- *Corresponding Author:
- Shweta Agarwa
Department of Pharmaceutical Sciences, Faculty of Technology and Engineering, Kalabhavan, M. S. University of Baroda, Vadodara-390 001, India
E-mail: shweta_ag26@rediffmail.com
| Date of Submission | 04 September 2014 |
| Date of Revision | 26 January 2015 |
| Date of Acceptance | 20 November 2015 |
| Indian J Pharm Sci 2015; 77(6):705-714 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Mucoadhesive tablets have emerged as potential candidates for gastroretentive drug delivery providing controlled release along with prolonged gastric residence time. Gastroretentive mucoadhesive tablets could result in increased bioavailability due to prolonged gastric residence time. A hydrophilic matrix system was developed as mucoadhesion is achievable on appropriate wetting and swelling of the polymers used. The polymers were so chosen so as to provide a balance between swelling, mucoadhesion and drug release. The polymers chosen were hydroxypropyl methylcellulose K4M, chitosan, and Carbopol 934. The concentrations of these polymers used has a great impact on the physicochemical properties of the resulting formulation. The tablets were formulated using wet granulation method and tranexamic acid was used as the model drug. The prepared tablets were characterized for size, shape, appearance, hardness, friability, weight variation, swelling, mucoadhesion and in vitro drug release. Several batches of tablets were prepared by varying the ratio of hydroxypropyl methylcellulose K4M and Chitosan. The batches having a greater ratio of chitosan showed higher rate of swelling, greater erosion, less mucoadhesion and faster release rate of the drug whereas the batches having greater ratio of hydroxypropyl methylcellulose K4M showed lesser rate of swelling, less erosion, better mucoadhesion and a smaller drug release rate. The level of carbopol was kept constant in all the batches.
Keywords
Mucoadhesive, gastroretentive, in vitro drug release, swelling, hydroxypropyl methylcellulose K4M, chitosan, carbopol
Gastroretentive mucoadhesive tablet is a special type of controlled release drug delivery system, which offers a prolonged gastric residence time by mucoadhesion to the gastric mucosa. A drug that is released from the dosage form in a controlled manner in the stomach exits the stomach together with gastric fluids and has the whole surface area of the small intestine available for absorption [1]. Gastroretentive mucoadhesive delivery systems increase the residence time of the dosage form in the gastrointestinal tract and provide intimate contact between a dosage form and absorbing tissue, which may result in high drug concentration in a local area and hence high drug flux through the absorbing tissue [2]. This could result in increased bioavailability as it is widely acknowledged that the extent of gastrointestinal drug absorption is related to the contact time with the small intestinal mucosa.
A hydrophilic matrix system was formulated because mucoadhesion to the gastric mucosa was desired, which is achievable on appropriate wetting and swelling of the polymers used. Chitosan, hydroxypropyl methylcellulose (HPMC) and carbopol are the most commonly used polymers for mucoadhesion so they were chosen to provide mucoadhesion to the formulation. To understand the mechanism of mucoadhesion it is necessary to know about the composition and properties of mucus. Mucus is a viscous, gelatinous, translucent secretion produced by specialized goblet cells [3]. The major components of mucus are water (>95%), mucin glycoproteins, proteins, lipids and mucopolysaccharides [4]. Mucin glycoproteins are the most important structure forming component of mucus gel giving it gel-like, cohesive and adhesive properties [5]. Mucin glycoproteins are of exceptionally high molecular weight (2-14×106 g/mol) forming an entangled network of macromolecules associated with each other through noncovalent bonds. This molecular association is important for the structure of mucus and is responsible for its rheological property. Pendant sialic acid (pKa=2.6) and sulphate groups located on the glycoprotein molecules make mucin an anionic polyelectrolyte at neutral pH. Since the properties of mucus are the properties of its main constituent, mucin glycoproptein, a comprehensive study of the structure of mucin glycoprotein is important [4]. Mucin glycoproteins are made up of a single chain polypeptide backbone with two distinct regions [6]: First, A heavily glycosylated central protein core to which many large carbohydrate side chains are attached, mainly by O-glycosidic linkages and one or two terminal peptide regions where there is little glycosylation. These regions are usually referred to as ‘naked protein’ region [7].
Mucin is stored in both submucosal and goblet cells, wherein the negative charges of the mucin glycoprotein are shielded by calcium ions, allowing for molecules to be tightly packed. When mucin is released into the lumen, outflux of calcium exposes the negative charges resulting in electrostatic repulsion and an approximately 400-fold expansion of molecules. These expanded mucin chains entangle with each other and undergo noncovalent interactions like hydrogen, electrostatic and hydrophobic bonding leading to the formation of viscoelastic gel [8].
Chitosan is a cationic polysaccharide, which is produced by deacetylation of chitin, the most abundant polysaccharide in the world, next to cellulose [9]. Chitosan is reported to have good mucoadhesive properties and also provides paracellular permeation enhancement by opening the tight junctions of the intestinal epithelium [10]. The primary amino functional groups of chitosan and the sialic acid and sulphonic acid substructures of mucus interact ionically, producing mucoadhesion [11-13]. In addition to this, hydrogen bonding between the hydroxyl and amino groups of chitosan and mucus also takes place. The linearity of chitosan molecules facilitates interpenetration due to sufficient chain flexibility and thus enhances its mucoadhesive property [14]. It also works to control the release of drug [15].
HPMC was chosen because of its controlled release as well as good mucoadhesive properties. It is a nonionic polymer, so its mucoadhesion does not depend on the pH of the medium, thus making it adhere at any pH [16]. The mucoadhesion of HPMC is attributable to the formation of physical (including hydrogen) bonds with the mucus components. It possesses a large number of hydroxyl groups that are responsible for adhesion [17]. Also it is economical and easily available in various grades [18]. The grade of HPMC that has been used is K4M. Carbopol has been used as it is an excellent mucoadhesive and it also provides binding to the tablet [19]. Excellent mucoadhesive characteristics are exhibited by carbopol due to strong hydrogen bonding with mucin [20].
The objective of the present investigation was to study the effect of various polymers and their concentration on the drug release characteristics, mucoadhesiveness and other physicochemical properties of the tablet dosage form. Tranexamic acid was used as the model drug to study the release characteristics of the formulated tablet dosage form as it is freely soluble in water, is not hygroscopic and is chemically stable. Tranexamic acid is a haemostatic and antifibrinolytic. It shows its effect by inhibiting fibrinolysis in common haemorrhages. It is given in bleeding tendencies in which systemic hyperfibrinolysis is involved like leukaemia, apalstic anaemia, abnormal bleeding during or after an operation, menorrhagia [21]. Tranexamic acid is a synthetic analogue of amino acid lysine [22]. Its oral bioavailability is 30-50% of the ingested dose. It has a half-life of 3.3 h and a dosing frequency of 2-4 times daily with a high dose of approximately 1000 mg (2-3 tablets at a time) [23].
The present study was undertaken to prolong the gastric retention time by way of gastroretentive mucoadhesive tablets and to check the effect of variation in the concentration of different polymers on the release rate of the drug and the physicochemical properties of the dosage form.
Materials and Methods
Tranexamic acid was obtained as a gift sample from Mercury Laboratories, Baroda. Chitosan was obtained as a gift sample from Seiber Hegner, Mumbai. HPMC K4M was obtained as gift sample from Colorcon Asia, Goa. Carbopol 934, calcium hydrogen phosphate, talc, magnesium stearate were purchased from Loba-Chemie, Mumbai. All other chemicals were of analytical reagent grade and were used as received.
Preparation of gastroretentive mucoadhesive tablets of tranexamic acid
Chitosan, HPMC K4M and carbopol 934 were used as the polymers in formulating the gastroretentive mucoadhesive tablets of tranexamic acid. A tablet of weight 750 mg was prepared using wet granulation method. The formula given in Table 1, was used for the preparation of tablets. Total polymer percentage was kept constant at 41% but the ratio of HPMC K4M and chitosan was varied in different batches of tablets prepared. Percentage of Carbopol was kept constant at 1%. Talc and magnesium stearate were used as glidant, lubricant and anti-adherant. The various ratios of HPMC and chitosan used in preparing different batches of tablets are given in Table 2.
| Ingredients used | Weight of ingredientsused (mg) | Percentage ofingredients used |
|---|---|---|
| Tranexamic acid | 375 | 50 |
| Chitosan+HPMC K4M | 300 | 40 |
| Carbopol 934 | 7.5 | 1 |
| Calcium hydrogen | 56.25 | 7.5 |
| phosphate (dihydrate) | ||
| Magnesium stearate | 7.5 | 1 |
| Talc | 3.75 | 0.5 |
Table 1: Formula for preaparation of tablets of tranexamic acid
| Batchnumber | HPMC: chitosanratio | Quantity of eachpolymer (mg) |
|---|---|---|
| C300 | 0:1 | H=0, C=300 |
| H300 | 1:0 | H=300, C=0 |
| H1:1 | 1:1 | H=150, C=150 |
| H1:3 | 1:3 | H=75, C=225 |
| H1:5 | 1:5 | H=50, C=250 |
| H1:7 | 1:7 | H=37.5, C=262.5 |
| H3:1 | 3:1 | H=225, C=75 |
| H5:1 | 5:1 | H=250, C=50 |
| H7:1 | 7:1 | H=262.5, C=37.5 |
Table 2: Ratio and quantity of Hydroxypropyl Methylcellulose and chitosan used in various formulations
The polymers present served as binders. All the ingredients were accurately weighed and the polymers (HPMC and chitosan) were weighed according to Table 2, sieved and mixed in a mortar. Water (11- 12 ml) was added as the granulating agent to form a moist, damp mass of the mixture. This moist mass was passed through sieve no. 16 to form granules. The wet granules were dried in a hot air oven at a temperature of 50-55o for 2 h. The dried granules were passed through sieve no. 18 and weighed amount of talc and magnesium stearate mixed at this point.
Evaluation of granules
The resulting granules were evaluated for flow property by determining the angle of repose, Hausner ratio and Carr’s compressibility index (Table 3). The bulk density and tapped density of the granules was also determined.
| Batch number | Angle of repose | Bulk density (g/ml) | Tapped density (g/ml) | Compressibility index (%) | Hausner ratio |
|---|---|---|---|---|---|
| C300 | 27.48 ± 0.04 | 0.416 ± 0.06 | 0.499 ± 0.05 | 16.63 ± 0.07 | 1.199 ± 0.06 |
| H300 | 25.79 ± 0.05 | 0.411 ± 0.03 | 0.478 ± 0.04 | 14.01 ± 0.04 | 1.163 ± 0.05 |
| H1:1 | 26.21 ± 0.12 | 0.412 ± 0.05 | 0.486 ± 0.05 | 15.22 ± 0.05 | 1.179 ± 0.04 |
| H1:3 | 26.10 ± 0.11 | 0.423 ± 0.06 | 0.489 ± 0.07 | 13.49 ± 0.03 | 1.156 ± 0.07 |
| H1:5 | 26.54 ± 0.06 | 0.404 ± 0.07 | 0.468 ± 0.07 | 13.67 ± 0.08 | 1.158 ± 0.06 |
| H1:7 | 26.78 ± 0.07 | 0.418 ± 0.11 | 0.492 ± 0.09 | 15.04 ± 0.08 | 1.177 ± 0.07 |
| H3:1 | 25.89 ± 0.04 | 0.415 ± 0.10 | 0.482 ± 0.06 | 13.90 ± 0.08 | 1.161 ± 0.06 |
| H5:1 | 26.03 ± 0.05 | 0.425 ± 0.09 | 0.491 ± 0.08 | 13.44 ± 0.07 | 1.155 ± 0.07 |
| H7:1 | 25.67 ± 0.03 | 0.409 ± 0.07 | 0.474 ± 0.08 | 13.71 ± 0.04 | 1.158 ± 0.05 |
Table 3: Evaluation of Granules of different formulations
Angle of repose was determined by fixed funnel method. In this 5 g of the granules were allowed to flow down a funnel fixed 4 cm from the horizontal surface. The radius and height of the heap of granules formed was measured and angle of repose calculated using the formula, tan θ=h/r, where θ=angle between the horizontal surface and the heap, h=height of the heap in cm and r=radius of heap in cm.
Bulk density was determined by pouring 10 g of granules in a 100 ml measuring cylinder and tapping the cylinder twice on a flat surface. The volume occupied by the granules was noted as bulk volume and bulk density calculated by using the formula, Db=M/ Vb, where, Vb=bulk volume in ml and M=mass of granules in gram.
Tapped density was determined using the bulk density apparatus. Ten gram of the granules were put in a 100 ml measuring cylinder and the initial volume noted down. The measuring cylinder was then tapped till no further change in volume was observed. This volume was also noted down as tapped volume and tapped density was calculated by using the formula, Dt=M/Vb-Vt, where, Dt=tapped density, M=mass of granules, Vb=bulk volume of granules and Vt=tapped volume of granules. The Carr’s compressibility index was calculated using the formula, Carr’s index (%)=Dt-Db/Dt×100 and Hausner ratio was calculated by the formula, H=Dt /Db.
The granules were then compressed into tablets on 8 station rotary tablet compression machine using 16/32 round dies and matching flat bevel edge punches with a bisecting bar on the upper punch. The different batches of tablets prepared were then characterized for their physical appearance, hardness, friability, weight variation, drug content, in vitro drug release using dissolution testing, mucoadhesive strength and swelling index.
Also, control batches of various formulations were prepared without the drug. In the control batches the drug was replaced with an equal amount of diluent and the same procedure and same tool set at the same setting was used for the compression of tablets. The various physicochemical properties like mucoadhesion, hardness, friability, weight variation, swelling index were also determined for the control batches and compared statistically with that of the batches containing the drug.
Physical properties of tablets
Physical appearance was ascertained by visually observing the tablets for their shape, colour, dimensions. The dimensions of the tablet i.e. the diameter and thickness were determined using vernier calipers. Hardness was determined using Monsanto hardness tester. Hardness was determined for 3 tablets of each batch. Roche friabiliator was used for checking the friability of the tablets. A preweighed sample of 10 tablets of each batch was placed in the friabiliator for 4 min at 25 rpm. After 100 revolutions the tablets were dusted and reweighed and friability determined using the formula, Friability=W1-W2/W1×100, W1=initial weight of tablets and W2=final weight of tablets after 100 revolutions. Weight variation was carried out as per Indian Pharmacopoeia 1996 using 10 tablets.
Drug content
Twenty tablets of tranexamic acid were weighed and average weight was calculated. The tablets were powdered and weight equivalent to 125 mg of tranexamic acid was added to a 25 ml volumetric flask and shaken with 0.1 N HCl to dissolve the drug. The drug solution was then filtered and the filtrate was used as the stock solution. Solutions of suitable drug concentration were prepared and analyzed for drug concentration and content by the colorimetric method given by Agarwal and Murthy [24]. Four determinations were carried out for drug content.
In vitro drug release
In vitro drug release studies were done to compare the drug release profile of different batches of tablets and to study the effect of change in the ratio of HPMC and chitosan on the release of drug. Also the kinetics and mechanism of release of drug from the formulated tablets were studied by fitting the dissolution data in various release kinetic models like zero order, first order, Higuchi, Hixon-Crowell, Korsmeyer-Peppas and Weibull distribution. R2 values were calculated for the various models and release kinetics determined.
In vitro drug release studies were carried out in USP XXII paddle type apparatus. One tablet was introduced in each of the six dissolution bowls. Nine hundred milliliter of 0.1 N HCl was used as the dissolution medium and the paddle was rotated at 50 rpm. Temperature was maintained at 37±0.5° throughout the dissolution studies. Samples (5 ml) was withdrawn at 1 h interval for 12 h and filtered through grade I Whatman filter paper to remove any undissolved particulate matter. The withdrawn volume was replaced with fresh dissolution medium to maintain sink conditions. The withdrawn samples were then analyzed for the drug content by the colorimetric method of Agarwal and Murthy [24]. Drug released at different intervals of time was then calculated from the data obtained.
Mucoadhesive strength
Ex vivo method based on the measurement of tensile strength by modified balance method was used for the determination of mucoadhesive strength of tablets. Stomach mucosa of rats was used for the test after cleaning it thoroughly and stabilizing it in 0.1 N HCl for 30 min 0.1 N HCl was used as the medium to dip the surface of mucosa and tablet. Five gram weight was used on the left pan for 10 min to make the tablet stick to the mucosa. After 10 min, additional weights were added in the form of water droplets from a burette on the right side till the tablet detached from the mucosa. Mucoadhesive strength was calculated by subtracting 5 g from the total weight used. The test was carried out on 3 tablets of each batch and result was calculated as mean±SD.
Swelling index
The swelling state of the polymer is considered crucial for its mucoadhesive behavior. For determining the swelling index, weighed tablets were placed on glass slides and then these glass slides were put in petri dishes containing 20 ml of 0.1 N HCl such that the upper surface of tablet was immersed in the liquid medium. The glass slides were taken out at regular intervals of time, superfluous moisture removed and the tablets reweighed. The following formula was used for the determination of swelling index, SI=Wt-Wo/Wo, where Wt=weight of tablet at time t, and Wo=weight of tablet at time 0. This can be multiplied by 100 to get the result in terms of percentage.
Statistical analysis
All statistical analysis was carried out with the help of software Graphpad Prism version 6. Comparison between the physicochemical properties of the control batches and batches containing drug was done by multiple t-test (by calculating P values for all batches) with confidence limit set at 95%. Whereas comparison of the physicochemical properties (like hardness, friability, weight variation, assay and drug release) of different batches of tablets (having drug) with different polymer concentrations was done by calculation of P-value by one way ANOVA at 95% confidence interval. For each physicochemical property the calculated P-value was compared with the tabulated P-value to check whether there was a significant difference or not.
Results and Discussion
The mucoadhesive gastroretentive tablets of tranexamic acid were prepared by wet granulation method according to the formula (Table 1) and the various ratios of polymers given in Table 2. The prepared granules for all the batches were evaluated for flow property by determining their angle of repose, Carr’s compressibility index and Hausner ratio. The results of angle of repose range from 25.67-27.48° indicating excellent flow. The results of Carr’s compressibility index lie between 13.44-16.63 indicating good to fair flow property and the results of Hausner ratio range from 1.155 to 1.199 indicating good to fair flow. All the results of flow property evaluation have been tabulated in Table 3. All the above parameters were also calculated for the granules of all the control batches and no significant difference was found between the flow property of control batches and the batches having drug.
The tablets of all batches were assessed for shape and size, hardness, friability, weight variation, drug content, mucoadhesive strength and swelling index. The results of characterization have been shown in Tables 4-6. Table 7 shows the characterization results for the control batches (The batches prepared without the drug but having same polymer composition).
| Batch number | Hardness* | Friability* | Weight | Assay†,a |
|---|---|---|---|---|
| (kg) | (%) | variation† (mg) | (mg) | |
| C300 | 7.0 ± 0.61 | 0.30 ± 0.11 | 755.3 ± 2.52 | 382.15 ± 2.88 |
| H300 | 9.0 ± 0.52 | 0.22 ± 0.10 | 753.0 ± 1.24 | 378.62 ± 3.96 |
| H1:1 | 8.8 ± 0.44 | 0.23 ± 0.09 | 748.8 ± 1.12 | 374.60 ± 2.42 |
| H1:3 | 7.4 ± 0.29 | 0.28 ± 0.08 | 754.5 ± 2.32 | 370.12 ± 3.68 |
| H1:5 | 7.8 ± 0.31 | 0.26 ± 0.10 | 754.8 ± 2.41 | 380.4 ± 4.02 |
| H1:7 | 8.1 ± 0.48 | 0.25 ± 0.06 | 749.1 ± 1.15 | 386.72 ± 3.42 |
| H3:1 | 8.4 ± 0.57 | 0.24 ± 0.06 | 752.4 ± 1.42 | 373.55 ± 3.89 |
| H5:1 | 8.2 ± 0.45 | 0.25 ± 0.05 | 754.6 ± 1.71 | 374.78 ± 2.76 |
| H7:1 | 8.4 ± 0.50 | 0.25 ± 0.07 | 748.6 ± 2.13 | 381.67 ± 3.82 |
Table 4: Results of hardness, friability, weight variation and assay
| Batch number | Mucoadhesive strength (g) |
|---|---|
| C300 | 7.0 ± 0.72 |
| H300 | 14.5 ± 0.67 |
| H1:1 | 8.5 ± 0.61 |
| H1:3 | 6.9 ± 0.74 |
| H1:5 | 4.8 ± 0.63 |
| H1:7 | 4.6 ± 0.75 |
| H3:1 | 11.0 ± 0.57 |
| H5:1 | 12.5 ± 0.55 |
| H7:1 | 12.5 ± 0.59 |
Table 5: Mucoadhesive strength of tablets
| Batch number | 60 min | 120 min | 150 min | 180 min | 210 min | 240 min |
|---|---|---|---|---|---|---|
| C300 | 14.9 ± 0.69 | 18.4 ± 0.62 | 19.6 ± 0.54 | - | - | - |
| H300 | 10.9 ± 0.62 | 17.5 ± 0.58 | 20.3 ± 0.62 | 22.3 ± 0.48 | 24.5 ± 0.49 | 25.1 ± 0.51 |
| H1:1 | 6.8 ± 0.32 | 8.2 ± 0.41 | 9.1 ± 0.45 | 10.8 ± 0.36 | - | - |
| H1:3 | 5.9 ± 0.44 | 8.0 ± 0.44 | 8.3 ± 0.33 | - | - | - |
| H1:5 | 5.3 ± 0.26 | 6.9 ± 0.24 | 7.6 ± 0.31 | - | - | - |
| H1:7 | 8.2 ± 0.28 | 11.0 ± 0.34 | 11.1 ± 0.36 | - | - | - |
| H3:1 | 6.8 ± 0.22 | 9.9 ± 0.25 | 10.7 ± 0.30 | 12.9 ± 0.34 | 14.6 ± 0.28 | 15.9 ± 0.37 |
| H5:1 | 10.9 ± 0.27 | 15.6 ± 0.36 | 17.9 ± 0.31 | 19.6 ± 0.42 | 22.7 ± 0.38 | 23.3 ± 0.43 |
| H7:1 | 7.2 ± 0.36 | 10.6 ± 0.29 | 11.8 ± 0.31 | 13.1 ± 0.40 | 14.4 ± 0.36 | 15.3 ± 0.24 |
Table 6: Swelling Index of tablets at different time intervals
| Batchnumber | Hardness* | Friability* | Weight variation† | Mucoadhesion* | Swelling |
|---|---|---|---|---|---|
| (kg) | (%) | (mg) | (g) | index* | |
| C300 | 7.2 ± 0.56 | 0.28 ± 0.10 755.3 ± 2.52 | 6.9 ± 0.60 | 19.7 ± 0.51 | |
| H300 | 9.1 ± 0.42 | 0.23 ± 0.12 753.0 ± 1.24 | 14.6 ± 0.54 | 25.0 ± 0.50 | |
| H1:1 | 8.6 ± 0.48 | 0.24 ± 0.08 748.8 ± 1.12 | 8.5 ± 0.42 | 10.6 ± 0.30 | |
| H1:3 | 7.5 ± 0.30 | 0.27 ± 0.08 754.5 ± 2.32 | 6.8 ± 0.66 | 8.4 ± 0.32 | |
| H1:5 | 7.7 ± 0.22 | 0.27 ± 0.09 754.8 ± 2.41 | 4.6 ± 0.50 | 7.5 ± 0.28 | |
| H1:7 | 8.0 ± 0.46 | 0.24 ± 0.07 749.1 ± 1.15 | 4.5 ± 0.48 | 11.2 ± 0.32 | |
| H3:1 | 8.4 ± 0.51 | 0.25 ± 0.07 752.4 ± 1.42 | 11.2 ± 0.52 | 15.8 ± 0.27 | |
| H5:1 | 8.1 ± 0.41 | 0.25 ± 0.06 754.6 ± 1.71 | 12.4 ± 0.54 | 23.0 ± 0.36 | |
| H7:1 | 8.3 ± 0.43 | 0.26 ± 0.07 748.6 ± 2.13 | 12.6 ± 0.51 | 15.1 ± 0.28 | |
Table 7: Characterization of Control Batches
On statistically analysing the physicochemical properties of control batches and batches containing the drug, no significant difference was found between them as the calculated P value was greater than the tabulated P value (0.05) in all the cases. This indicates that the drug does not have any effect on the physicochemical properties of the formulated tablets. The results of statistical analysis have been tabulated in Table 8.
| Batchnumber | Hardness | Friability | Weightvariation | Mucoadhesion | Swellingindex |
|---|---|---|---|---|---|
| C300 | 0.6971 | 0.8271 | 0.6532 | 0.8623 | 0.8254 |
| H300 | 0.8083 | 0.9170 | 0.5518 | 0.8503 | 0.8203 |
| H1:1 | 0.6229 | 0.8925 | 0.5272 | 1.0 | 0.5008 |
| H1:3 | 0.6993 | 0.8857 | 0.9644 | 0.8698 | 0.7254 |
| H1:5 | 0.6722 | 0.9037 | 0.9572 | 0.6888 | 0.6996 |
| H1:7 | 0.8073 | 0.8601 | 0.8045 | 0.8552 | 0.7373 |
| H3:1 | 1.000 | 0.8601 | 0.7570 | 0.6767 | 0.7245 |
| H5:1 | 0.7901 | 1.0 | 0.9366 | 0.8332 | 0.4066 |
| H7:1 | 0.8057 | 0.8696 | 0.8577 | 0.8351 | 0.4007 |
Table 8: Statistical comparison of physicochemical parameters of control batches with batches having drug
Statistical analysis was also carried out for comparison of the physicochemical properties (like hardness, friability, weight variation, assay and drug release) of different batches of tablets (having drug) with different polymer concentrations. The results of statistical analysis have been tabulated in Table 9.
| Parameter analysed | Tabulated | Calculated | Significant difference |
|---|---|---|---|
| P* | P* | ||
| Hardness | 0.05 | 0.0020 | Yes |
| Friability | 0.05 | 0.9696 | No |
| Weight variation | 0.05 | 0.9202 | No |
| Assay | 0.05 | 0.6190 | No |
| Cumulative percentage of drug release at 8 h | 0.05 | 0.0001 | Yes |
Table 9: Statistical analysis of characterization parameters of different formulations by one-way analysis of variance
The hardness of all the batches of tablets was found to be between 7-9 kg. On Statistical comparison of hardness of tablets with different polymer composition, a significant difference was found among them as the calculated P value (0.0020) was less than the tabulated P value (0.05). This indicates that the polymer composition affects the hardness of tablets, although no regular trend was seen in the increase or decrease of hardness on changing the polymer composition. Friability was less than 0.5% for all the batches. The calculated P value (0.9696) for friability was found to be greater than the tabulated value (0.05) on statistical analysis by one way ANOVA, showing no significant difference among the batches with respect to friability. All the batches formulated were found to be within the limits of weight variation as RSD in each case is less than 6% as required by USP/NF 24. Statistical comparison of weight variation of batches with different polymer composition by one way ANOVA showed no significant difference among them as the calculated P value (0.9202) was found to be greater than the tabulated P value (0.05) at 95% confidence limit (Table 9).
All the batches passed the assay test as the results were within limits (355.25-394.75 mg). Statistical analysis of the values of assay of different batches did not show significant difference among them as the calculated P value (0.6190) was greater than the tabulated P value (0.05) (Table 9). The diameter of tablets of all the batches was found to be 12.7 mm and thickness varied between 5.3-5.4 mm, similar dimensions were also found for tablets of control batches.
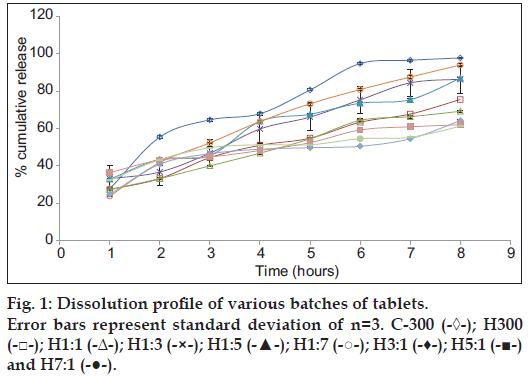
The results of in vitro dissolution studies have been given in Table 10 and the release profile in fig. 1. It was observed that the release of drug varied greatly on changing the polymer-polymer ratio. This is also evident from the statistical comparison of results of % cumulative release at 8 h of different batches, as the calculated P value (0.0001) was less than the tabulated value (0.05). The drug release in hydrophilic matrix tablets occurs through the hydrophilic gel barrier formed around the tablets, and the drug release rate depends on the formation and viscosity of gel layer [25], the extent of swelling and erosion of the polymer chains. Erosion occurs as the polymer chain becomes more hydrated, diluting the gel formed and gradually the gel gets so diluted that disentanglement concentration is reached resulting in erosion.
| Time (h) | C300 | H300 | H1:1 | H1:3 | H1:5 | H1:7 | H3:1 | H5:1 | H7:1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27.7 ± 0.89 | 27.3 ± 0.59 | 27.3 ± 0.59 | 33.1 ± 0.42 | 32.9 ± 0.29 | 23.8 ± 0.32 | 24.8 ± 0.48 | 36.4 ± 0.51 | 32.4 ± 0.55 |
| 2 | 55.4 ± 0.72 | 33.2 ± 0.62 | 33.2 ± 0.62 | 36.7 ± 0.36 | 43.5 ± 0.25 | 41.8 ± 0.22 | 40.9 ± 0.53 | 43.1 ± 0.56 | 43.3 ± 0.64 |
| 3 | 64.6 ± 0.66 | 44.4 ± 0.46 | 40.0 ± 0.41 | 46.8 ± 0.22 | 45.5 ± 0.44 | 52.3 ± 0.43 | 46.5 ± 0.52 | 44.8 ± 0.69 | 49.7 ± 0.39 |
| 4 | 67.9 ± 0.73 | 51.0 ± 0.63 | 46.8 ± 0.82 | 59.7 ± 0.41 | 63.8 ± 0.63 | 63.7 ± 0.52 | 48.9 ± 0.70 | 48.2 ± 0.47 | 51.3 ± 0.47 |
| 5 | 80.6 ± 0.57 | 54.8 ± 0.68 | 54.5 ± 0.77 | 66.1 ± 0.39 | 67.6 ± 0.58 | 73.2 ± 0.71 | 49.7 ± 0.65 | 52.4 ± 0.37 | 51.3 ± 0.53 |
| 6 | 94.7 ± 0.48 | 63.3 ± 0.71 | 64.3 ± 0.81 | 75.3 ± 0.76 | 73.5 ± 0.54 | 80.8 ± 0.64 | 50.5 ± 0.39 | 59.2 ± 0.42 | 54.5 ± 0.78 |
| 7 | 96.5 ± 0.53 | 67.7 ± 0.44 | 66.3 ± 0.75 | 84.4 ± 0.70 | 75.4 ± 0.77 | 87.5 ± 0.59 | 54.5 ± 0.44 | 60.9 ± 0.68 | 55.3 ± 0.49 |
| 8 | 97.7 ± 0.45 | 75.5 ± 0.36 | 69.2 ± 0.63 | 86.3 ± 0.82 | 86.9 ± 0.59 | 93.9 ± 0.75 | 64.2 ± 0.62 | 62.0 ± 0.73 | 61.2 ± 0.47 |
Table 10: Percent Cumulative Drug Release at various time intervals
On comparing the release of drug from C300 and H300 after 2 h it is seen that the release is greater from C300 (approximately 55%), although at the end of 1 h, release is almost the same. Same release at the end of 1 h could be attributed to swelling of both the polymers initially with no erosion. But by the end of 8 h, higher erosion rate of chitosan results in greater release of the drug. Higher erosion rate of chitosan is also supported by swelling index studies in which the C300 tablets kept slipping from the slide at the end of 2 h showing poor and weak gel strength. At the end of 8 h, C300 gives 97.7% release, whereas H300 gives 75.5% release. Visual observation of the tablets at the end of 8 h also shows C300 tablet to be very small in size as compared to H300 tablet also supporting the high erosion rate of chitosan and thus pointing towards a higher disentanglement concentration of the polymer chains. Comparison of release profile of H1:1, H1:3, H1:5, H1:7 shows that as the ratio of chitosan is increased in the tablets, the final release increases. This may also be due to the higher rate of erosion of chitosan. Initially (at the end of 1 h), it is observed that the release of drug from H1:7 is less as compared to H1:1, H1:3, H1:5 batches although the ratio of chitosan used is higher. This may be due to the fact that although chitosan swelled rapidly but its erosion may not have started so soon. This may have resulted in increasing the diffusional pathlength of the drug molecules, due to swelling of the polymer and formation of gel layer around the tablet, thus giving lesser release and a different release pattern from overall release pattern. Diffusional pathlength of H1:5, H1:3, H1:1 batches is less due to lesser chitosan, thus giving higher initial release (in the 1st h, when erosion has not started). Thus release pattern in the 1st h is dominated by the diffusion of the drug molecules rather than by erosion of the polymer chains. The 1st h data shows H1:1 release to be less than H1:3 release, although according to the above explanation it should be higher. This could be attributed to the higher gel strength of the gel formed around the tablets, which resists the movement of drug although the pathlength is less, due to the presence of higher content of HPMC. Content of HPMC in H1:1 batch is double of what is present in H1:3 batch (H1:1 HPMC=150 mg, H1:3 HPMC=75 mg). The size of the tablet left after 8 h is in the order H1:1>H1:3>H1:5>H1:7, clearly showing that the rate of erosion of the tablet is directly proportional to the amount of chitosan used in the formulation.
On comparing the release profile of batches H3:1, H5:1, H7:1, it is observed that release increases from H3:1 to H5:1 batch but is less in H7:1 batch as compared to H5:1 batch. This could be because H3:1 contains greater amount of chitosan, which swells rapidly without eroding initially thus increasing the path to be traversed by the drug to get released and so giving lesser release. H5:1 batch contains lesser chitosan as compared to H3:1 batch and so shows lesser initial swelling, resulting in smaller diffusional pathlength for the drug molecules and thus giving a higher release. The anomalous behavior of H7:1 can be explained on the basis of higher gel strength of HPMC. In H7:1 batch, although the diffusional pathlength for the drug molecules is less as compared to H3:1 and H5:1 batches, due to the presence of very small amount of chitosan, but the presence of large amount of HPMC K4M gives a high gel strength, thus resisting the release of drug molecules. At the end of 8 h, it is observed for these batches that as the ratio of HPMC increases, the percentage of drug released decreases. This is because greater the amount of HPMC, lesser the erosion of the tablet and lesser is the release.
Thus on the basis of release rate studies it can be said that the initial release of the drug is governed by the diffusional pathlength of the drug molecule and the gel strength of the gel formed around the tablet; diffusional pathlength being contributed mainly by the swelling of chitosan and gel strength by HPMC. The latter part of the release is governed by the erosion of the polymers (predominantly that of chitosan).
The R2 values obtained after fitting the dissolution data in various release kinetic models for each batch are given in Table 11. From the R2 values obtained it is seen that Higuchi model is being followed by C300, H1:1, H1:3, H1:5 and H3:1 batches. Batches H300 and H7:1 follow Weibull model and H1:7 and H5:1 follow Hixon-Crowell and Korsmeyer models, respectively. The diffusional exponent (n) of Korsmeyer-peppas equation was calculated as the R2 value for this model for all the batches was greater than 0.9. Table 12 gives the mechanism of release and diffusional exponent from Korsmeyer-peppas equation The calculated diffusional exponent (n) shows anomalous transport for C300, H300, H1:1, H1:3, H1:5, H1:7 batches indicating drug release through diffusion and relaxation or erosion of the polymer matrix.
| Batch number | Hixon- Crowell | Korsmeyer | Weibull | Zero order | First order | Higuchi |
|---|---|---|---|---|---|---|
| C300 | 0.974 | 0.952 | 0.955 | 0.895 | 0.943 | 0.981 |
| H300 | 0.984 | 0.991 | 0.993 | 0.943 | 0.989 | 0.991 |
| H1:1 | 0.966 | 0.972 | 0.954 | 0.920 | 0.978 | 0.989 |
| H1:3 | 0.983 | 0.952 | 0.921 | 0.938 | 0.976 | 0.985 |
| H1:5 | 0.961 | 0.962 | 0.933 | 0.900 | 0.957 | 0.985 |
| H1:7 | 0.995 | 0.994 | 0.984 | 0.950 | 0.964 | 0.990 |
| H3:1 | 0.836 | 0.903 | 0.912 | 0.777 | 0.859 | 0.943 |
| H5:1 | 0.831 | 0.956 | 0.942 | 0.747 | 0.866 | 0.935 |
| H7:1 | 0.763 | 0.941 | 0.948 | 0.692 | 0.797 | 0.911 |
Table 11: R2 values for different release kinetic models
| Batch number | Diffusional exponent (n) | Release mechanism |
|---|---|---|
| C300 | 0.584 | Anomalous diffusion |
| H300 | 0.619 | Anomalous diffusion |
| H1:1 | 0.482 | Anomalous diffusion |
| H1:3 | 0.512 | Anomalous diffusion |
| H1:5 | 0.466 | Anomalous diffusion |
| H1:7 | 0.651 | Anomalous diffusion |
| H3:1 | 0.380 | - |
| H5:1 | 0.263 | - |
| H7:1 | 0.268 | - |
Table 12: Mechanism of release and diffusional exponent from korsmeyer-peppas equation
Mucoadhesive strength for different batches of tablets is given in Table 5. The mucoadhesive strength ranges from 14.5 to 4.6 g. The results clearly indicate that the batch having the maximum amount of HPMC shows the maximum mucoadhesive strength (14.5±0.67). On comparing the results of batches H1:1, H1:3, H1:5, H1:7, it is seen that as the ratio of chitosan increases, mucoadhesive strength decreases. Comparison of results of H3:1, H5:1, H7:1 show that as the ratio of HPMC increases, mucoadhesive strength also increases. Leveling takes place at H5:1 as at H7:1 no further increase in mucoadhesive strength takes place on increasing the ratio of HPMC. The mucoadhesive strength of C300 batch is greater than H1:5 and H1:7 batch, which contain a small amount of HPMC but less than the batches containing substantial amount (that is at least 50% HPMC of the total amount of variable portion of polymer) of HPMC. This suggests that presence of HPMC in larger proportions is necessary to achieve good mucoadhesion.
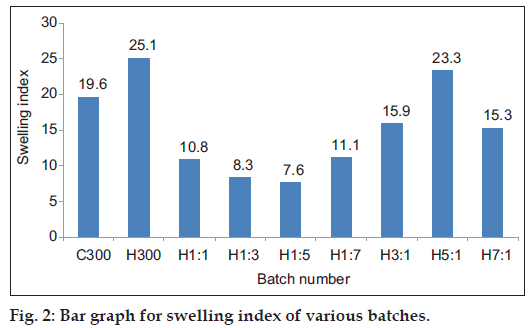
The results of swelling index have been tabulated in Table 6 and graphically represented in fig. 2. The initial swelling (at 1 h) has been greatest for C300 batch(14.9±0.69), which contains maximum chitosan but no HPMC alongwith the other ingredients. These tablets also kept slipping from the glass slide showing that the gel formed on hydration of the polymer did not have good adhesion, probably due to overhydration resulting in the formation of slippery weak gel. Although the initial swelling index of H300 batch was less (10.9±0.62) than C300 batch but it showed the maximum swelling index (25.1±0.51). Also the tablets adhered well to the glass slides and a layer of gel was seen adhering to the sides of the tablets. This suggests that the gel formed was firm and there was no overhydration, making the tablets adhere well to the slides. The C300 batch showed increase in swelling till 150 min, whereas the H300 batch showed an increase till 240 min suggesting that the disentanglement of the polymer chains was faster in chitosan than in HPMC. Comparing the swelling index of H1:1, H1:3, H1:5 and H1:7 batches shows that as the HPMC content is decreased the final swelling index also shows a decrease. Although H1:7 batch is an exception in the sense that the ratio of HPMC:chitosan is the least, still its final swelling index (11.1±0.36) is the highest. This could probably be due to the very low content of HPMC, making it behave like C300 batch. H3:1, H5:1, H7:1 batches showed increase in swelling for a longer period of time i.e. till 240 min, probably due to higher HPMC content. This could be because the HPMC polymer chain disentanglement was slow, thus slowing the rate of erosion of the tablet. It has been observed that as the content of HPMC is increased in H3:1, H5:1 and H7:1 batches, the final swelling also increases. The H7:1 batch shows a final swelling of 15.3±0.24, which is less than H5:1 batch. This anomalous behavior oh H7:1 batch could not be explained. The results of swelling index suggest that a higher proportion of HPMC prolongs the swelling time and also the swelling index whereas a higher ratio of chitosan gives rapid swelling but smaller swelling index for lesser time. From the studies performed the inferences that can be drawn about the characteristics of the polymers used have been given in Table 13.
| Polymer | Swelling rate | Erosion | Release of drug | Mucoadhesion |
|---|---|---|---|---|
| Chitosan | High | High | High | Low |
| HPMC K4M | Low | Low | Low | High |
Table 13: Inferences drawn from the studies performed
Thus based on the above results H1:1 and H1:3 were considered the best batches as they showed optimum swelling, mucoadhesive strength and in vitro drug release characteristics. They had an optimum blend of both the polymers i.e. HPMC and chitosan, which optimized the swelling, mucoadhesion, erosion and drug release characteristics of the tablets. Chitosan showed rapid swelling, high erosion, faster drug release and low mucoadhesion whereas HPMC showed slow but greater swelling, lesser erosion of tablet, slower drug release and high mucoadhesion; a combination of both the polymers gives a formulation with well balanced and desired characteristics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Venkataraman S, Davar N, Chester A, Kleiner L. An overview of controlled release systems. In: Wise DL, editor. Handbook ofPharmaceutical Controlled Release Technology. New York: MarcelDekker Inc.; 2000. p. 431-65.
- Khar RK, Ahuja A, Ali J. Mucoadhesive drug delivery. In: Jain NK,editor. Controlled and Novel Drug Delivery System. 1st ed. New Delhi:CBS Publishers and Distributors; 1997. p. 353-80.
- Bansil R, Turner B. Mucin structure, aggregation, physiologicalfunctions and biomedical applications. Curr Opin Colloid Interface Sci2006;11:164-70.
- Capra R, Baruzzi A, Quinzani L, Strumia M. Rheological, dielectricand diffusion analysis of mucin/carbopol matrices used in amperometricbiosensors. Sens Actuators B 2007;124:466-76.
- Marriott C, Gregory NP. Mucus physiology and pathology. In:Lanaerts V, Gurny R, editors. Bioadhesive Drug Delivery Systems.Florida: CRC Press; 1990. p. 1-24.
- Fiebrig I, Harding S, Rowe A, Hyman S, Davis S. Transmissionelectron microscopy studies on pig gastric mucin and its interactionswith chitosan. Carbohydr Polym 1995;28:239-44.
- Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymericplatforms for controlled drug delivery. Eur J Pharm Biopharm2009;71:505-18.
- Willits RK, Saltzman WM. Synthetic polymers alter the structure ofcervical mucus. Biomaterials 2001;22:445-52.
- He P, Davis S, Illum L. In vitro evaluation of the mucoadhesiveproperties of chitosan microspheres. Int J Pharm 1998;166:75-88.
- Ward PD, Tippin TK, Thakker DR. Enhancing paracellular permeabilityby modulating epithelial tight junctions. Pharm Sci Technolo Today2000;3:346-358.
- Rossi S, Ferrari F, Bonferoni MC, Caramella C. Characterization ofchitosan hydrochloride-mucin interaction by means of viscosimetric andturbidimetric measurements. Eur J Pharm Sci 2000;10:251-7.
- Bernkop-Schnürch A. Mucoadhesive systems in oral drug delivery. DrugDiscov Today Technol 2005;2:83-7.
- Hassan EE, Gallo JM. A simple rheological method for the in vitroassessment of mucin-polymer bioadhesive bond strength. Pharm Res1990;7:491-5.
- El-Kamel A, Sokar M, Naggar V, Al Gamal S. Chitosan and sodiumalginate-based bioadhesive vaginal tablets. AAPS PharmSci 2002;4:E44.
- Ravi Kumar MN. A review of chitin and chitosan applications. ReactFunct Polym 2000;46:1-27.
- Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: Thenext generation. J Pharm Sci 2000;89:850-66.
- Majithiya RJ, Raval AJ, Umrethia ML, Ghosh PK, Murthy RS.Enhancement of mucoadhesion by blending anionic, cationic andnonionic polymers. Drug Deliv Technol 2008;8:40-5.
- Available from: http://www.colorcon.com/products-formulation/allproducts/polymers-controlled-release/hydrophilic-matrix-tablets/methocelcr.[Last accessed on 2015 Jan 26].
- Singla AK, Chawla M, Singh A. Potential applications of carbomer inoral mucoadhesive controlled drug delivery system: A review. Drug DevInd Pharm 2000;26:913-24.
- Fefelova NA, Nurkeeva ZS, Mun GA, Khutoryanskiy VV.Mucoadhesive interactions of amphiphilic cationic copolymers basedon [2-(methacryloyloxy)ethyl]trimethylammonium chloride. Int J Pharm2007;339:25-32.
- British Pharmacopoeia. Vol. 2. London: HMSO Publication; 2002. p.2279.
- Lee JY, Hahn PM, Van Dijk JP, Reid RL. Treatment of menorrhagiawith tranexamic acid. J Soc Obstetrics Gynaecol Can 2000;22:794-8.
- European Medicines Agency. Committee for Proprietary MedicinalProducts (CPMP) Opinion Following an Article 10 Referral: CYKLO-f(tranexamic acid). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Cyklo-f/human_referral_000097.jsp&mid=WC0b01ac0580024e9a#. [Last accessed on2015 Jan 26].
- Agarwal S, Murthy RS. A novel and sensitive colorimetric method forestimation of tranexamic acid in bulk and in pharmaceutical dosageform. Int J Res Pharm Biomed Sci 2012;3:947-50.
- Emeje MO, Kunle OO, Ofuefule SI. The effect of molecular size ofcarboxymethyl cellulose on the rate of hydration, matrix erosion and drug release. Drug Deliv Technol 2005;5:56-60.