- *Corresponding Author:
- M. Nappinnai
Department of Pharmaceutics, C. L. Baid Mehta College of Pharmacy, Thoraipakkam, Chennai - 600 096, India.
E-mail: mnappinnai@gmail.com
| Date of Submission | 16 May 2006 |
| Date of Revision | 2 April 2007 |
| Date of Acceptance | 8 July 2007 |
| Indian J Pharm Sci 2007, 69 (4): 511-514 |
Abstract
Microspheres of diltiazem hydrochloride were formulated using combination of polyethylene glycol 6000 and Eudragit RS 100 and Eudragit RS 100 alone by solvent evaporation and non-solvent addition methods with an aim to prolong its release. Six formulations prepared by using different drug to polymer ratios, were evaluated for relevant parameters and compared with marketed SR capsules. Depending upon the drug to polymer ratio, the entrapment, loading and encapsulation were found to range between 77.45±0.22 to 91.08±0.62% , 34.76±0.15 to 52.46±0.25% and 66.09±0.19 to 82.7 ±0.57%, respectively. The microspheres were spherical, discrete and compact and size distribution was between 4 to 24 µm. In vitro studies were carried out at different pH for a period of 12 h and compared with marketed formulation. As similarity factor f2 was 92.8 for FVI, it was subjected to further study. Formulations prepared using the combination of the retardants exhibited first order of drug release and zero order for preparations containing Eudragit RS 100 alone. The analysis of regression values of Higuchi plot and Korsmeyer-Peppas plot and "n" values of Korsmeyer-Peppas model suggested a combination of diffusional and dissolutional mechanism indicating the drug release from the formulations was controlled by more than one process. Drug polymer interaction was absent as evidenced by FT-IR and DSC thermograms. In vivo pharmacokinetic study of the formulation proved that prolongation of drug release was obtained by formulating as microspheres.
Keywords
Diltiazem hydrochloride, microspheres, Eudragit RS 100, PEG 6000, in vitro, in vivo evaluation.
Diltiazem hydrochloride, a calcium channel blocker is available as sustained release preparation and literature reports many different sustained release formulations [1]. Eudragit RS 100 has not been reported in preparation of diltiazem hydrochloride microspheres and its suitability was evaluated in this work. PEG 6000 is generally associated with solubilisation but at high concentrations may act as retardant [2]. In this work a combination of both the materials was evaluated.
Materials and Methods
Diltiazem hydrochloride was obtained as gift sample from Mano pharmaceuticals, Chennai. Eudragit RS 100 was purchased from Colorcon Asia, Mumbai. All other chemicals used were of analytical grade. Twelve male white rabbits weighing about 2.8 kg to 3.1 kg were used in the in vivo study.
Formulation
All the formulations were prepared according to the formulae given in Table 1. Solvent evaporation method was adopted for preparing formulations I, II, III which contains both Eudragit RS 100 and PEG 6000. A homogenous mixture of the polymers was made in acetone. Diltiazem hydrochloride was then added to the polymer solution. The resulting mixture was then poured in liquid paraffin while stirring continuously. Stirring was continued for 1 h, until acetone evaporated completely. The microspheres formed were collected by filtration, washed 4-5 times with petroleum ether and dried at room temperature for 24 h2. Coacervation-phase separation by the addition of non-solvent was followed for formulations IV, V, VI. Eudragit RS100 was dissolved in warm toluene to get homogenous polymer solution. The drug was then added to the polymer solution and mixed thoroughly with the aid of mechanical stirrer for 10 min. Coacervation was then induced by the addition of petroleum ether slowly over a period of 20 min while stirring at the same speed. After rigidisation, the encapsulated product was collected by filtration and dried at room temperature for 24 h3.
| Formulation | Drug:PEG6000: Eudragit RS 100 |
Drug entrapment (%) Mean ± SD |
Drug loading (%) Mean ± SD |
Drug encapsulation (%) Mean ± SD |
|---|---|---|---|---|
| F-I | 1:0.25:0.25 | 77.45 ± 0.22 | 51.63 ± 0.15 | 66.09 ± 0.19 |
| F-II | 1:0.50:0.50 | 83.06 ± 0.61 | 41.53 ± 0.30 | 74.76 ± 0.54 |
| F-III | 1:0.75:0.75 | 86.96 ± 0.40 | 34.76 ± 0.15 | 79.96 ± 0.35 |
| FIV | 1:0.00:0.50 | 78.72 ± 0.38 | 52.46 ± 0.25 | 68.73 ± 0.33 |
| FV | 1:0.00:1.00 | 85.33 ± 0.30 | 42.66 ± 0.15 | 77.65 ± 0.27 |
| FVI | 1:0.00:1.50 | 91.08 ± 0.62 | 36.53 ± 0.15 | 82.70 ± 0.57 |
Table 1: Polymer Concentrations And Results For Parameters Evaluated
Determination of drug entrapment, loading and encapsulation efficiency
An aliquot of 100 mg of micro spheres was triturated with distilled water. The volume was made up to 100 ml with distilled water and was sonicated for 2 h. It was then filtered to remove debris and the absorbance was measured by using Shimadzu UV/Vis spectrophotometer (UV-1601) at 236 nm. Quantitative estimation of diltiazem hydrochloride was calculated by using equation obtained by linear regression analysis of the calibration data of diltiazem hydrochloride in distilled water [4]. Results are shown in Table 1. The drug loading in microspheres was estimated using the formula, L=Qm/Wm×100, where L is the percentage of loading of microspheres, Wm is the weight of the microspheres; Qm is the quantity of the drug present in Wm of microspheres. The amount of drug encapsulation in the microspheres was determined using the formula, E=Qp/Qt×100, where E is the percentage of encapsulation of microspheres; Qp is the product of drug content per g of microspheres and yield of microspheres in g [5]. Results are shown in Table 1.
In vitro release studies
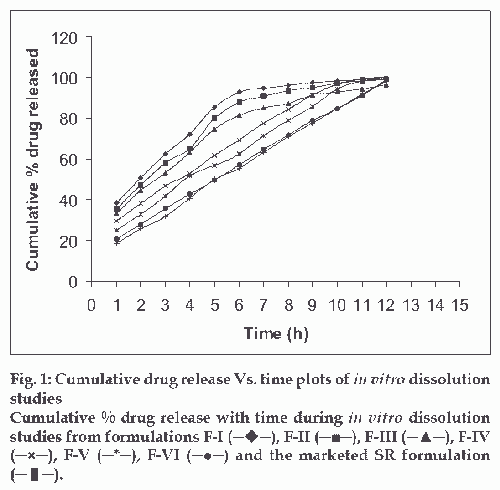
In vitro release profile of diltiazem hydrochloride from the preparations was examined in pH 1.2 buffer from 0-2 h, in pH 4.5 phosphate buffer from 2 to 4 h and in phosphate buffer pH 7.2 from 4 to 12 h using the rotating basket method specified in USP XXI at 100 rpm. Microspheres equivalent to 90 mg of drug were placed in the basket and the medium was maintained at 37±0.5o C. An aliquot of 10 ml were withdrawn periodically at intervals of one h and same volume of fresh medium was replaced. The concentration of the drug released at different time intervals was determined by measuring the absorbance at 236 nm [6]. Graphical representation of result is indicated in fig. 1.
Similarity factor
The dissolution data was subjected for determining f 2 values by using the formula, f2=50×log {1+ (1/n)Ετ=1 n(Rt-Tt)2}-0.5 ×100
Release Kinetics
Data obtained from dissolution studies was fitted to various kinetic equations. The kinetic models used were a zero order equation [5] (Q=Qo-kot), first order equation6 (Ln Q=Ln Qo - k1t) and Higuchi’s equation [7] (Q=kh t½) ½), Korsmeyer-Peppas equation [3] log Qt vs.log t, where Qt, is the cumulative amount of drug released at time t and Q0 is the initial amount of drug present in microspheres. k0 is the zero order release rate constant, k1 is the first order release rate constant, and kh is the diffusion rate constant. [8]
Particle size analysis and scanning electron microscopy (SEM)
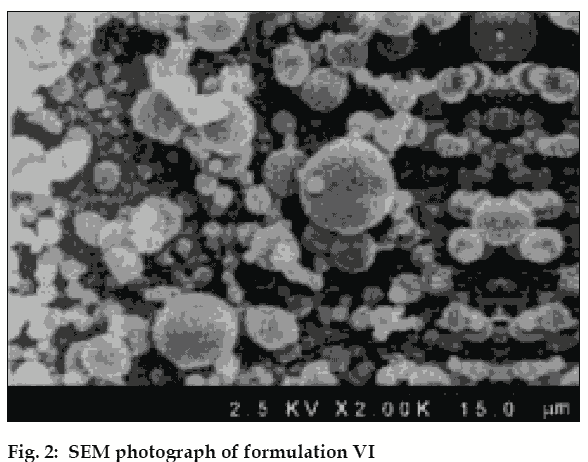
Particle size analysis was carried out by optical microscopy. About 200 microspheres were selected randomly and their size was determined using optical microscope fitted with a standard micrometer scale. For F VI exhibiting best in vitro release profile SEM was carried out [3].
FT-IR analysis and DSC
Infrared spectra of F-VI were recorded on Bomem MB-II FT-IR spectrometer. DSC thermogram of F-VI was recorded on a scanning calorimeter equipped with a thermal analysis data system (Perkin-Elmer DSC-7) [3].
In vivo release studies
Study was approved by IAEC (Approval Number: XI-8, CLBMCP-2004-2005). Male white rabbits weighing about 2.8 kg to 3.1 kg were used in this study. The animals were housed under standard environmental conditions (23±2û; 55±5% relative humidity; 12 h light/dark cycle). Prior to oral administration the rabbits were starved for 24 h and are allowed free access to tap water only. The animals (12) were divided into two groups of 6 each. To group I (standard group), marketed diltiazem hydrochloride capsule was administered. To the group II (test group) prepared microspheres (Formulation- VI) was administered. The capsule/microspheres were administered to the rabbit by gastric intubation method [9] . Blood samples (1 ml) were withdrawn from the marginal ear vein of the rabbit at regular interval of 2 h for period of 12 h. The plasma samples were separated by centrifugation and assayed for diltiazem hydrochloride by high performance liquid chromatography reported elsewhere [9]. Results of in vivo tests given in Table 2.
| Parameters | Marketed preparation | F-VI |
|---|---|---|
| Cmax(ng/ml) | 259.3 ± 2.19 | 261.5 ± 1.34 |
| Tmax(h) | 6.0 | 6.0 |
| AUC0-α ng-h/ml) | 1294.8 ± 17.69 | 1274.5 ± 7.5 |
| AUMC0-α(ng-h×h/ml) | 8221 ± 90.16 | 7966.6 ± 32.01 |
Table 2: In vivo study– pharmacokinetic Parameters
Results and Discussion
The drug entrapment was maximum in F-VI (91.08±0.62%), drug loading was maximum in F-IV (52.46±0.25%), and drug encapsulation was maximum in F-VI (82.70±0.57%). Dissolution test results show that increase in polymer concentration of Eudragit RS 100, decreased rate of drug release from the microspheres, whereas increasing PEG 6000 actually increased the drug release. Comparison of dissolution pattern of test formulations with marketed diltiazem hydrochloride sustained release capsules, showed that F-VI exhibited similar release pattern of marketed SR capsules. The f 2 value was 92.8. Log percentage of drug remaining versus time curves exhibited straight line for the formulations (I, II, III) and confirmed that the release rates followed first order. Cumulative percentage of drug release versus time curves exhibited straight line for the formulations (IV, V, VI) and confirmed that the release rates followed zero order kinetics. Cumulative percentage of drug release versus square root of time curves shows linearity and it proves that all the formulations follows Higuchi model, suggesting that diffusion may be the mechanism of drug release. The correlation values of Higuchi′s plot of formulation VI and diltiazem hydrochloride SR capsule were found to be 0.9697 and 0.9616, respectively. Log cumulative percentage of drug release versus log time curves shows high linearity and it proves that all the formulations follow Korsmeyer-Peppas model. The correlation values of Korsmeyer-Peppas plot of formulation VI and diltiazem hydrochloride marketed SR capsule were found to be 0.9864 and 0.9877, respectively. The slope values of Korsmeyer-Peppas plot of formulation VI and diltiazem hydrochloride marketed SR capsule were found to be 0.6448 and 0.0.7016, respectively. The diffusion exponent of release profile (slope) has a value of 0.6447 (n>0.5), which indicates a zero order release controlled by non Fickian diffusion .The analysis of regression values of Higuchi plot and Korsemeyer-Peppas plot and “n” values of Korsmeyer- Peppas model shows a combination of diffusional and dissolutional mechanism indicating the drug release from the formulations was controlled by more than one process. The particle size of F-VI was found to be in the range of 4 μm to 24 μm. The surface morphology of prepared microspheres observed under a scanning electron microscopy, showed good spherical geometry as evidenced by the photographs (fig. 2). The microspheres were discrete, spherical and uniform. Determination of interaction between drug and polymer were performed using FT-IR and by DSC for F-VI. FT-IR spectra study showed no change in the fingerprint of pure drug spectra, thus confirming absence of drug to polymer interactions (Figures may be reproduced on request). A sharp endotherm was observed for diltiazem hydrochloride at 213.17°. This melting endotherm was also observed for F IV at 212.32°, indicating absence of drug to polymer interactions. In vivo result analysis of pharmacokinetic parameters revealed that tmax of reference and test formulations were the same (tmax 6 h).The observed values for Cmax were 259.3±2.19 and 261.5±1.34 ng/ml for reference and F-VI respectively. The observed values for AUC0-α were 1294.8±17.67 and 1274.5±7.5 ng-h/ml for reference and F-VI, respectively. The observed values for AUMC0-α were 8221±90.16 and 7966.6±32.01 ng-h(h/ml) for reference and F VI, respectively. Statistical analysis by performing t-test (p<0.05) proved that there was significant difference between test formulation and reference. From the results it was observed that formulation VI was suitable for sustained / prolonged therapeutic effect. The microspheres showed same mean residence time (MRT) when compared with marketed diltiazem hydrochloride SR capsule. From the present study it may be concluded that diltiazem hydrochloride can be formulated as prolonged/sustained release drug delivery system (microspheres) with Eudragit RS 100.
References
- Bhalerao SS, Lalla JK, Rane MS. Study of processing parameters influencing the properties of diltiazem hydrochloride. J Microencap 2001;18:299-307.
- Bhalerao SS, Lalla JK, Rane MS. A study of electrokinetic and stability property of suspension of diltiazem hydrochloride microcapsule. Indian Drugs 2001;38:464-7.
- Chowdary KP, Ramesh KV. Studies on microencapsulation of diltiazem. J Pharm Sci 1993;55:52-4.
- Reddy MN, Shriwaikar AA. Rosin, a polymer for microencapsulation of diltiazem hydrochloride for sustained release by emulsion-solvent evaporation technique. Indian J Pharm Sci 2000;62:308-10.
- Saravanan M, Dhanraj MD, Sridhar SK, Ramachandran S, Sam SK, Rao SG. Preparation, characterization and in vitro release kinetics of ibuprofen polystyrene microspheres. Indian J Pharm Sci 2004;66:287-92.
- Pandey VP, Manavalan R, Sundarrajan T, Ganesh KS. Formulation and release characteristics of sustained release diltiazem hydrochloride tablets. Indian J Pharm Sci 2003;65:44-8.
- Ishikawa T, Watanabe Y, Takayama K, Endo H, Matsumoto M. Effect of hydroxypropylmethylcellulose (HPMC) on the release profiles and bioavailability of a poorly water-soluble drug from tablets prepared using macrogol and HPMC. Int J Pharm 2000;202:173-8.
- Higuchi T. Mechanism of sustained-action medication -theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Wagner JG. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci 1969;58:1253-7.