- *Corresponding Author:
- E. Robins
Direction Technique, Valois SAS, Route des Falaises, 27100 Le Vaudreuil, France
E-mail: emmanuelle.robins@valois.com
| Date of Acceptance | 26 October, 2007 |
| Indian J Pharm Sci,2007, 69 (5): 722-724 |
Abstract
Introduction
A hydrofluoroalkane (HFA) based budesonide formulation was developed so that 220 µg of budesonide per shot would exit the valve over 200 doses. This formulation was designed to be physically and chemically stable and it would give reproducible aerosol performances [1-4]. The stability of the resulting metered dose inhaler was also evaluated under accelerated storage conditions (40°/75%RH) up to 6 mo. The aim was also to match the innovator Pulmicort® CFC (chloroß uorocarbon) product in terms of in vitro performances.
Materials and Methods
Excipients selected for evaluation were polyethylene glycol 300 (at levels of 0.15 to 5% w/w, supplied by Fluka, France) and ethanol (at levels of 0.2 to 2% w/w, supplied by Prolabo, France) [4]. The amount of micronised budesonide (supplied by Aarti Healthcare Ltd, India) introduced into the formulation vessel was such that the appropriate dose of budesonide would be delivered to the patient. Pressurised metered dose inhalers (pMDIs) were prepared by introducing the HFA budesonide suspension formulation as a one step filling process, through a metering valve previously crimped onto a standard anodised aluminium canister (supplied by Presspart, Great-Britain).
To evaluate the homogeneity of the dispersion by visual inspection, formulations were filled into glass bottles. Promising formulations were evaluated with regard to drug stability upon storage, delivered dose uniformity (DDU) over 10 actuations at 28.3 l/min and particle size distribution using a Next Generation Impactor at 30 l/min.
Valve types and materials combined with various actuator outlet orifice diameters were also evaluated. A first set of results was obtained with a DF30/50 RCU valve having polyacetal plastic components and nitrile rubber and thermoplastic elastomer gaskets combined with a 0.7 mm outlet orifice diameter actuator. A second set of results was obtained with a DF316/50 RCU valve with polyacetal plastic components and nitrile rubber and thermoplastic elastomer gaskets combined with a 0.5 mm outlet orifice diameter actuator.
Results and Discussion
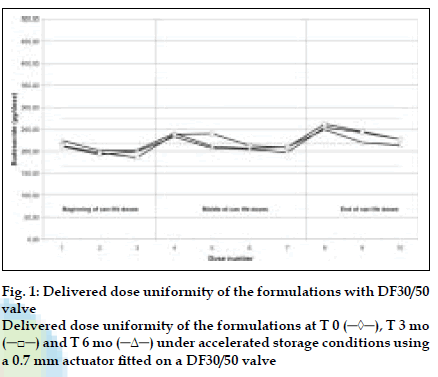
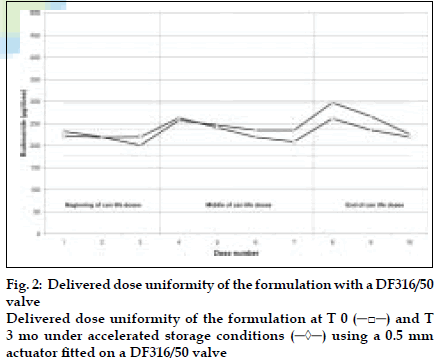
DDU results obtained with a DF30/50 valve fitted with a 0.7 mm outlet orifice diameter actuator are shown in (fig. 1) and those obtained with a DF316/50 valve fitted with a 0.5 mm outlet orifice diameter actuator are shown in (fig. 2). DDU data is summarised in Table 1 and conforms to the current FDA MDI/DPI guidance document for Dose Uniformity. Fine Particle Fraction (FPF) results are summarised in Table 2.
| DF30 valve (n=10) | DF316 valve (n=2) | |
|---|---|---|
| 0.7 mm actuator | 0.5 mm actuator | |
| TO | 217±22 µg | 208±23 µg |
| T3 (40*/75%RH) | 223±25 µg | 223±20 µg |
| T6 (40° /75%RH) | 218±15 µg | Not determined |
Table 1: Delivered Dose Uniformity Through Can Life Depending On Used Valve Type And Outlet Orifice Size Of The Actuator
| DF30 valve (n=3) 0.7 mm actuator | DF316 valve (n=2) 0.5 mm actuator | Pulmicort CFC (n=3) as supplied actuator | ||||
|---|---|---|---|---|---|---|
| FPF (%) | MMAD (µm) | FPF (%) | MMAD (µm) | FPF (%) | MMAD (µm) | |
| TO | 17±2 | 5.06±0.02 | 19±2 | 5.06±0.05 | 14±1 | 5.46±0.11 |
| T3 (40' /75%RH) | 16±1 | 5.28±0.03 | 18±1 | 5.29±0.11 | Not determined | |
| T6 (40° /75%RH) | 14±2 | 5.36±0.04 | Not determined | Not determined | ||
Table 2: Aerodynamic Particle Size Distribution Depending On Used Valve Type And Outlet Orifice Size Of The Actuator
During this study, PEG 300 at levels less than 0.5% w/w was found to ensure good product performances and valve functioning throughout the MDI units’ life. There was no sign of valve sticking. Ethanol at levels less than 1% w/w helped dissolution of the PEG300 without causing significant solubilisation of budesonide, which could lead to problems of chemical degradation. The level of ethanol selected also helped to decrease the propensity for rapid formation of coarse flocks. This formulation was readily redispersible and avoided irreproducible dosing of the drug. The stable suspension of particulate budesonide was aided by employing a mixture of HFA propellants closely matching the density of the micronised budesonide.
Valois offers a fully developed budesonide HFA formulation. It includes a suspension formulation stable for at least 6 mo under 40°/75%RH storage conditions, together with the selected appropriate valve, canister and actuator.
Acknowledgements
The authors would like to thank Presspart for supplying the canisters.
References
- Purewal T, Formulation of metered dose inhalers, Metered dose inhalers technology, Buffalo Grove: Interpharm Press; 1998; 1-8.
- Atkins P, Baker PN, Mathiesen D, The design and development of inhalation drug delivery systems. Pharmaceutical Inhalation Aerosol Technology. New York: Marcel Dekker; 1992; 168-71.
- Vervaet C, Byron PR. Drug-surfactant-propellant interactions in HFAformulations. Int J Pharm, 1999; 186:13-30.
- Hickey AJ. Lenfant C. editors. Inhalation aerosols, Physical and Biological Basis. 2nd ed. Lung Biology in Health and Disease 2007; 221.