- *Corresponding Author:
- P. D. Anumolu
Gokaraju Rangaraju College of Pharmacy, Osmania University, Hyderabad-500 090, India
E-mail: panindrapharma@yahoo.co.in
| Date of Submission | 10 February 2016 |
| Date of Revision | 17 November 2015 |
| Date of Acceptance | 11 December 2014 |
| Indian J Pharm Sci, 2016;78(1):166‑169 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
A simple, rapid, specific and highly sensitive spectrofluorimetric method has been developed for the quantification of dabigatran etexilate mesylate in bulk and capsule dosage form. A linear relationship was found between fluorescence intensity and concentration in the range of 0.01-1.0 μg/ml in dimethyl sulphoxide as solvent at an emission wavelength of 391 nm after excitation at 334 nm, with a good correlation coefficient (0.989). The detection and quantification limits were found to be 0.005 and 0.015 μg/ml, respectively. The proposed method was applied for dabigatran etexilate mesylate capsules, results reveal with percentage recovery of 102% and percentage relative standard deviation values were found to be less than 2 for accuracy and precision studies. The proposed method was validated for linearity, range, accuracy, precision, limit of detection and quantification according to International Conference on Harmonization guidelines. Statistical analysis of the results revealed high accuracy and good precision. The suggested procedures could be used for the determination of dabigatran etexilate mesylate in bulk and capsule dosage form in quality control laboratories of industries as well as in academic institutions.
Keywords
Dabigatran etexilate mesylate, spectrofluorimetry, validation
Dabigatran etexilate mesylate (DAB) chemically ethyl 3-(1{2 [({4 [amino ({ [(hexyloxy) carbonyl] imino}) methyl] phenyl} amino) methyl]-1-methyl- 1H-1,3-benzodiazol-5-yl}-N-(pyridin-2-yl)formamido) propanoate is a selective anticoagulant, used as stoke and systemic embolism in patient with atrial fibrillation. Literature survey revealed that, few methods were reported for quantification of DAB by UV/Vis spectrophotometry [1,2] and RP-HPLC [3,4]. To the best of our knowledge, no method reported on the use of spectrofluorimetry for the quantification of DAB. Spectrofluorimetry has assumed a major role in drug analysis because of its greater sensitivity and selectivity than absorption spectrophotometry [5-11]. Hence, we aimed to develop and validate a simple, precise, accurate, selective and high sensitive spectrofluorimetric method for the estimation of DAB in pharmaceutical dosage form.
Dabigatran etexilate mesylate (DAB) pure drug was obtained as gift samples from Neuland Laboratories Limited, Hyderabad, India. Brand name is Pradaxa 110 mg, Boehringer Ingelheim Pharma, India. Methanol was purchased from Qualigens, Mumbai. Hydrochloric acid, glacial acetic acid, sodium hydroxide, DMSO, DMF, acetonitrile and ethanol was purchased from S.d. fine-Chem Ltd., Mumbai, India. The fluorescence spectra and measurements were recorded using Shimadzu RF-5301 PC Spectrofluorophotometer, equipped with 150 watt Xenon arc lamp, 1 cm nonfluorescence quartz cell was used, connected to RFPC software.
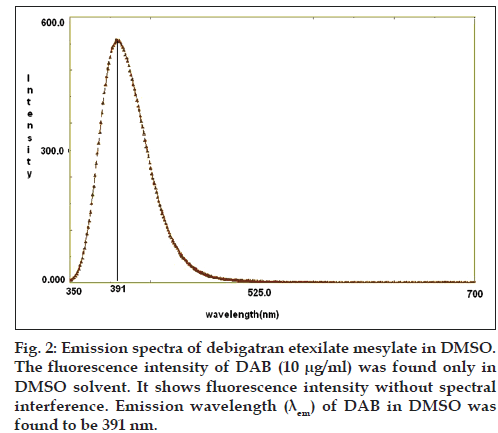
DAB molecule contains polycyclic aromatic systems like pyridine ring and benzodiazolin ring, in which more π electrons are available to exhibit fluorescence (fig. 1). For spectrofluorimetric method purpose, various solvents were investigated such as acetonitrile, methanol, DMSO, ethanol, DMF and different buffer systems. The fluorescence intensity of DAB was found only in DMSO solvent. Hence, we selected DMSO as solvent for quantification of DAB in bulk and pharmaceutical formulation at emission wavelength 391 nm after excitation wave length 334 nm as shown in fig. 2.
Standard DAB (10 mg) was weighed and transferred into 10 ml volumetric flask and dissolved in dimethyl sulphoxide (DMSO). The flask was shaken and volume was made up to the mark with DMSO. Further the stock solution was diluted with DMSO to get final concentration of 10 μg/ml and fluorescence intensity quantified by spectrofluorimeter.
The calibration curve was constructed by plotting the analyte fluorescence intensity against the concentration (μg/ml). Calibration curve was evaluated by its correlation coefficient. The stability of the drug was measured in DMSO and DAB was found to be stable for 72 h.
For the assay, twenty capsules of marketed formulation (Pradaxa) each containing 110 mg of DAB were taken and accurately weighed. Capsules were emptied and weight of granules was calculated by subtracting weight of each empty shell from each capsule weight. Average weight was determined and an accurately weighed quantity of granules equivalent to 10 mg of DAB was transferred into 10 ml volumetric flask and dissolved in 5 ml of DMSO and sonicated for 10 min. Then the volume is made up to mark with the DMSO and filtered through whatman filter paper. Further the filtrate was diluted with DMSO to get final concentrations within linearity range for quantification of DAB. Results were in good agreement with the label claim of the drug and reported in Table 1. The proposed method validated according to ICH guidelines [12].
| Parameter | Value |
|---|---|
| Excitation wavelength (nm) | 334 |
| Emission wavelength (nm) | 391 |
| Range (µg/ml) | 0.01–1.0 |
| Limit of detection (μg/ml) | 0.005 |
| Limit of quantification (μg/ml) | 0.015 |
| Correlation coefficient (r2) | 0.987 |
| Slope (m) | 154.1 |
| Intercept (c) | 12.37 |
| Regression equation | Y=154.1x+12.37 |
The equation of the regression line was found to be Y=154.1x+12.37 with a correlation coefficient of 0.987, revealed that DAB was showing linear relationship between concentration (μg/ml) and fluorescence intensity. DAB was linear in the range of 0.01–1.0 μg/ml. The LOD and LOQ of the method were found to be 0.005 and 0.015 μg/ml respectively, which indicate the sensitivity of the method. DAB: Dabigatran etexilate mesylate, LOD: limit of detection, LOQ: limit of quantification
Table 1: Optimum Conditions Of Proposed Method
A linear calibration plot for the method was obtained over the calibration range of 0.01–1 μg/ml at an emission wavelength of 391 nm. The equation of the regression line was found to be Y=154.1x+12.37 with a correlation coefficient of 0.987. Optimum conditions of proposed method mentioned in Table 2. The percentage recoveries were conducted at three different levels (80, 100 and 120%) as per standard addition method and results of recovery were found to be in the range of 98.8-102.4%, indicates that the method is accurate. Results from precision expressed in terms of %RSD, found to be less than 2. No significant differences between intraday and interday precision, which indicated that the method is reproducible and reliable. The results of limit of detection (LOD) and limit of quantification (LOQ) revealed that proposed method was highly sensitive than previous methods. The % assay in commercial formulations was found to be in the range 98-102% by the proposed method and % RSD was less than 2, which indicates the accuracy of the proposed method. The results of the assay (Table 1) indicated that the method is specific for the assay of DAB without interference from excipients used in pharmaceutical formulations.
| Formulation | DAB | ||
|---|---|---|---|
| Label claim(mg/ml) | Amount found (mg) (Amount±SD) | % RSD | |
| Pradaxa 110 mg | 110 | 110.9 ±0.152 | 0.137 |
The amount of DAB found in formulation pradaxa was 10 mg. These amounts were within the limits. The % RSD for both formulations was <1, which indicates the accuracy of the proposed method, n=3. DAB: Dabigatran etexilate mesylate, RSD: relative standard deviation, SD: standard deviation.
Table 2: Analysis Of Formulation
It is concluded that the contemplated method was found to be highly sensitive from the LOD and LOQ values compared to the previously reported methods and simple for the quantification of DAB in pure form and pharmaceutical dosage form. The assay values were in good agreement with their respective label claim, which suggested no interference of formulation excipients in the estimation. The results obtained from validation proved that; the proposed method was scientifically sound. These advantages encourage that the proposed method can be routinely employed in quality control for analysis of DAB in tablet formulation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ankit P, Kumar S, Kumar SA, Aarti Z, Seth AK. Spectrophotometric method for estimation of dabigatranetexilate in bulk and its pharmaceutical dosage form.Int J Pharm Sci 2014;5:31-9.
- Anumolu PD, Archana G, Sunitha G, Rachel PK, Harika H, Sowndarya NS. Simplistic application of 3-methyl-2-benzothiazolinehydrazone (MBTH) an oxidative coupling chromogenic reagent for quantification of metaxalone and dabigatranetexilatemesylate bulk drug and their dosage forms. Pharm Anal Acta 2015;6:1-5.
- Pradeep GS, Chandewar AV. Validated stability-indicating high performance liquid chromatographic assay method for the determination of Dabigatranetexilatemesylate. Res J Pharm Bio ChemSci 2014;5:1637-44.
- Sekhar Reddy BR, VijayaBhaskar RN. Stability indicating RP-HPLC method for estimation of dabigatran in pure and pharmaceutical dosage forms. South Pac J Pharm Bio Sci 2014;2:80-92.
- Fawazia I, Mohie KS, Manal I. Validated stability-indicating spectrofluorimetric methods for the determination of ebastine in pharmaceutical preparations. Chem Cent J 2011;11:1-14.
- Mesut A, Emrah K. Validated spectrofluorimetric method for the determination of sunitinib malate, dyecomplexation approach for a noval anticancer drug. Acta Pharm Sci 2010;52:469-85.
- Mohamed W, Fathallah FB. Synchronous fluorescence spectrofluorimetric method for the simultaneous determination of metaprolol and felodipine in combined pharmaceutical preparation. Chem Cent J 2011;70:489-93.
- Panikumar AD, Sirisha N, Haripriya A, Babu RS, Subrahmanyam CV. First derivative synchronous spectrofluorimetric quantification of telmisatran/amlodipine besylate combination in tablets. Dhaka Univ J Pharm Sci 2013;12:35-40.
- Sunitha G, Bhagirath R, Alapati VR, Ramakrishna K, Subrahmanyam CV, Anumolu PD. Fluorimetric quantification of brimonidine tartrate in eye drops. Indian J Pharm Sci 2013;75:730-2.
- Panikumar DA, Sunitha G, Bagirath R, Santoshi VP, Archana G. Zero-crossing point derivative simultaneous spectrofluorimetric method for quantification of nebivolol hydrochloride and valsartan combination in tablets.Int J Pharm Sci Rev Res 2014;27:164.
- Kavitha A, Vijaya DD, Himabindu S, Panikumar DA. Forced degradation studies, quantification and in-vitrodissolution studies oftadalafil by spectroflourimetry. Asian J Pharm Clin Res 2013;6:326-9.
- International Conference on Harmonization. Harmonized Tripartite Guideline, Validation of Analytical of Procedures, Text and Methodology, Q2 (R1); 2005.