- *Corresponding Author:
- Rajani Mathur
Department of Pharmacology, Delhi Institute of Pharmaceutical Sciences and Research, Pushp Vihar, New Delhi-110 017, India
E-mail: mathurajani@yahoo.com
| Date of Submission | 4 January 2011 |
| Date of Revision | 28 April 2011 |
| Date of Acceptance | 29 April 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 303-306 |
Abstract
Tridax procumbens L. (Compositae) is a common weed that grows in the rice fields of India. Traditionally the juice from leaves of Tridax procumbens has been used for healing dermal wound. However, in experimental studies, equivocal pro and anti-healing action of T. procumbens has been demonstrated. The present study evaluates the effect of topical ointment formulation of the leaf juice of T. procumbens using excision wound model in mice. Excision wounds (4 mm, i.d.) were inflicted on depilated back of mice. Ointment formulation of TP (50 mg of either 1 or 4 mg/g) was applied twice daily for 4 days on the dermal wound. Similarly, control group was treated with VEGF ointment (50 mg of 1 μg/g). The parameters observed were re-epithelization, vascularity, fibroblast number, collagen content. The healing exerted by TP (1 mg/g) was comparable to VEGF (1 μg/g). On the other hand, TP (4 mg/g) induced inflammation, edematous tissue and decreased vascularity. Taken together, the results imply that TP possesses dose dependent pro-healing potential, and its high dose exerts inflammatory reaction.
Keywords
Excision wound, Tridax procumbens, topical ointment formulation, VEGF, wound healing
Dermal wound is a common pathologic condition and may be defined as any break in the integrity of the skin. It is associated with high degree of morbidity due to blood loss, pain, edema, inflammation and loss of functionality. Cutaneous wound are characterized by migration and proliferation of fibroblasts, endothelial and epithelial cells, deposition of connective tissue, angiogenesis, re-epithelization, and finally contraction of wound [1].
Proper healing of wounds is essential for restoration of disrupted anatomical continuity and disturbed functional state. Impaired healing of open wounds is one of the troublesome complications that have been recognized for many years. It is debatable, whether systemic drugs can hasten healing in a nutritionally and endocrinally normal individual. In such cases, the basic principles of wound healing that include minimizing tissue damage, debriding nonviable tissue, maximizing tissue perfusion and oxygenation, proper nutrition, and a moist wound healing environment prove to be most useful [2]. Thus, a drug that can enhance vascularization, re-epithelization, and collagenation, when applied topically, should prove ideal.
Herbs belonging to the Compositae family have been described in ancient Indian medicine system for the management of wounds. Traditionally in India, the fresh juice of Tridax procumbens leaves have been used as one of the most popular remedy for dermal wounds [3]. In excision wound model, systemic administration (intraperitoneal) of juice from leaves of T. procumbens has been implicated with both pro and antihealing properties [4,5]. In order to clearly establish its activity, the present study was undertaken to evaluate the effect, if any, of topical ointment formulation of juice from leaves of T. procumbens on paradigms of dermal wound healing.
Fresh leaves of T. procumbens were collected from the residential campus of All India Institute of Medical Sciences, New Delhi, India. All reagents were purchased from S. D. Fine Chemicals, Mumbai, India. The study protocol for using animals was approved by the standing animal ethics committee of All India Institute of Medical Sciences, New Delhi, India and all experiments were conducted in accordance with the guidelines laid down by the same. Male Swiss albino mice (20-35 g) were used in the study and maintained under standard laboratory conditions of housing, food and water.
Fresh leaves of the plant T. procumbens were collected, cleaned and wiped dry. They were ground fine in a mortar, without adding water or any other vehicle. The juice was strained through clean muslin, and further filtered through Whatman No.1 filter paper. The filtrate was centrifuged at 1000 rpm for 15 min at 4° (Sorvall® RC-5B, DuPont Company, USA). The supernatant was lyophilized to yield dry powder, which was labeled as TP. The yield of TP as calculated from fresh leaves was 2% w/w.
Using standard reagents and protocols the CM was subjected to phytochemical evaluation to test the presence of alkaloids (Mayer’s, Hager’s, Wagner’s and Dragendorff’s test), reducing sugars (Fehling’s test), deoxysugars (Keller-kiliani test), steroids (Liberman- Buchard test) and tannins (FeCl3 test) [6].
The ointment base was developed by mixing PEG 4000 and PEG 400 in the formulating ratio of 2:3 with continuous triturating and heating. Further, 100 μl of distilled water was added to the ointment base. The lyophilized T. procumbens leaf powder was formulated into the base to attain TP in the final concentrations of 1 and 4 mg/g. Similarly, ointment base containing VEGF in the concentration of 1 mg/g of base was prepared. The formulated ointments were stored at 4°, till further use.
Under pentobarbitone anaesthesia (60 mg/kg, ip), two excision wounds were punched on depilated back of each mouse, using skin borer (4 mm, i.d). The day of surgery was accounted as day zero. Drug was applied topically from the day following the surgery for total duration of 4 days. The animals of treatment group received the drug twice daily. The quantity of ointment was standardized, such that constant volume approximating 50 mg base was applied over both wounds, each time. Animals in the positive control group received equal volume (50 mg base) of VEGF ointment (1 mg/g). The mice of the vehicle control group received ointment base only (50 mg) for the experiment duration.
On the 5th day the newly formed tissue was carefully excised from the mice back under anesthesia. Wound biopsies were fixed in 10% formalin solution and sections (4 mm) were cut and stained with haematoxylin and eosin. Sections were histopathologically assessed under light microscope and graded in respect of re-epithelization, vasculartity and fibroblast content.
In the other biopsy collagen content was estimated by Sircol reagent kit (Biocolor INC, UK). Acid soluble collagen was estimated using collagen-Type I as a standard. Briefly, punched skin was mixed with 500 μl of 0.5M acetic acid and homogenized and centrifuged at 19,000 rpm for 30 min. To a 10 μl aliquot of supernatant, 90 μl of 0.5M acetic acid and 1 ml of Sircol reagent was added (Sircol collagen assay kit, Biocolor, UK) and vortexed for 30 min. It was re-centrifuged at 19,000 rpm for 30 min. The supernatant was decanted and the pellet was reconstituted in 2 ml of 0.5M NaOH. The color complex was measured at 540 nm (Beckman DU640, USA).
The data obtained from excision wound model were analyzed for statistical significance using Student t-test (unpaired) and P<0.05 considered significant.
Following the battery of qualitative tests conducted for the presence of alkaloids, carbohydrates, saponins, glycosides (C and O) and steroid, the extract tested positive for steroids, saponins, glycoside (O type) and carbohydrates, which is in agreement with earlier reports [7].
Wounds treated with TP (1 mg/g) and VEGF (1 μg/g) exhibited marked dryness and there was no visual sign of inflammation or any pathological fluid oozing out from the wounds as compared to vehicle treated wounds. In contrast, wounds treated with TP (4 mg/g) were wet and soft to touch.
In excision wound model of mice the collagen content was estimated by using sircol kit and the values are tabulated in Table 1. Wounds treated with VEGF (1 μg/g) and TP (1 mg/g) exhibited a significant increase of 47 and 33.81%, respectively in collagen levels as compared to vehicle control group (P<0.05). However, the collagen content of wounds treated with TP (4 mg/g) was markedly less by 29%, as compared to control wounds.
| Groups | Collagen (µg) |
|---|---|
| Vehicle control | 34.3 ± 3.2 |
| VEGF (1 mg/g) | 50.45 ± 5.1* |
| TP (1 mg/g) | 45.9 ± 2.8* |
| TP (4 mg/g) | 23.06 ± 3.5 |
| *P<0.05 vs control |
* P<0.05 vs control
Table 1: Collagen Content Of Dermal Sections From Mice Of Different Groups At 5 Days Post Wounding In Excision Wound Model
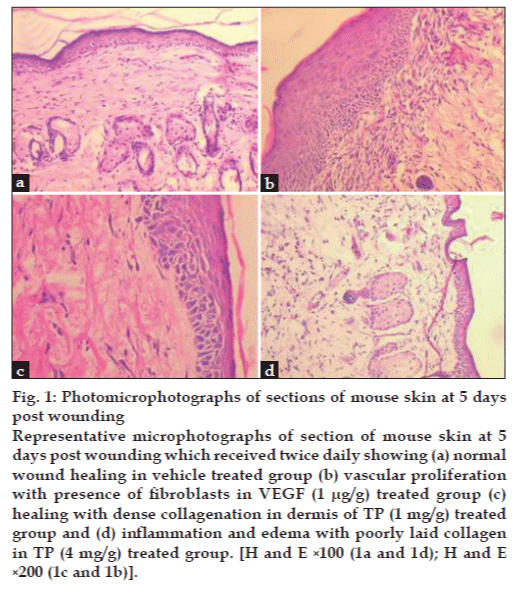
Histopathological evaluation of dermal section revealed that as compared to vehicle control group, topical application of TP (1 mg/g) on dermal wounds increased the infiltration of inflammatory cells, fibroblasts and re-epithelization with moderate vascularity (figs. 1a and c). Treatment with VEGF (1 μg/g) led to marked increase in vascularity, fibroblast number and re-epithelization (fig. 1b and Table 2). However, treatment with TP (4 mg/g) elicited inflammatory response as indicated by ulcerous and edematous epithelium in the histopathology sections (fig. 1d).
Figure 1: Photomicrophotographs of sections of mouse skin at 5 days post wounding Representative microphotographs of section of mouse skin at 5 days post wounding which received twice daily showing (a) normal wound healing in vehicle treated group (b) vascular proliferation with presence of fibroblasts in VEGF (1 μg/g) treated group (c) healing with dense collagenation in dermis of TP (1 mg/g) treated group and (d) inflammation and edema with poorly laid collagen in TP (4 mg/g) treated group. [H and E ×100 (1a and 1d); H and E ×200 (1c and 1b)].
| Group | Re-epithelization | Fibroblast number | Vascularity |
|---|---|---|---|
| Vehicle control | ++ | + | + |
| VEGF (1 mg/g) | ++ | +++ | +++ |
| TP (1 mg/g) | + | ++ | ++ |
| TP (4 mg/g) | + | + | ++ |
| + Slight; ++ Moderate; +++ Marked |
+ Slight; ++ Moderate; +++ Marked
Table 2: Relative Grading Of Dermal Sections On Histology Findings At 5 Days Post Wounding In Excision Wound Model In Mice
Wound healing is a complex multiphase process that involves a chain of well-orchestrated biochemical and cellular events. The process can be broadly classified in three stages- inflammation, proliferation and remodeling. The participation of various inflammatory cells is crucial for repair process. These cells promote migration and proliferation of endothelial cells, leading to neovascularization[1]. The proliferative phase is characterized by angiogenesis, collagen deposition, granulation tissue formation, epithelization and wound contraction. Finally the fibroblasts grow and form extracellular matrix as part of tissue remodeling. These interlinked events are controlled by specific growth factors and cytokines at site of injury[8]. Impaired wound healing causes morbidity for patient and may lead to complication like- dehiscence and chronic wound healing ulcer[9]. Currently, the mainstay of treatment modality is steroid application with supportive antibiotics, which is fraught with unwanted side effects. Therefore, there is a need to develop therapeutic agents, which augment healing process.
The dermal wound healing property of fresh leaves of Tridax procumbens in the regional literature is well documented [4,10]. The practice common amongst the farmers of Indian villages is to crush the fresh leaves and let the juice cover the dermal wound. Attempts have been made to corroborate the traditional use of leaves of T. procumbens by way of pharmacological assays [4]. In excision wound model, juice of fresh leaves of T. procumbens was administered intraperitoneally to rabbits. An equivocal response was elicited wherein enhanced re-epithelization of wound was recorded on one hand, and retardation of scar contraction and granulation, on the other [4]. It was postulated that extract of T. procumbens is essentially prohealing but also has corticotropic influence (as evidenced by increase in adrenal weight and decrease in thymus weight in rat experiments). The corticotropic effect might indirectly retard the healing process by enhancing endogenous secretion of cortical hormones, which are known to inhibit all the phases of wound healing [5].
Therefore, the present study was conducted to unequivocally explore the effect of T. procumbens on dermal wound healing. The study was designed to investigate the effectiveness of topical formulation of T. procumbens leaves in experimentally induced dermal wound in mice. Topical formulations are preferred choice for healing dermal wounds as they are locally well absorbed to produce pharmacodynamic action effectively [11]. Secondly, topical formulations help to circumvent adverse events associated with systemic administration of the drug and it was hypothesized that the reported antihealing effects of T. procumbens could be attenuated by this approach. In addition, this approach is in tandem with the traditional use of this medicinal plant involved topical application and is cited to be effective.
We report here that as a topical formulation, TP (1 mg/g) was found to be effective in healing dermal wound and its pharmacodynamic effect was comparable to VEGF (1 μg/g) treatment. TP (1 mg/g) acted by stimulating collagen synthesis, which has been reported to be an essential step in faster healing of wound [12]. Further, histopathological evaluation of dermal wounds indicated fibroblast proliferation accompanied with neovascularization in the TP (1 mg/g) treated group. Wound healing in any tissue follows a predictable sequence of events with the aim to restore damage tissue as closely as possible to its normal state. This study clearly demonstrates that TP augments proliferation and remodeling stages of wound healing. However, this is a dose dependent phenomenon, as the higher dose exhibited antihealing property, which is in confirmation with earlier reported observations[4]. Further studies are required to delineate the mechanism underlying the anti-healing effects of Tridax procumbens.
Acknowledgements
We thank Dr. Shiladit Sengupta, MIT, USA for the kind gift of VEGF.
References
- Clark RAF. Cutaneous wound repair. New York: Oxford University; 1991. p. 576.
- Barua CC, Talukdar A, Begum SA, Sarma DK, Fathak DC, Barua AG, et al. Wound healing activity of methanolic extract of leaves of Alternanthera brasiliana Kuntz using in vivo and in vitro model. Indian J Exp Biol 2009; 47:1001-5.
- Upadhyay B, Parveen, Dhaker AK, Kumar A. Ethnomedicinal and ethnopharmaco-statistical studies of Eastern Rajasthan, India. J Ethnopharmacol 2010;129:64-86.
- Diwan PV, Tilloo LD, Kulkarni DR. Influence of Tridax procumbens on wound healing. Indian J Med Res 1982;75:460-4.
- Diwan PV, Tilloo LD, Kulkarni DR. Steroid depressed wound healing and Tridax procumbens . Indian J Physiol Pharmacol 1983;27:32-6.
- Trease GE, Evans WC. A textbook of pharmacognosy. Oxford: ELSB Baillere Tindal; 1987. p. 1055.
- Kasture AV, Wadodka SG. Preliminary phytochemical study of Tridax procumbens Linn. Indian J Pharmacol 1971;33:96.
- Bennet NT, Schultz GS. Growth factors and wound healing: Biochemical properties of growth factors and their receptor. Am J Surg 1993;165:728-37.
- Goodson, Hunt TK. Wound healing and diabetic patient. Sur GynecolObst 1979;149:600-8.
- Udupa SL, Udupa AL, Kulkarni DR. Influence of Tridax procumbens on lysyl oxidase activity and wound healing. Planta Med 1991;57:325-7.
- Shukla A, RasikAM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica . J Ethnopharmacol 1999;65:1-11.
- Maquart FX, Bellon G, Gillery P, Wegrowski Y, Borel JP. Stimulation of collagen synthesis in fibroblast culture by a triterpene extract from Centella asiatica . Connect Tissue Res 1990;24:107-20.