 nm. From the overlaid UV spectra, suitable wavelength considered for monitoring the drugs was 255 nm. Standards were dissolved in methanol and injected separately for HPLC analysis, and the responses (peak areas) were recorded at 255 nm. It was observed that there was no interference from the mobile phase or baseline disturbance, and these three drugs absorbed well at 255 nm. Also, no significant peaks were observed from the formulation matrix, indicating no interference from the matrix of the formulation. It was, therefore, concluded that 255 nm was the most appropriate wavelength for analysis of all the three drugs with suitable sensitivity.
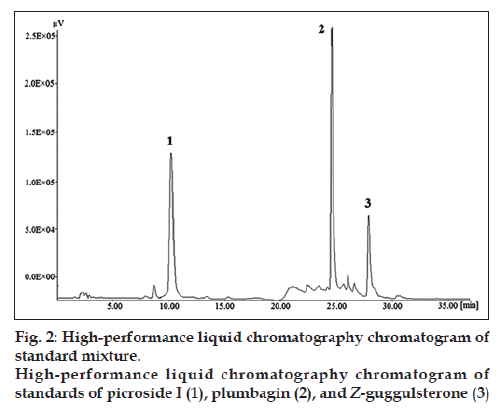
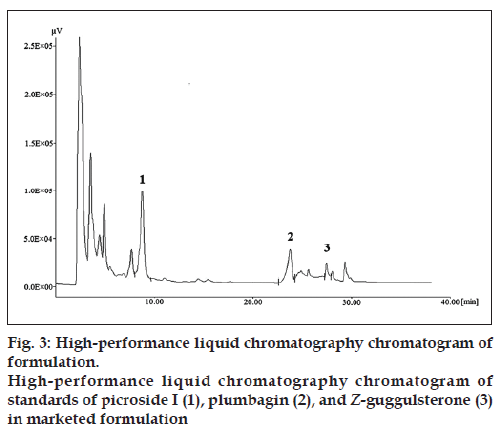
The chromatogram of the standard drug with concentration of 100 μg/ml (fig. 2) revealed good separation of the selected marker constituents. Chromatographic peaks were identified by comparing their retention times under the same operating conditions. Chromatograms obtained from the methanol extract of the polyherbal formulation (tablet) showing picroside I, plumbagin, and Z‑guggulsterone are shown in the figure (fig. 3).
The method was validated for linearity, accuracy, precision, repeatability, selectivity, and specificity study. All the validation studies were carried out by replicate injection of the sample and standard solutions.
Linearity was determined for the three drugs, picroside I, plumbagin, and Z‑guggulsterone separately by plotting a calibration graph of peak area against the respective concentration. From the calibration curve, it was clear that picroside I had linearity between 200 and 1000 μg/ml and plumbagin had a range between 1 and 8 μg/ml, whereas Z‑guggulsterone had a range between 1 and 10 μg/ml.
The linear regression equation for the three drugs found was as follows; picroside I: y=9104x‑39549, r2=0.998; plumbagin: y=74918x+10615, r2=0.980; Z‑guggulsterone: y=64278x+12485, r2=0.991, where y is peak area and x is concentration.
Accuracy and precision of the developed method were carried out as per ICH norms. Accuracy of the method was confirmed by doing a recovery study at three different concentration levels by replicate analysis (n=3). The results of the accuracy study are reported in Table 1. From the recovery study, it was clear that the method is accurate for quantitative estimation of picroside I, plumbagin, and Z‑guggulsterone in tablet dosage form because all the statistical results were within the acceptance range (i.e., RSD% <2.0).
| Drug | Amount taken (µg/ml) | Amount added (µg/ml) | Mean % recovery ± SDa | % RSD |
|---|---|---|---|---|
| Picroside I | 180 | 200 | 98.33 ± 0.395 | 0.4 |
| 180 | 160 | 100.21 ± 0.38 | 0.379 | |
| 180 | 120 | 99.75 ± 0.97 | 0.972 | |
| Plumbagin | 4 | 6 | 99.84 ± 0.69 | 0.69 |
| 4 | 5 | 101.78 ± 0.46 | 0.447 | |
| 4 | 3 | 98.95 ± 0.59 | 0.601 | |
| Z-guggulsterone | 3 | 7 | 101.13 ± 1.17 | 1.158 |
| 3 | 4 | 99.85 ± 0.96 | 0.961 | |
| 3 | 2 | 102.27 ± 1.01 | 0.987 |
Table 1: Result Of Recovery Study
Precision was determined by studying the repeatability and intermediate precision. Repeatability result indicates precision under the same operating conditions over a short interval time and interassay precision. standard deviation, coefficient of variance, and standard error were calculated for the three drugs. The results are mentioned in Table 2. Intermediate precision was carried out by doing intra and interday precision studies. In the intraday study, the concentrations of the three drugs were calculated on the same day at an interval of one hour. In the interday study, the concentrations of drug contents were calculated on three different days, and the study expressed within laboratory variation on different days. In both intra and interday precision studies for the methods, RSD values were not more than 2.0%, which indicates good intermediate precision (Table 2). The developed method was precise for quantitative study because the precision study was found statistically significant (RSD % <3.0 and SD <1.0 for intra and interday studies).
| Parameters | Picroside I | Plumbagin | Z-guggulsterone |
|---|---|---|---|
| Precision (% RSD) | |||
| Intradaya | |||
| Rt | 1.09 | 1.158 | 0.691 |
| Pa | 0.539 | 2.75 | 2.031 |
| Interdaya | |||
| Rt | 0.152 | 0.351 | 0.117 |
| Pa | 0.684 | 2.705 | 1.554 |
| Limit of detection (μg/ml) | 2.7 | 0.09 | 0.099 |
| Limit of quantification (μg/ml) | 9.002 | 0.31 | 0.33 |
Table 2: Result of intraday and interday Precision, lod, and loq study
LOD and LOQ studies were carried out to evaluate the detection and quantitation limits of the method to determine the presence of any impurities by using the following equations: LOD=3.3 σ/S and LOQ=10 σ/S, where σ is the standard deviation and S is the slope of the curve. The results are given in Table 2.
To check the selectivity of the developed method, solutions of the three drugs were injected into the system, and three sharp peaks for picroside I, plumbagin, and Z‑guggulsterone were obtained at retention times of 9.52, 24.33, and 27.70 min, respectively with reference to the placebo solution. Specificity of the method was assessed by comparing the chromatogram obtained from the standard drugs (fig. 2) with the chromatogram obtained from the tablet solutions (fig. 3). Because the retention time of the standard drugs and the retention time of the three drugs in sample solutions were the same, the method was specific. The developed method was specific and selective as no interference of excipients was found.
The results showed that the method was suitable for the simultaneous estimation of picroside I, plumbagin, and Z‑guggulsterone. The amount of picroside I, plumbagin, and Z‑guggulsterone present in the marketed formulation was found to be 0.83, 0.019, and 0.016%, respectively.
The proposed method is advantageous as it showed good resolution with respect to peak symmetry, reproducibility, efficiency, and separation of marker compounds. Moreover, no peaks of other constituents present in the formulation were found to interfere with that of the marker constituent, indicating no interference. Also, quantification of compounds by high‑performance thin‑layer chromatography (HPTLC) is less sensitive as compared to HPLC. This method therefore has significance in terms of sensitivity and selectivity as compared to other methods. The only drawback of this method is its long run time. However, no chromatographic or spectrometric method has been reported for the simultaneous estimation of picroside I, plumbagin, and Z‑guggulsterone in combined dosage form. Moreover, the method shows separation of heterologous compounds, that is, glycosides and steroids which are present in many of the marketed herbal formulations. Considering the advantages, this method can potentially be applied to estimate these marker compounds in combination not only to facilitate standardization of polyherbal formulations but also for use in scientific and commercial applications.
A validated HPLC method for the simultaneous quantification of picroside I, plumbagin, and Z‑guggulsterone has been established. It has been shown that the developed method achieved accuracy, reproducibility, repeatability, linearity, precision, and selectivity, which prove the reliability of the method. The method enabled accurate, sensitive, and reproducible quantification of these marker constituents in a polyherbal formulation. It enabled rapid quantitation of many samples in routine and quality control analysis of tablet formulation. The same solvent was used throughout the experimental work, and no interference of any excipient matrices was found. The result shows that the method could find practical application as a quality control tool for the simultaneous estimation of three drugs from their combined dosage form in a quality control laboratory.
Acknowledgements
The authors are thankful to the University Grants Commission (UGC), India for the financial assistance provided during the research work.
References
- Indian Pharmacopoeia. 6th ed., vol. 3. Ghaziabad: The Indian Pharmacopoeia Commission; 2010. p. 2516-7.
- Handa SS. Perspective of Indian medicinal plant in management of liver disorder. New Delhi: Indian Council of Medicinal Research; 2008. p. 245-70.
- Indian Herbal Pharmacopoeia. Vol. 1. New Delhi: A Joint Publication of Regional Research Laboratory and Council of Scientific and Industrial Research; 1998. p. 106-12.
- Chaturvedi GN, Singh RH. Jaundice of infectious hepatitis and its treatment with an indigenous drug-Picrorhiza kurroa (A review of 30 cases and clinical trial). J Res Indian Med 1966;1:1-14.
- Krishnamurthi A. The Wealth of India, Raw Materials. New Delhi: Council of Scientific Industrial Research; 2005. p. 49-50.
- Gupta RK. Botanical explorations in the Bhillangna valley of the erstwhile Tehri Garhwalstate-II. J Bombay Nat HistSoc1956-1957;54:878-86.
- The Ayurvedic Pharmacopoeia of India. 1st ed., vol. 2, part 1.New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homeopathy; 1999. p. 85-7.
- Weinges K, Kloss P, Henkels WD. Natural products from medicinal plants XVII. Picroside-II, a new 6-vanilloyl-catapol from Picrorhiza kurroa Royle and Benth. Justus Liebigs Ann Chem 1972;759:173-82.
- Weinges K, Kunstler K. Natural products from medicinal plants, XXII. Note on the isolation and elucidation of the constitution of a new picroside from PicrorhizakurrooaRoyle and Benth. Justus LiebigsAnn 482 Indian Journal of Pharmaceutical Sciences July - August 2013the plant family Burseraceae. Planta Med 1993;59:12-6.
- Kumar V, Mehrotra N, Lal J, Gupta R. Pattern profiling of the herbal preparation picroliv using liquid chromatography–tandem mass spectrometry. J Chromatogr A 2004;1045:145-52.
- Stuppner H, Wagner H. Minor iridoid and phenol glycosides of Picrorhizakurrooa. Planta Med 1989;55:467-9.
- Stuppner H, Wagner H. New cucurbitacin glycosides from Picrorhiza kurroa. Planta Med 1989;55:559-63.
- Chiu NY, Chang KH. The Illustrated Medicinal Plants of Taiwan.5th ed., vol. 2. Taipei: SMC Publishing Inc; 2003. p. 152.
- De Paiva SR, Marques SS, Figueiredo MR, Kaplan MA.Plumbaginales: A pharmacological approach. Florestae Ambiente 2003;10:98.
- The Ayurvedic Pharmacopoeia of India. 1st ed., Vol. 1, part 1. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and homeopathy; 1999. p. 38,40,99-100.
- Sidhu GS, Sankaram AV. New biplumbagin and 3-chloroplumbagin from Plumbago zeylanica. Tetrahedron Lett 1971;26:2385-8.
- Lin LC, Yang LL, Chou CJ. Cytotoxic naphthoquinones and plumbagic acid glucosides from Plumbago zeylanica. Phytochemistry 2003;62:619-22.
- Dinda B, Hajra AK, Das SK. Chemical constitutents of Plumbagoindica roots. Indian J Chem 1998;37B:672-5.
- Sankaram AV, Srinivasarao A, Sidhu GS. Chitranone a new binaphthaquinone from Plumbago zeylanica.Phytochemistry 1976;15:237-8.
- Tezuka M, Takahashi C, Kuroyanagi M, Satake M, Yoshihira K, Natori S. New naphthoquinones from Diospyros. Phytochemistry1973;12:175-83.
- Gunaherath GM, Gunatilaka AA, Sultanbawa MU, Salasubramaniam S.1,2 (3)Tetrahydro-3,3-biplumbagin anaphthalenone and other constituents from Plumbago zeylanica. Phytochemistry 1983;22:1245-7.
- Sankaram AV, Rao AS, ShooleryJN. Zeylanone and Isozeylanone, two novel quinonesfrom Plumbago zeylanica. Tetrahedron 1979;35:1777-82.
- Indian Pharmacopoeia. 6th ed., vol. 3. Ghaziabad: The Indian Pharmacopoeia Commission; 2010. p. 2504-6.
- Latha BP, Reddy IR, Ismail SM, Vijaya T. Medicinal plants and their derivatives as potential source in treatment of obesity. Asian J Exp Biol Sci 2010;1:719-27.
- Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C. Gugulipid, an extract of Commiphorawhighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav 2007;86:797-805.
- Gujral ML, Saxena K, Tangri KK,Amma MK, Roy AK. Antiarthritic and antiinflammatory activity of gum guggul (Basalsamodenron mukul Hook). Indian J Physiol Pharmacol 1960;40:267-73.
- Duwiejua M, Zeitlin IJ, Waterman PG, Chapman J, Mhango GJ, Proven GJ. Antiinflammatory activity of resins from some species of
- Singh BB, Mishra LC, Vinjamury SP, Aquilina N, Singh VJ, Shepard N. The effectiveness of Commiphora mukul for osteoarthritis of the knee: An outcomes study. AlternTher Health Med 2003;9:74-9.
- Leeman-Neill RJ, Wheeler SE, Singh SV, Thomas SM, Seethala RR, Neill DB, et al. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis 2009;30:1848-56.
- Chander R, Rizvi F, Khanna AK, Pratap R. Cardioprotective activity of synthetic guggulsterone (e and z-isomers) in isoproterenol induced myocardial ischemia in rats: A comparative study. Indian J ClinBiochem 2003;18:71-9.
- Chander R, Khanna AK, Pratap R. Antioxidant activity of Guggulsterone: The active principle of Gugulipid from Commiphora mukul. J Med Arom Plant Sci 2004;24:370-4.
- Mester M, Mester L, Nityanand S. Inhibition of platelet aggregation by guggulu steroids. Planta Med 1979;37:367.
- Deng R. Therapeutic effects of guggul and its constituent guggulsterone. Cardiovasc Drug Rev 2007;25:375-90.
- Siddiqui MJ. Guggul: An excellent herbal panacea. Asian J Pharm Health Sci 2011;1:35-9.
- Patil VD, Nayak UR, Dev S. Chemistry of Ayurvedic crude drugs–I: Guggulu (resin from Commiphora mukul)–1: Steroidal constituents. Tetrahedron 1972;28:2341-52.
- Bajaj AG, Dev S. Chemistry of Ayurvedic crude drugs–V: Guggulu (resin from commiphora mukul)–5 some new steroidal components and, stereochemistry of guggulsterol-I at C-20 and C-22. Tetrahedron 1982;38:2949-54.
- Dev S. Guggultetrols: A new class of naturally occurring lipids. PureApplChem 1989;61:353-6.
- Patil VD, Nayak UR, Dev S. Chemistry of ayurvedic crude drugs–III: Guggulu (resin from Commiphora mukul)-3 long-chain aliphatic tetrols, a new class of naturally occurring lipids. Tetrahedron 1973;29:1595-8.
- ICH, Q2A, Hamonised Tripartite Guideline, Test on Validation of Analytical Procedures, IFPMA, In: Proceedings of the International Conference on Harmonization, Geneva, March, 1994.
- ICH, Q2B, Hamonised Tripartite Guideline, Validation of Analytical Procedure: Methodology, IFPMA, In: Proceedings of the International Conference on Harmonization, Geneva, March 1996.
- Katekhaye SD, Shinde PB, Laddha KS. Isolation and HPLC method development for filixic acid PBP from Dryopteris filix-mas.Int J Phytopharm 2011;1:1-7. Chem 1977;1977:1053-7.