- *Corresponding Author:
- G. Zhao
National Research and Development Center for Coarse Cereal Processing, Chengdu University, Chengdu 610 106, People’s Republic of China
E-mail: zhaogang@cdu.edu.cn
| Date of Submission | 26 September 2013 |
| Date of Revision | 19 December 2014 |
| Date of Acceptance | 11 November 2015 |
| Indian J Pharm Sci, 2015;77(6):661-667 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The rutin, quercetin concentrations, antioxidant activity, and aroma compounds in different commercial tartary buckwheat tea were analyzed in our study. Results revealed that the materials and the processing protocol affected the chemical composition and activity of tartary buckwheat tea. Rutin and quercetin concentrations, antioxidant activity were significantly different in various kinds of tartary buckwheat tea, where the whole bran tea and the whole plant tea had the lower rutin, but higher quercetin concentrations and higher antioxidant activity. The whole embryo tea had the converse results. There was strong correlation between quercetin concentration and antioxidant activity (r>0.98, P<0.05). Meanwhile, Twenty eight different aroma compounds in tartary buckwheat tea were identified by gas chromatography-mass spectrometry. Those compounds were mainly composed of pyrazine, aldehydes, fatty acids and ketones. The main type of aroma compounds in different tartary buckwheat tea were similar, but their relative contents were different. The implications to the quality control of buckwheat tea were extensively discussed.
Keywords
Tartary buckwheat tea, rutin, quercetin, antioxidant, aroma compounds, gas chromatography-mass spectrometry
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn), belongs to the Polygonaceae family, has been widely planted around the world as food and drinks [1]. Great values of buckwheat from the nutriological and pharmacological views had been reported [2]. Buckwheat contains many beneficial components including flavonoids, phenolic compounds, D-fagomine, fagopyrins, and D-chiro-inositol [3-5]. Our previous research also found that trace emodin was existed in tartary buckwheat and its products [6]. Such components have been reported to help control blood glucose and blood pressure levels [7-9]. Moreover, buckwheat has antioxidant, antitumor activity [10,11]. Great efforts are paid to the development of buckwheat products recently, since the values of buckwheat are received more and more attention from consumers. Many new buckwheat products are introduced every year such as tea, wine, cracks, sauce, noodles, vinegar and capsules. Among these products, tartary buckwheat tea was one of the most popular buckwheat products in China, Japan, South Korea and European countries. Our previous research indicated tartary buckwheat tea contains many beneficial trace elements [12], and buckwheat tea is a good supply of essential elements.
According to the difference of raw material, tartary buckwheat tea mainly includes tartary buckwheat whole bran tea, tartary buckwheat whole plant tea and tartary buckwheat whole embryo tea. Whole bran tea is made from tartary buckwheat bran; the material of whole plant tea mainly includes buckwheat bran, flour, flower, stem and root. Germinating seeds were the mainly material of whole embryo tea. Beside, the processing protocol of these three kinds of tartary buckwheat tea is also different. Whole embryo tea is mainly made using five manufacturing processes including soaking, steaming, drying, dehulling, and roasting. To process whole bran tea and whole plant tea, buckwheat material was grounded, mixed with water, extruded after blending, then dehydrated, dried and roasted. Some reports indicated activity and chemical properties of buckwheat and its products were affected by different processing method [13-15]. Rutin-degrading enzyme, contained in buckwheat [16,17], was one of the most important factors that affected the properties of buckwheat products through changing the composition of flavonoids [14,18]. Rutin and quercetin are the mainly flavonoids in tartary buckwheat tea, its concentration affected by the processing method, which can change the activity of the rutin-degrading enzyme, thus, the quality of these tartary buckwheat tea should be different and need to be defined.
On the other hand, the flavor among these three kinds of tartary buckwheat tea was also different. The volatile aromatic compounds of tartary buckwheat and its products have been investigated recently. A total of 48 compounds were quantified and their odor activity values (OAV) were calculated in tartary buckwheat and its milling fraction by Janes [19]. Qin and others made a study on aroma of tartary buckwheat tea and identified 77 compounds, including furanoids, aldehydes and pyrazines using three extraction methods [20], but none of them compared the difference of volatile aromatic compounds in various tartary buckwheat tea.
While great efforts are paid to the market development of tartary buckwheat tea, the quality control of buckwheat tea is scarce currently. Flavonoid is one of the most important quality control fators in tartary buckwheat tea, but not defined in various products. Herein, we focused on describing the differences of rutin, quercetin content, antioxidant activity and aroma compounds composition in different kinds of tartary buckwheat tea, to provide a reference for improving the quality standards of tartary buckwheat tea, and to give some useful imformation to the consumer.
Materials and Methods
Tartary buckwheat teas were obtained from local supermarkets (Table 1). The teas were dried, shattered and then passed a 40 mesh screens sieve. Rutin and quercetin standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). 1,1-diphenyl- 2-picrylhydrazyl radical (DPPH•), 2,2'-azinobis- (3-ethylbenzthiazoline-6-sulfonate) (ABTS) was purchased from Sigma, methanol (high performance liquid chromatography (HPLC) grade) was purchased from Fisher Scientific Co., (USA). All others chemicals and solvents used in the study were of analytical grade.
| Code number | Brand | Trade name | Producing area |
|---|---|---|---|
| S1 | Yixiangren | Tartary buckwheat whole bran tea | Sichuan |
| S2 | Yixiangren | Tartary buckwheat whole plant tea | Sichuan |
| S3 | Yixiangren | Tartary buckwheat whole embryo tea | Sichuan |
| S4 | Sanjiang | Tartary buckwheat whole bran tea | Sichuan |
| S5 | Sanjiang | Tartary buckwheat whole plant tea | Sichuan |
| S6 | Sanjiang | Tartary buckwheat whole embryo tea | Sichuan |
Table 1: Sources Of The Commercial Buckwheat Tea Samples
Determination of the flavonoid content
The determination method was according to our previously reported method [21] with slight modification. The dried powder was passed through the 40 mesh screens. The extraction was performed by mixing 0.5 g of sample with 25 ml of methanol:water (80%, v/v) solution in one conical flask under sonication for 30 min at room temperature. Extract was passed through 0.45 μm filter and then placed in a HPLC autosampler vial for immediate HPLC analysis. The HPLC system was equipped with two LC-10ATvp pumps and a SPD-M10Avp diode-array detector (Shimadzu, Japan). A Diamonsil C18 column (4.6 mm×250 mm, 5 μm) was used. Separation was performed by using a mixture of acetonitrile and distilled water containing 0.3% H3PO4, with a gradient elution: 0–8 min (20% acetonitrile), 8–13 min (20–40% acetonitrile), 13–29 min (40% acetonitrile), 29–29.1 min (40–20% acetonitrile), and 29.1–30 min (20% acetonitrile). The flow rate was set at 1.0 ml/min, and UV detection at 365 nm. The temperature of the column was set at 30°, and the sample injection volume was 20 μl. Identification of rutin and quercetin were achieved by comparing the retention time of samples with those of the standards. Rutin and quercetin were quantified by using external standard method.
1,1-diphenyl-2-picrylhydrazyl radical scavenging activity assay
The method described by Guo et al. was used with slight modification to assess the DPPH radical scavenging activity of buckwheat teas extracts [22]. One milliliter of the appropriate dilution of extract or control was mixed with 4.0 ml of 0.124 mg/ml DPPH radical solution. The mixture was shaken vigorously and left to stand at room temperature for 30 min in the dark. The mixture was measured spectrophotometrically at 517 nm. A standard of Trolox was run using several concentrations ranging from 17.08 μmol/l to 273.25 μmol/l. A standard curve was then prepared by plotting the percentage (%) of free radical scavenging activity of Trolox versus its concentration. The final result was expressed as μmol Trolox equivalent antioxidant capacity in 1 g of sample (μmol Trolox eq./gDW). Data was reported as mean±SD for at least three replicates.
2,2’-azinobis-(3-ethylbenzthiazoline-6-sulfonate)•+ scavenging activity assay
The method was according to previously reported method [22] with sligfht modification. ABTS•+ was dissolved and adjusted to 5 mmol/l with phosphate buffer (pH 7.4), after oxidizing with manganese dioxide, left to stand under ambient temperature for one night. The final reaction mixture contained 3.0 ml of ABTS•+ with an absorbance of 0.7 adjusted by phosphate buffer (pH 7.4) at 734 nm and 200 μl of the appropriate dilutions of extracts or 200 μl of distilled water for the control. The absorbance at 734 nm was measured after a reaction time of 1 min. The final result was expressed as μmol Trolox eq./gDW that were calculated using a standard curve prepared with Trolox. Data was reported as mean±SD for at least three replicates.
Aroma compounds analysis
The volatile compounds of tartary buckwheat tea were extracted using solid-phase microextraction (SPME). DVB/CAR/PDMS SPME 50/30 μm fiber was used (Supelco, USA). Tartary buckwheat tea powder (3 g) was put into a 25 ml glass vial, crimped, and kept at 80° for 40 min. The SPME fiber was inserted into the headspace and the compounds were sampled for 60 min. The fiber was subsequently inserted into the injector port of a gas chromatograph and desorbed for 5 min under 260°.
The samples were analyzed using a gas chromatograph (HP6890) equipped with a mass spectrometric detector (HP5973). The volatile compounds were separated with a HP-5 MS capillary column (30 m×0.25 mm×0.25 um), Agilent, USA. Oven temperature for SPME injection was programmed from 50° at 10°/min to 200°, then at 15°/min to 290°. The injector temperatures were set at 260°. MS conditions: Electron impact ionization mode, temperature of ion source was 250º, total ion current (TIC) was recorded.
Statistical analysis
All treatments were performed in triplicate, and the results were represented by their mean values and the standard deviations (SD). The data were submitted to analysis of variance to detect significant differences and the correlation analysis was performed with SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Results and Discussion
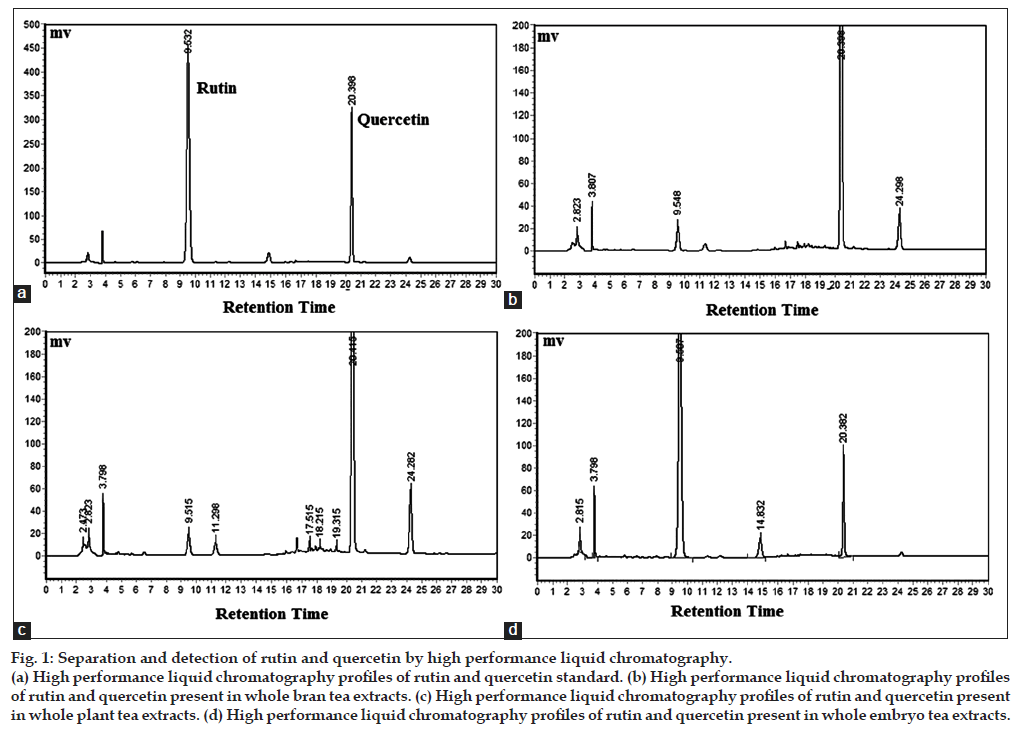
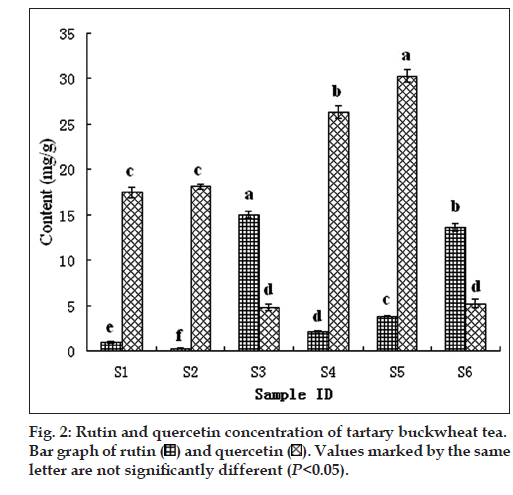
There were mainly 3 categories of commercial buckwheat teas in our study, including tarary buckwheat whole plant tea, tarary buckwheat whole bran tea and tarary buckwheat whole embryo tea. Here, we analyzed the commercial products from Yixiangren and Sanjiang (Table 1). As shown in fig. 1, UV enabled the detection of rutin and quercetin as a distinct single peak with a retention time of 9.532 min and 20.398 min, respectively. As listed in fig. 2, the flavonoid concentrations of buckwheat teas were different significantly. The whole bran tea (S1, S2) and the whole plant tea (S4, S5) had the lower rutin concentrations (0.96, 0.23, 2.09, and 3.83 mg/g, respectively) but had the higher quercetin concentrations (17.41, 18.04, 26.26, and 30.29 mg/g, respectively). The whole embryo tea (S3, S6) had the converse results, 15.03 mg/g of rutin and 4.78 mg/g of quercetin in S3; 13.62 mg/g of rutin and 5.22 mg/g of quercetin in S6. This result may be cause by the rutin-degrading enzymes contained in tartary buckwheat. Mixing with water in the processing of whole bran tea and whole plant tea caused rutin-degrading enzymes in buckwheat flour to easily be accessible to rutin, which consequently was degraded into quercetin. These results are in a good agreement with those reported by Suzuki et al [23].
Figure 1: Separation and detection of rutin and quercetin by high performance liquid chromatography. (a) High performance liquid chromatography profiles of rutin and quercetin standard. (b) High performance liquid chromatography profiles of rutin and quercetin present in whole bran tea extracts. (c) High performance liquid chromatography profiles of rutin and quercetin present in whole plant tea extracts. (d) High performance liquid chromatography profiles of rutin and quercetin present in whole embryo tea extracts.
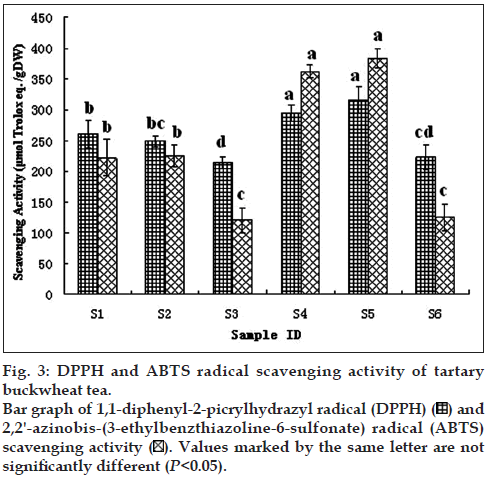
The DPPH radical scavenging activity was expressed as μmol trolox eq./gDW. As shown in fig. 3, the DPPH radical scavenging capacity of buckwheat teas was different significantly. The whole bran tea and the whole plant tea (S1, S2 and S4, S5) had the higher scavenging capacity (261.07, 249.15 and 214.24, 294.44 μmol trolox eq./gDW, respectively) than that of the whole embryo tea (S3, S6, 214.24, 223.74 μmol trolox eq./gDW, respectively).
Figure 3: DPPH and ABTS radical scavenging activity of tartary buckwheat tea. Bar graph of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulfonate) radical (ABTS) scavenging activity
and 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulfonate) radical (ABTS) scavenging activity . Values marked by the same letter are not significantly different (P<0.05).
. Values marked by the same letter are not significantly different (P<0.05).
The ABTS•+ scavenging activity of tartary buckwheat was presented in fig. 3. It also resulted in the similar pattern to the DPPH radical scavenging capacity. The whole bran tea and the whole plant tea (S1, S2 and S4, S5) had the higher scavenging activity (222.75, 225.33, 362.5, 383.8 μmol trolox eq./gDW, respectively) than that of the whole embryo tea (S3, S6, 120.46, 125.44 μmol trolox eq./gDW, respectively).
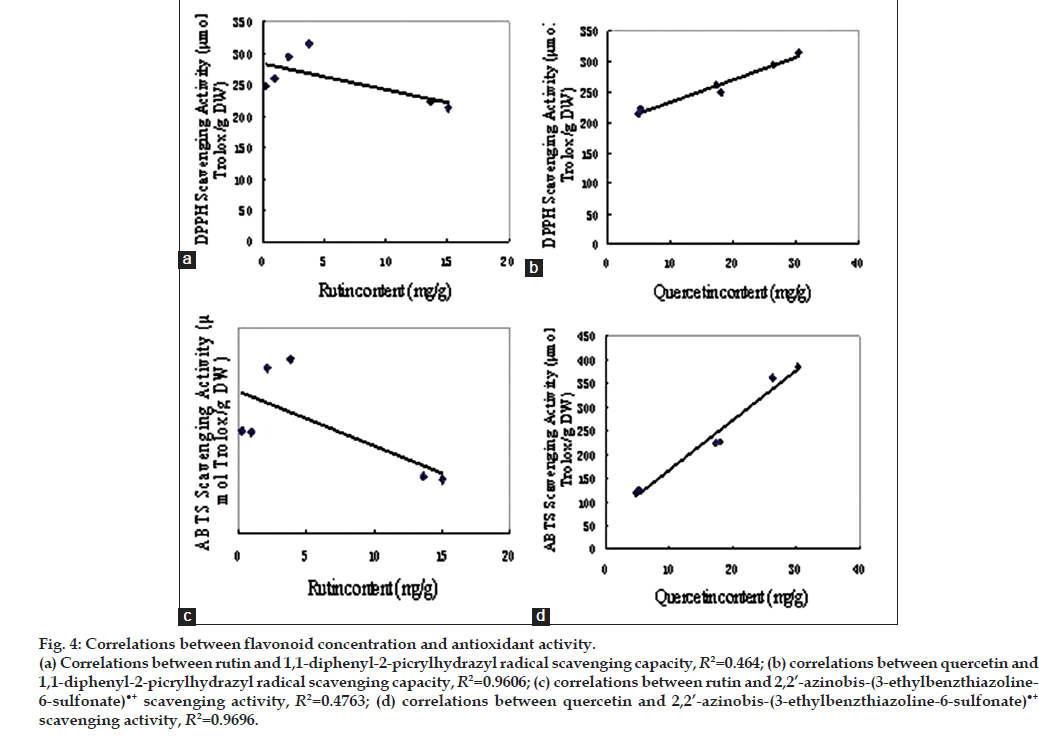
The correlation analysis between rutin, quercetin concentration and DPPH radical scavenging capacity, ABTS•+ scavenging activity were presented in fig. 4. The positive/negative correlations between the two variables are indicated by the correlation coefficients (r) and partial correlation coefficient (Pr). When the absolute value of r is close to 1, there is strong correlation. Pr was the correlation coefficient of the dependent variable with the independent variables after eliminating the correlation between the independent variables. The r value of DPPH radical scavenging capacity, ABTS•+ scavenging activity with rutin were −0.6812, −0.6901, respectively, when with quercetin were 0.9801, 0.9847, respectively (P<0.05), indicated that it is strong correlation between DPPH radical scavenging capacity, ABTS•+ scavenging activity and quercetin. The Pr of DPPH radical scavenging capacity, ABTS•+ scavenging activity with rutin were 0.7387 (P>0.05), 0.7915 (P>0.05), respectively, when with quercetin were 0.9831, 09891, respectively (P<0.05), had the similar result of the r analysis.
Figure 4: Correlations between flavonoid concentration and antioxidant activity. (a) Correlations between rutin and 1,1-diphenyl-2-picrylhydrazyl radical scavenging capacity, R2=0.464; (b) correlations between quercetin and 1,1-diphenyl-2-picrylhydrazyl radical scavenging capacity, R2=0.9606; (c) correlations between rutin and 2,2’-azinobis-(3-ethylbenzthiazoline- 6-sulfonate)•+ scavenging activity, R2=0.4763; (d) correlations between quercetin and 2,2’-azinobis-(3-ethylbenzthiazoline-6-sulfonate)•+ scavenging activity, R2=0.9696.
The antioxidant activity of tartary buckwheat and its products was affected by the concentration and composition of flavonoids and other phenols. A report suggests that rutin and quercetin were the major antioxidative constituents [24]; Li’s report [25] indicated that there was strong correction between rutin concentration and DPPH radical scavenging activity. Yang’s study found that the correlation was obvious between antioxidant and phenols but not flavonoids by linear regression equation [26]. A report showed that the antioxidative activity of pure quercetin is higher than that of rutin [27]. In our study, there were significantly different in the antioxidant activity of different kinds of tartary buckwheat, and the correlation of quercetin concentration with antioxidant activity was strong. The r value and Pr value of antioxidant activity correlation with rutin were less than that of antioxidant activity correlation with quercetin, and were not significant. These result indicated that, quercetin may be the major antioxidative constituent in tartary buckwheat tea. Although the whole bran tea and the whole plant tea had the higher quercetin concentration and antioxidant activity, the solubility of quercetin in boiling water was far lower than that of rutin, thus, the utilization of flavonoids in tartary buckwheat tea needs further research.
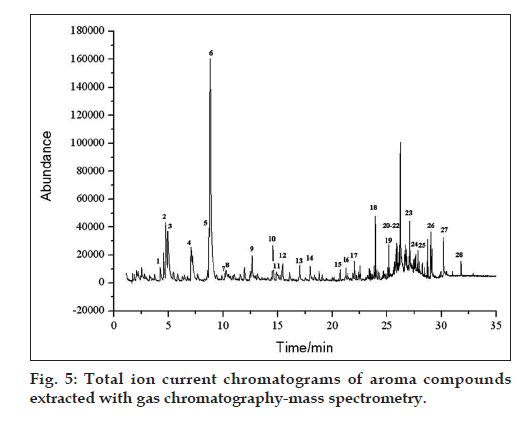
SPME technique has many advantages, and was widely used in the fields of medicine, food, agriculture and environment. Large amounts of organic solvent are not required, and the operation is convenient and accurate. We select this technique in our study, three kinds of tartary buckwheat teas (S1, S2, S3) were analyzed and twenty eight different compounds were identified by comparison of their mass spectra with those recorded in the NIST 2002 spectra library. Typical TIC chromatograms of aroma compounds extracted with SPME shown in fig. 5. Only the result of quality >85% have been listed in Table 2. Those compounds were mainly composed of pyrazine, aldehydes, fatty acids and ketones. The kinds of compounds contained in different tartary buckwheat tea were similar, but their relative content was different. There were 42.67% of aldehydes, 3.64% of fatty acids content in S1, 21.69% of aldehydes, <0.5% of fatty acids content in S2 and 31.15% of aldehydes, 9.52% of fatty acids content in S3.
| Number | RT | Compounds | Relative | ||
|---|---|---|---|---|---|
| contents (%) | |||||
| S1 | S2 | S3 | |||
| 1 | 4.51 | Butyl acetate | 2.19 | 1.47 | 1.44 |
| 2 | 4.74 | 2-methylpyrazine | 5.58 | 4.34 | 3.08 |
| 3 | 4.94 | 3-furancarboxaldehyde | 6.63 | 3.36 | 6.38 |
| 4 | 7.06 | 2,5-dimethyl pyrazine | 2.93 | 2.90 | 7.99 |
| 5 | 8.71 | Benzaldehyde | 2.83 | 2.42 | 2.71 |
| 6 | 8.81 | 5-methyl furfural | 25.8 | 18.9 | 8.78 |
| 7 | 10.17 | 2-ethyl-6-methylpyrazine | 0.65 | 0.39 | 0.69 |
| 8 | 10.24 | 2-ethyl-5-methylpyrazine | - | 0.92 | 3.06 |
| 9 | 12.67 | 4-hydroxy-2,5-dimethyl-3 (2H) | 2.42 | 2.36 | - |
| furanone | |||||
| 10 | 14.57 | Nonanal | 2.43 | 1.38 | 2.17 |
| 11 | 14.88 | Maltol | - | 0.85 | - |
| 12 | 15.46 | N-methylpyrrole-2-carboxaldehyde | 1.55 | 1.73 | - |
| 13 | 17.02 | 2-nonenal | 1.49 | - | - |
| 14 | 17.98 | Naphthalene | 0.96 | 0.68 | - |
| 15 | 20.73 | Dimethyl adipate | 0.61 | 0.54 | 0.74 |
| 16 | 21.23 | 2-decenal,(2Z)- | 0.68 | - | - |
| 17 | 22.02 | Methyl-naphthalen | 0.93 | 0.86 | 1.04 |
| 18 | 23.93 | Tetradecane | 1.56 | 1.38 | 1.35 |
| 19 | 25.19 | Pentadecane | 0.87 | 1.38 | 1.09 |
| 20 | 25.69 | Naphthalene, 2,3,6-trimethyl- | 1.11 | 2.25 | 1.75 |
| 21 | 25.93 | 4-methyl-2,5-dimethoxybenzaldehyde | 1.90 | 3.35 | 1.65 |
| 22 | 26.14 | 1-hexadecene | 1.20 | - | 2.35 |
| 23 | 27.08 | Heptadecane | 1.74 | 1.60 | 2.77 |
| 24 | 27.13 | 2,6,10,14-tetramethylpentadecane | 1.82 | 2.00 | 1.32 |
| 25 | 28.23 | 2-pentadecanone, 6,10,14-trimethyl- | 0.88 | 0.25 | - |
| 26 | 29.01 | Hexadecanoic acid | 1.40 | 3.25 | - |
| 27 | 30.18 | Oleic acid | 2.24 | 6.27 | - |
- means <0.1%
Table 2: Compounds Found In Three Kinds Of Tartary Buckwheat Tea
In Qin’s report [20], 77 compounds were identified in tartary buckwheat tea using three extract method, among them, 8 compounds had the highest odor activity value (OAV≥10). Five of this 8 compounds were detected in our sdudy: 2,5-dimethyl pyrazine, 2-ethyl-5-methylpyrazine, 4-hydroxy-2,5-dimethyl- 3(2H) furanone, nonanal and maltol. The composition and content of aroma compounds milling fractions of tartary buckwheat were shown difference in Janes’s report [19]. In our study, as discussed previously, the relative content of these compounds were also different in different kinds of tartary buckwheat tea, indicated the raw materials was an important factor affected the content of aroma compounds, this will cause the difference of the odor in the tea. Salicylaldehyde, which is the most characteristic compound of common buckwheat [28], was not found in samples of tartary buckwheat tea analyzed in this study.
In summary, the present study was carried out to compare the rutin and quercetin concentrations, antioxidant activity, and aroma compounds in different commercial tartary buckwheat tea. Results indicated that raw materials and machining process of tartary buckwheat tea have important influence on its chemical composition and activity. Quercetin may be the major antioxidative constituents in tartary buckwheat tea, and should be seriously concerned in future. Our results will stimulate more interest on the quality evaluation of tartary buckwheat tea.
Financial support and sponsorship
This research was financially supported by Special Fund for Agro-scientific Research in the Public Interest (201303069), China Agriculture Research System (CARS-08-B-3, CARS-08-D-3) and Research Funds of Key Laboratory of Food Processing in Sichuan Province (13-S04).
Conflicts of interest
There are no conflicts of interest.
References
- Wijngaard HH, Arendt EK. Buckwheat. Cereal Chem 2006;83:391-401.
- Christa K, Soral-Smietana M. Buckwheat grains and buckwheat products – Nutritional and prophylactic value of their components – A review. Czech J Food Sci 2008;26:153-62.
- Ikeda K. Buckwheat: Composition, chemistry, and processing. Adv Food Nutr Res 2002;44:395-434.
- Amézqueta S, Galán E, Vila-Fernández I, Pumarola S, Carrascal M, Abian J, et al. The presence of D-fagomine in the human diet from buckwheat-based foodstuffs. Food Chem 2013;136:1316-21.
- Yang N, Ren G. Determination of D-chiro-Inositol in tartary buckwheat using high-performance liquid chromatography with an evaporative light-scattering detector. J Agric Food Chem 2008;56:757-60.
- Peng LX, Wang JB, Hu LX, Zhao JL, Xiang DB, Zou L, et al. Rapid and simple method for the determination of emodin in tartary buckwheat (Fagopyrumtataricum) by high-performance liquid chromatography coupled to a diode array detector. J Agric Food Chem 2013;61:854-7.
- Lin LY, Peng CC, Yang YL, Peng RY. Optimization of bioactive compounds in buckwheat sprouts and their effect on blood cholesterol in hamsters. J Agric Food Chem 2008;56:1216-23.
- Yao Y, Shan F, Bian J, Chen F, Wang M, Ren G. D-chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. J Agric Food Chem 2008;56:10027-31.
- Kim DW, Hwang IK, Lim SS, Yoo KY, Li H, Kim YS, et al. Germinated Buckwheat extract decreases blood pressure and nitrotyrosineimmunoreactivity in aortic endothelial cells in spontaneously hypertensive rats. Phytother Res 2009;23:993-8.
- Zhao G, Peng LX, Wang S, Hu YB, Zou L. HPLC fingerprint Antioxidant properties study of buckwheat. J IntegrAgric 2012;11:1111-8.
- Guo X, Zhu K, Zhang H, Yao H. Purification and characterization of the antitumor protein from Chinese tartary buckwheat ( FagopyrumtataricumGaertn.) water-soluble extracts. J Agric Food Chem 2007;55:6958-61.
- Huang YF, Peng LX, Liu Y, Zhang ZF, Lu LY, Zhao G. Evaluation of essential and toxic element concentrations in different parts of buckwheat. Czech J Food Sci 2013;31:249-55.
- Zhang M, Chen H, Li J, Pei Y, Liang Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT Food SciTechnol 2010;43:181-5.
- Yoo S, Yoo J, Kim Y, Inglett GE, Lee S. Reduction of rutin loss in buckwheat noodles and their physicochemical characterization. Food Chem 2012;132:2107-11.
- Qin P, Wu L, Yao Y, Ren, G. Changes in phytochemical compositions, antioxidant and α-glucosidase inhibitory activities during the processing of tartary buckwheat tea. Food Res Int 2013;50:562-7.
- Yasuda T, Nakagawa H. Purification and characterization of the rutin-degrading enzymes in tartary buckwheat seeds. Phytochemistry 1994;37:133-6.
- Zheng YD, Luo QL, Zhou ML, Wang DZ, Zhang YD, Shao JR, et al. Isolation and screening of strains producing high amounts of rutin degrading enzymes from Fagopyrumtataricum seeds. J Basic Microbiol 2013;53:181-7.
- Vogrincic M, Timoracka M, Melichacova S, Vollmannova A, Kreft I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J IntregrAgric 2010;58:4883-7.
- Janeš D, Prosen H, Kreft S. Identification and quantification of aroma compounds of tartary buckwheat (FagopyrumtataricumGaertn.) and some of its milling fractions. J Food Sci 2012;77:C746-51.
- Qin P, Ma T, Wu L, Shan F, Ren G. Identification of tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. J Food Sci 2011;76:S401-7.
- Peng LX, Zou L, Zhao JL, Xiang DB, Zhu P, Zhao G. Response surface modeling and optimization of ultrasound-assisted extraction of three flavonoids from tartary buckwheat (Fagopyrumtataricum). Pharmacogn Mag 2013;9:210-5.
- Guo XD, Ma YJ, Parry J, Gao JM, Yu LL, Wang M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011;16:9850-67.
- Suzuki T, Honda Y, Funatsuki W, Nakatsuka K. Purification and characterization of flavonol 3-Glucosidase, and its activity during ripening in tartary buckwheat seeds. Plant Sci 2002;163:417-23.
- Yao YP, Tian CR, Cao W. Anti-oxidative constituents of ethanol of extract from buckwheat seeds by HPLC-Electro-Spray MS. AgricSci Chin 2008;7:356-62.
- Li HP, Wang M, Cai Y, Wang PK, Wang AH, Lu SJ, et al. Phenols extraction and antioxidant capacity of tartary buckwheat in liangshan region. J Anhui AgricSci 2010;38:5097-100.
- Yang HY, Chai Y, Huang ZM, Huang M. Effects of extraction solvents and methods on antioxidant activity of tartary buckwheat bran extracts. J Chin Inst Food SciTechnol 2011;11:28-32.
- Lu X, Wang L, Wei H, Yang ZQ, Wang W. Structure-activity relationship of flavonoids in antioxidant activity. Food Sci 2006;27:233-7.
- Janeš D, Kreft S. Salicylaldehyde is a characteristic aroma component of buckwheat groats. Food Chem 2008;109:293-8.