- Corresponding Author:
- P. V. Dangre
Department of Pharmaceutics, Kamla Nehru College of Pharmacy, Butibori, Nagpur-441 108, India
E-mail: pankajdangre8@gmail.com
| Date of Submission | 01 November 2014 |
| Date of Revision | 19 October 2015 |
| Date of Acceptance | 29 November 2015 |
| Indian J Pharm Sci 2015;77(6):764-770 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Antibiotics and antiinflammatory agents are the primary and main therapeutic categories in the treatment of otitis media. One of the simpler and feasible approaches of minimizing the problem of repeated use and subsequent resistance is development of sustained release formulation. Therefore, the present investigation aimed to develop a sustained release in situ gel formulation containing combination of broad spectrum antibiotic and antiinflammatory agents for the management of otits media. The prolonged release is achieved by phase transition of Poloxamer 407 (in situ) from sol to gel at a physiological temperature in combination with viscosity imparting agent Natrasol 250 and Klucel HF. The formulation P3N3 (19% w/v Poloxamer 407, 1.5% w/v Natrasol 250) and P3K3 (19% w/v Poloxamer 407, 1.5% w/v Klucel HF) showed mucoadhesive strength 37.17±0.32×103 and 38.12±0.13×103 dyne/cm2, respectively, and gel strength 2.1 and 2 cm, respectively. Both these formulations indicated good drug content and viscosity besides a good gelling ability. The in vitro diffusion has demonstrated prolongation of release of both the drugs over a period of 8 h.
Keywords
Otitis media, in situ gel, Poloxamer 407, phase transition
Otitis media (OM) is the accumulation of fluids in the middle ear, with or without the symptoms of inflammation. The infection is caused by dysfunction or obstruction of eustachian tube and is most commonly diagnosed in children under the age of two [1]. OM is a major health problem and occurs with high incidence and prevalence in both developed and developing countries [2]. The microbiology of OM differs with Streptococcus pneumoniae, Heamophilus influenzae, Moraxella catarrhalis, the most commonly isolated pathogens responsible for majority of infections [3]. The burden of otitis media is particularly heavy for children in the areas of the world in which access of medical care is limited [4]. Infants with severe and recurrent OM with effusion are at a risk of problems in behavior and development of speech, language and cognitive ability [5]. In addition to this otitis media is responsible each year for more than 50 000 deaths in children younger than 5 y of age [6]. Among the south-Asian countries, a prevalence rate in India is high (7.8%) [7].
Treatment modalities for otitis media infection include use of antibiotics and pain relievers in the forms of ear drops, ointments, insufflations. The surgical procedure is only for the cases where the medication is non-responsive. It includes insertion of small ventilation tube in the ear drum to improve air flow and fluid back up in the middle ear.
One of the limitations of intra tympanic (IT) drug delivery is that drug is rapidly lost from the middle ear by a number of processes. Ear drops have drawback of shorter residence time in ear, while semisolid preparations have disadvantages such as difficulty in application. The middle ear mucosa is ciliated, which aids the removal of fluids from the compartment. In order to provide a prolonged delivery of drug to the cochlea, it is therefore necessary to control the drug loss from the middle ear, specifically drug loss down the eustachian tube and clearance from the middle ear mucosa. This can be achieved by formulating a solution, which undergoes phase transition at a body temperature and forms in situ gel after administration. A typical composition of such otic gel may include thermosensitive gelling polymer such as Poloxamer 407 [8].
In the present research work, otic in situ gel containing the combination of a broad spectrum antibiotic e.g. ciprofloxacin and antiinflammatory drug e.g. dexamethasone was formulated. In situ forming systems are liquid aqueous solution before administration, but gel under physiological conditions. There are several mechanisms that lead in situ gel formation [9]. The major advantage associated with in situ gel is the possibility of administrating accurate and reproducible quantities compared to the already formed gel [10].
An in situ gelling system undergoes phase transition and is strong enough to withstand shear forces in the tympanic cavity and have long residence time in the ear. In situ gel along with its ability to release the drug in sustained manner lowers the systemic absorption of drug, thereby reduces the need for frequent administration and thus improves patient compliance.
Ciprofloxacin hydrochloride is having better safety profile, broad spectrum activity and short half life (3-5 h), justifying the need of sustained drug delivery [11,12]. Among the topical antibiotics, topical fluroquinolones are more effective than other types of antibiotics in OM [13-15]. Dexamethasone sodium phosphate is an antiinflammatory drug having better safety profile and help in rapid resolution of otorrhea [16]. The Poloxamer was used as temperature sensitive gelling polymer, Klucel HF (Hydroxyl propyl cellulose) and Natrasol 250M (Hydroxy ethyl cellulose) were added to build up the viscosity of the gel.
Materials and Methods
Ciprofloxacin hydrochloride was generous gift from Zim Laboratories Ltd., Nagpur, India, dexamethasone sodium phosphate was received as gift from Unijules Pvt. Ltd., Nagpur, India. Klucel HF (hydroxypropyl cellulose) and Natrosol 250M (hydroxyethyl cellulose) was provided by Signet Chemical Corporation, Mumbai, India. All other chemicals and solvents used were of analytical grade.
Formulation of medicated in situ gels
A typical formulation of medicated in situ gel consisted of ciprofloxacin hydrochloride 0.3% w/v, dexamethasone sodium phosphate 0.1% w/v, benzalkonium chloride 0.01% v/v, thermosensitive gelling polymer Poloxamer 407 (17-19% w/v), viscosity modifiers Klucel HF, Natrosol 205M (0.5, 1, 1.5% w/v) and distilled water as solvent (Table 1). The formulations were prepared by dispersing the Poloxamer in sterile water with continuous mixing, addition of viscosity modifiers fallowed by weighed quantities of drugs. The mixture was store overnight at 4o to allow complete hydration of polymer. To the above solution, benzalkonium chloride was added with continuous mixing until a clear solution.
| Formulations | Poloxamer 407 (% w/v) | Natrosol 250M (% w/v) | Klucel HF (% w/v) |
|---|---|---|---|
| P1N1 | 17 | 0.5 | |
| P1N2 | 17 | 1.0 | |
| P1N3 | 17 | 1.5 | |
| P2N1 | 18 | 0.5 | |
| P2N2 | 18 | 1.0 | |
| P2N3 | 18 | 1.5 | |
| P3N1 | 19 | 0.5 | |
| P3N2 | 19 | 1.0 | |
| P3N3 | 19 | 1.5 | |
| P1K1 | 17 | 0.5 | |
| P1K2 | 17 | 1.0 | |
| P1K3 | 17 | 1.5 | |
| P2K1 | 18 | 0.5 | |
| P2K2 | 18 | 1.0 | |
| P2K3 | 18 | 1.5 | |
| P3K1 | 19 | 0.5 | |
| P3K2 | 19 | 1.0 | |
| P3K3 | 19 | 1.5 |
All these bases contained ciprofloxacin hydrochloride 0.3% w/v, dexamethasone sodium phosphate 0.1% w/v, benzalkonium chloride 0.01% v/v. The final volume was made up to 10 ml using distilled water
Table 1: Composition of formulations
Estimation of drug content
The contents of ciprofloxacin hydrochloride and dexamethasone sodium phosphate in various formulations were estimated by UV spectrophotometric method. For this, 0.1 ml of each of the otic formulation was taken and diluted up to 100 ml with distilled water. The samples were analysed spectrophotometrically at λmax of ciprofloxacin hydrochloride and dexamethasone sodium phosphate i.e. 271 nm and 242 nm, respectively. The concentration of drugs in samples was determined from a previously developed and validated simultaneous method [17].
Sol-gel transition temperature
For this, about 2-3 ml of each of the sol formulation was taken in glass test tubes. The test tube was exposed to gradually increasing temperature in the range of 32º-38º (increment of 1º every 5 min) and the temperature at which sol was transformed to semisolid gel was noted.
Gelling time
The gelling time was measured by using glass chamber tilted at an angle of 45º containing water, which was maintained at 37º±0.5°. The individual otic formulations (100-200 μl) were dropped on the outer surface of glass chamber and time required for phase transition was noted. The transition of solution to viscous gel was observed visually and numerical grades (Table 2) were assigned depending upon, time taken for gel formation and collapsing of gel structure.
| Observed gel properties and time for phase transition from sol to gel | Numerical scores |
|---|---|
| No phase transition | - |
| Phase transition after 90 s collapsed | + |
| of gel structure within 1–5 min | |
| Phase transition between 60–90 s | ++ |
| Phase transition within 60 s stability | +++ |
| of gel structure for 30 min |
Table 2: Gradation of in situ otic gel
Mucoadhesive strength
For determination of mucoadhesive strength of otic gel an apparatus was fabricated in the laboratory. The apparatus was modified dispensing balance consisting of central lever, on one arm with pan and other arm with glass vial. Another glass vial in inverted position was fixed to the wooden base of the balance using double sided adhesive tape. The membrane was fitted in the gap between lower surface suspended vial and upper surface of the fixed vial. Weighed quantities (0.5 g) of individual samples of otic gel formulations were applied to the base of inverted glass vial using double sided adhesive tape to secure the gel in position. The distance between two vials was adjusted in such a way that the gel sample remained adhered to mucosal membrane. Sufficient pressure was applied on both of the vials for 10 s to allow proper adhesion of gel to mucosa. A constant weight was added to the pan to pull the vial away from the vial. The weight required for detaching the two vials was noted down. The mucoadhesive force, expresses as the detachment stress in dyne/cm2. Detachment stress=mg/A, where, m is the weight required for detaching the two vials, g is the acceleration due to gravity, taken as 980 cm/s2, and A is the area of membrane exposed and is equal to A=πr2. (r- is the radius of the exposed membrane).
Gel strength
For this, 8 ml of each of the gel bases (sol phase) was transferred into 10 ml glass measuring cylinder. The cylinder was fixed to the burette stand using a three way clamp. A scale of 15 cm was placed at the back drop of the cylinder. A stainless steel ball bearing (6 mm diameter and 1.045 g weight) was dropped from height of 5 cm into the bulk of gel mass contained in the cylinder with proper care of not allowing the ball to touch the sides of cylinder. The distance travelled by the ball within 2 min was noted down to the nearest value in mm.
Viscosity measurement and rheological behaviour
The viscosity of both the phases of each formulation i.e. sol phase (at temperature below 10º) as well as that of the preformed gels (at temperature 35-37º) was noted using Brookfield viscometer (RVDV-II+Pro) with small sample volume adaptor. In case with sol phase, the specified volume of solution was transferred in sample cell, which was placed carefully within the adaptor. The guard leg was placed around the adaptor and the volume of sample was stirred slowly using a motor driven stirring spindle (S 27). The viscosity values were noted at different rpm. Viscosity of preformed gels was measured using the T-bar spindle (S 96) rotated within the container containing 6 g of gels. A typical run involved changing the angular velocity of spindle from 1 to 100 rpm. The viscosity values at each rpm were noted.
In vitro release of drugs
The recipient compartment (i.e., weighing bottle) was filled with phosphate buffer saline (PBS) pH 7.4 (3 ml). The pretreated synthetic membrane of appropriate diameter (2.0 cm) was mounted carefully on the rim of the Eppendorf’s tube (6.5 mm internal diameter), 0.2 ml of sample was placed in Eppendorf ’s in solution form and left to form gel then donor compartment placed in receptor compartment, which contained 3 ml PBS. The whole diffusion assembly was then placed in the thermostatically controlled magnetic stirrer at 37±0.5° and 70 rpm. About 0.1 ml quantities of aliquots were withdrawn carefully from the receptor compartment and were replaced immediately with the same volume of fresh PBS (maintained at 37±0.5°). The samples were diluted suitably and the content of drugs in each sample was estimated from the absorbance at 271 and 242 nm using the previously developed method.
Stability of selected in situ gel formulation
Formulations P3N3 and P3K3 were stored at 2-8o (refrigeration condition) in clean, dry, airtight container, kept away from light for 3 mo and evaluated for drug content, viscosity, gelation behaviour, gel strength and in vitro drug release.
Results and Discussion
The temperature dependent gelation of poloxamer solution could be explained as being a change in the micellar properties by temperature increases. Raising the temperature of poloxamer solution will be accompanied by an increase in the micellar aggregation number and a decrease in of critical micelle concentration, allowing the formation of more closely packed and a more viscous gel. Moreover, the conformational changes in the orientation of methyl groups in the side chains of poly(oxypropylene) polymer chains, constituting the core of micelle, with the expulsion of the hydrating water from the micelles will contribute to the gelation phenomenon [18]. The solutions containing Poloxamer 407 as gelling agent exhibited improved gelling ability i.e. reduced gelling time and phase transition temperature (Table 3) with increasing concentration of polymers. However, this correlation was evident only at polymer concentration >17% w/v and above. Moreover, the effect of increasing concentration of gelling polymer was more pronounced on gelling time than the transition temperature. The addition of Natrosol 250 M and Klucel HF in the solution was only for the purpose of a imparting a greater mechanical strength to form in situ gel by increasing their viscosity. The most effective combination of polymer were Poloxamer 18% w/v with Natrosol 250M (P2N3 with phase transition at 55 s), 19% (P3N1, N2, N3 with phase transition at 50, 55, 35 s) and Klucel HF (P3 K3 with phase transition at 59 s).
| Formulations | Gelling ability (grades) | Gelling time (s) | Gelling temperature (°) |
|---|---|---|---|
| P1N1 | - | - | - |
| P1N2 | - | - | - |
| P1N3 | - | - | - |
| P2N1 | + | 92 | 42 |
| P2N2 | ++ | 62 | 38 |
| P2N3 | +++ | 55 | 36 |
| P3N1 | +++ | 50 | 36 |
| P3N2 | +++ | 55 | 34 |
| P3N3 | +++ | 35 | 32 |
| P1K1 | - | - | - |
| P1K2 | - | - | - |
| P1K3 | - | - | - |
| P2K1 | + | 190 | 45 |
| P2K2 | + | 120 | 42 |
| P2K3 | + | 95 | 38 |
| P3K1 | + | 100 | 38 |
| P3K2 | ++ | 75 | 38 |
| P3K3 | +++ | 59 | 36 |
Table 3: Sol-Gel transition temperature and gelling ability/time
The contents of ciprofloxacin hydrochloride and dexamethasone sodium phosphate in the selected in situ gelling solution systems ranges between 96.39-100.87% (Table 4), which is acceptable since the compendia specification for the drug contents in gel is 90-110%.
| Formulations | Drug content* (% w/v) | Mucoadhesive strength* | Gel strength | |
|---|---|---|---|---|
| Ciprofloxacin hydrochloride | Dexamethasone sodium phosphate | (dyne/cm2×103) | Distance travelled (cm) | |
| P2N1 | 97.27 ± 0.41 | 98.54 ± 0.13 | 26.47 ± 0.23 | 2.6 |
| P2N2 | 98.23 ± 0.67 | 98.74 ± 0.53 | 27.12 ± 0.14 | 2.5 |
| P2N3 | 98.21 ± 0.82 | 98.76 ± 0.51 | 29.05 ± 0.13 | 2.2 |
| P3N1 | 98.19 ± 0.34 | 98.02 ± 0.62 | 30.12 ± 0.18 | 2.2 |
| P3N2 | 98.95 ± 0.31 | 97.78 ± 0.37 | 33.27 ± 0.17 | 2.1 |
| P3N3 | 99.81 ± 0.77 | 100.87 ± 0.51 | 37.17 ± 0.32 | 2.1 |
| P2K1 | 96.39 ± 0.46 | 97.39 ± 0.59 | 29.54 ± 0.22 | 2.3 |
| P2K2 | 96.42 ± 0.63 | 97.12 ± 0.47 | 31.68 ± 0.24 | 2.4 |
| P2K3 | 97.68 ± 0.85 | 98.02 ± 0.61 | 32.39 ± 0.08 | 2.2 |
| P3K1 | 98.21 ± 0.71 | 98.14 ± 0.67 | 33.17 ± 0.19 | 2.1 |
| P3K2 | 98.34 ± 0.56 | 98.51 ± 0.72 | 36.67 ± 0.16 | 2.1 |
| P3K3 | 99.18 ± 0.65 | 98.74 ± 0.81 | 38.12 ± 0.13 | 2.0 |
*Results are mean of three observations, ± standard deviation
Table 4: Evaluation of formulations
The gel strength, which is an indication for the viscosity of the solution at physiological temperature, was determined by distance travelled (cm) within 2 min, the stainless steel ball penetrate down the gel. The sufficient gel strength is required to prevent the leakage from the ear. The distance travelled by stainless steel ball has inverse relation to the gel strength; the lower the distance, higher will be the gel strength. The increase in polymer concentration leads to increase in gel strength of the formulations (Table 4).
Formulation should have sufficient mucoadhesive strength in order to retain the formulation within the applied area for sustained pharmacological action. The formulation P3K3 having maximum mucoadhesive strength (Table 4). There was corresponding increase in Muco-adhesive strength with increasing concentration of individual polymers. Mucoadhesive involves association of reactive functional groups of polymers with those of mucin layer lining the organ. However, the extent of association depends on the degree of freedom available for the polymer chains.
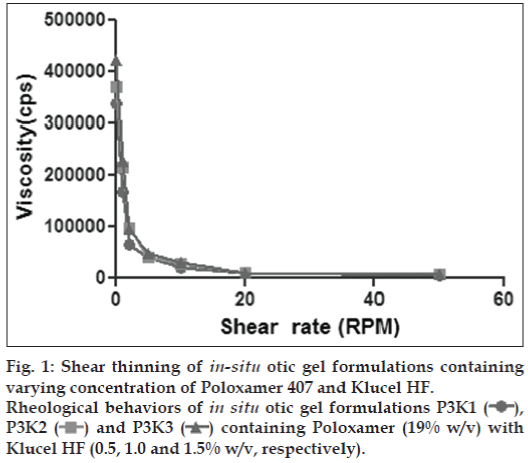
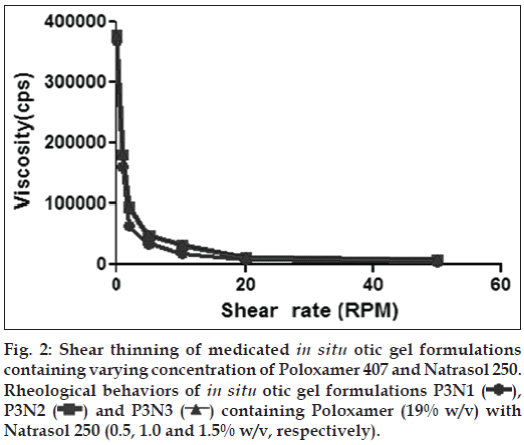
There are no specifications as such for viscosity of in situ gelling systems. The rise in viscosity of all formulations at physiological temperature was due to sol gel transformation. In the development of otic in situ gel, appropriate viscosity of formulation is important. The formulation must be administered easily as drops and get transformed into a viscous gel. These systems are expected to undergo falling viscosity at increasing shear rate. The in situ gel bases containing Poloxamer 407 in combination with viscosity modifiers naturally were more viscous than the bases. The sol phase then converted to gel bases by change in temperature. The in situ gels bases clearly indicated the shear thinning behaviour (figs. 1 and 2). As the concentration of viscosity increasing agent was increased the viscosity was also increased.
The in vitro drug release study was carried out for all formulated otic in situ gels containing ciprofloxacin hydrochloride and dexamethasone sodium phosphate. All batches showed a sustained drug release profile for 8 h.
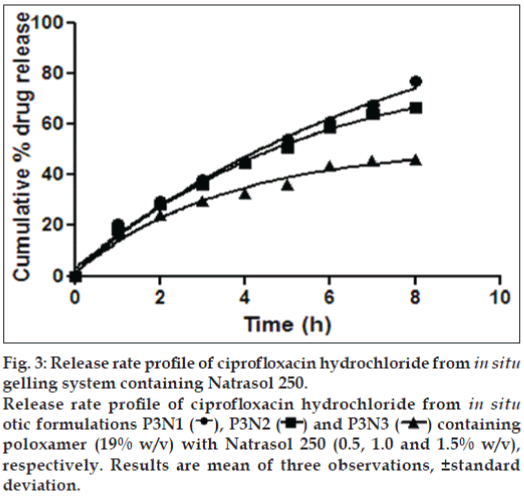
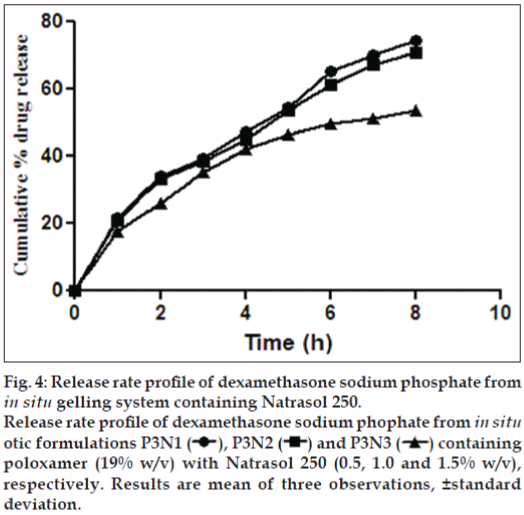
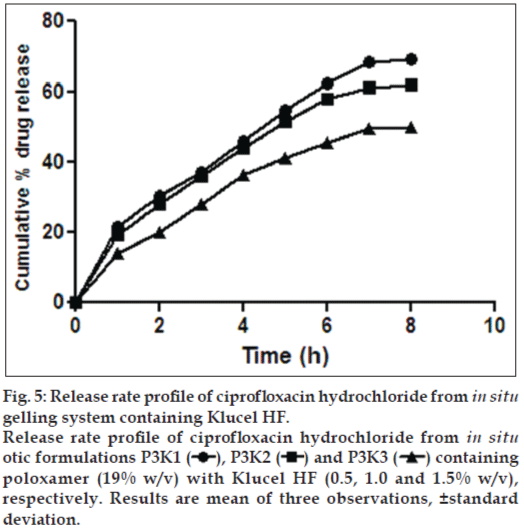
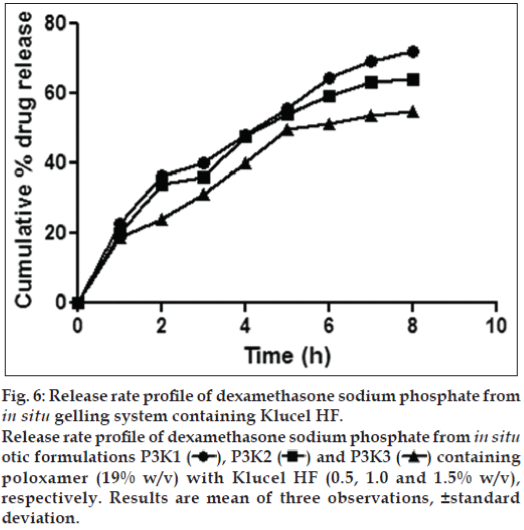
Formulations P3N3 showed 46.16±72 and 53.58±46% release of ciprofloxacin hydrochloride and dexamethasone sodium phosphate (figs. 3 and 4), respectively, whereas P3K3 indicate 50.15±27% of ciprofloxacin hydrochloride (fig. 5) and 54.97±33% of dexamethasone sodium phosphate (fig. 6) release for a period of 8 h. The R2 values for both the formulations were in good correlation for Higuchi, therefore release rate of drugs were in accordance of Higuchi kinetic release. It was noted that, the release of drug is not only affected by poloxamer concentration but also the concentration of viscosity modifiers. The drug release retarding effect of polymers could be attributed of their ability to increase overall product viscosity as well as their ability to distort the extramicellar aqueous channels of polymer micelles through which drug diffuses thereby delaying the drug release.
Figure 3: Release rate profile of ciprofloxacin hydrochloride from in situ gelling system containing Natrasol 250.
Release rate profile of ciprofloxacin hydrochloride from in situ otic formulations P3N1  , P3N2
, P3N2  and P3N3
and P3N3  containing poloxamer (19% w/v) with Natrasol 250 (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
containing poloxamer (19% w/v) with Natrasol 250 (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
Figure 4: Release rate profile of dexamethasone sodium phosphate from in situ gelling system containing Natrasol 250.
Release rate profile of dexamethasone sodium phophate from in situ otic formulations P3N1  , P3N2
, P3N2  and P3N3
and P3N3  containing poloxamer (19% w/v) with Natrasol 250 (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
containing poloxamer (19% w/v) with Natrasol 250 (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
Figure 5: Release rate profile of ciprofloxacin hydrochloride from in situ gelling system containing Klucel HF.
Release rate profile of ciprofloxacin hydrochloride from in situ otic formulations P3K1  , P3K2
, P3K2  and P3K3
and P3K3  containing poloxamer (19% w/v) with Klucel HF (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
containing poloxamer (19% w/v) with Klucel HF (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
Figure 6: Release rate profile of dexamethasone sodium phosphate from in situ gelling system containing Klucel HF.
Release rate profile of dexamethasone sodium phosphate from in situ otic formulations P3K1  , P3K2
, P3K2  and P3K3
and P3K3  containing poloxamer (19% w/v) with Klucel HF (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
containing poloxamer (19% w/v) with Klucel HF (0.5, 1.0 and 1.5% w/v), respectively. Results are mean of three observations, ±standard deviation.
Form the results of gelation behaviour, viscosity, gel strength, mucoadhesion, and in vitro drug release studies, formulations P3N3 and P3K3 were selected. Both the formulations showed the prolonged release of ciprofloxacin hydrochloride and dexamethasone sodium phosphate over a period of 8 h, and were stable with no adverse effect of temperature and humidity during the storage over 3 mo. The formulations suggest the management of otits media could be possible with in situ otic gelling system however, in vivo studies are needed to ascertain efficacy of the experimental formulation.
Acknowledgment
We thank Zim Laboratories Ltd., and Unijules Pvt. Ltd., Nagpur, India for providing ciprofloxacin hydrochloride and dexamethasone sodium phosphate, we also thankful to Signet Chemical Corporation, Mumbai, India for gifting Klucel HF and Natrosol 250M.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Dicks L, Knoetze H, Reenen C. Otitis media: A review, with a focus on alternative treatments. Probiotics AntimicroProt 2009;1:45-59.
- St Sauver J, Marrs CF, Foxman B, Somsel P, Madera R, Gilsdorf JR. Risk factors for otitis media and carriage of multiple strains of Haemophilusinfluenzae and Streptococcus pneumoniae. Emerg Infect Dis 2000;6:622-30.
- Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J 1992;11 8 Suppl:S7-11.
- Klein JO. The burden of otitis media. Vaccine 2000;19Suppl 1:S2-8.
- Vernon FL. Impact of otitis media on speech, language, behaviour and cognition. In: Rosenfeld RM, Bluestone CD, editors. Evidence-Based Otitis Media. Hamilton, Ontario: Decker; 1999. p. 353-73.
- Berman S. Otitis media in developing countries. Pediatrics 1995;96(1 Pt 1):126-31.
- World Health Organization. Chronic SuppurativeOtistis Media, Burden of Illness and Management Options. Child and Adolescent Healthand Development, Prevention of Blindness and Deafness. Geneva, Switzerland: WHO; 2004.
- Dangre PV, Kattekar KR, Shirolkar SV. Development and evaluation of in situ gelling otic formulation of chloramphenicol using poloxamer 407. Indo Am J Pharm Res 2013;3:8001-7.
- Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels – Review of temperature-sensitive systems. Eur J Pharm Biopharm 2004;58:409-26.
- Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev 2002;54:37-51.
- Cho KY, Chung TW, Kim BC, Kim MK, Lee JH, Wee WR, et al. Release of ciprofloxacin from poloxamer-graft-hyaluronic acid hydrogels in vitro. Int J Pharm 2003;260:83-91.
- Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm 2010;74:332-9.
- Podoshin L, Brodzki A, Fradis M, Ben-David J, Larboni J, Srugo I. Local treatment of purulent chronic otitis media with ciprofloxacin. Harefuah 1998;134:32-6.
- Fradis M, Brodsky A, Ben-David J, Srugo I, Larboni J, Podoshin L. Chronic otitis media treated topically with ciprofloxacin or tobramycin. Arch Otolaryngol Head Neck Surg 1997;123:1057-60.
- Miro N, The Spanish Study Group. Controlled multicenter study on chronic supurative otitis media treated with topical applications of ciprofloxacin 0.2% solutions in single dose container or combination of polymyxin-neomycin, and hydrocortisone susupensions. Otolaryngol Head Neck Surg 2000;123:617-23.
- Alper CM, Dohar JE, Gulhan M, Ozunlu A, Bagger-Sjobak D, Hebda PA, et al. Treatment of chronic suppurative otitis media with topical tobramycin and dexamethasone. Arch Otolaryngol Head Neck Surg 2000;126:165-73.
- Shahu PA, Potnis VV, Dangre PV, Thote LT. Development and validation of simultaneous spectrophotometric estimation of ciprofloxacin hydrochloride and dexamethasone in bulk drug and its formulation. Indo Am J Pharm Res 2013;3:7103-13
- Attwood D, Collett JH, Tait CJ. The micellar properties of (polyoxyethylene)-(polyxoxypropylene) co-polymer Pluronic F-127 in water and electrolyte solution. Int J Pharm 1985;26:25-33.