- *Corresponding Author:
- M. Sankar
Department of Biotechnology, Rajalakshmi Engineering College, Chennai-602 105, India
E-mail: sankar7950@gmail.com
| Date of Submission | 20 March 2014 |
| Date of Revision | 31 January 2015 |
| Date of Acceptance | 14 September 2015 |
| Indian J Pharm Sci 2015;77(5):556-562 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The present study is designed to evaluate the efficacy of heptoplus a polyherbal formulation as an oral supplementary agent for isoniazid and rifampicin induced hepatotoxicity in rats. 50 and 100 mg/kg of heptoplus supplement were fed orally to the rats along with isoniazid and rifampicin and compared to rats treated with 100 mg/kg Liv 52 standard drug. Rats treated with isoniazid and rifampicin suffered from severe oxidative stress by the virtue of free radicals induced lipid per oxidation. As a result abnormal index of serum biochemical markers for liver function and increased liver lysosomal enzymes activity was observed. However rats nourished with 100 mg/kg of heptoplus and Liv 52 protected the liver from oxidative damage by maintaining normal antioxidant profile status and restored normal serum liver biochemical markers. Increased liver lysosomal enzymes activity is prevented in the rats supplemented with heptoplus and Liv 52. Histopathological analysis also revealed severe vascular changes and lobular necrosis in the treatment of isoniazid and rifampicin. Heptoplus (100 mg/kg) and Liv 52 supplemented rats liver apparently revealed normal architecture of liver. This study confirms that heptoplus has liver protective activity against Isoniazid and Rifampicin induced liver injury in rats, in par with Liv 52.
Keywords
Isoniazid, rifampicin, oxidative stress, heptoplus, Liv 52
Tuberculosis (TB) is one of the major communicable diseases, and nearly 2 million people die every year [1]. TB is ranked seventh among all illness [2]. Isoniazid (INH) and rifampicin (RIF) are the two major regimens currently used for the treatment of TB for a period of 4 to 6 months [3], which may induce hepatotoxicity [4]. The incidence of hepatic dysfunction is more, when INH and RIF are used in combination [5]. In India the prevalence of hepatotoxicity is 11.6%, when compared to western countries where it is 4.3% [6]. Antituberculosis drug induced hepatotoxicity ranges from non specific elevation of transaminases to fulminant of liver failure [7]. The liver dysfunction is due to the synergistic effect of INH and RIF [8]. Hydrazine (HYZ) a metabolite of INH is converted to toxic compound by CYP450, which leads to hepatotoxicity. RIF, aggravates hepatotoxicity by inducing CYP450, as a result more toxic metabolites are generated from hydrazine [9]. In addition HYZ depletes the reserved glutathione (GSH) level in the liver, resulting in oxidative stress and cell death [10,11]. Since oxidative stress is one of the major attributing mechanisms for antituberculosis drug induced hepatotoxicity and liver damage [12], the present study is designed to study the effect of heptoplus as supplementary agent for INH and RIF induced liver dysfunction in rats.
Materials and Methods
Rifampicin and isoniazid were procured from sigma chemicals and all other chemicals used for this study were of analytical grade. Heptoplus a Tamil Nadu state drug licensing authority licensed (lic.no 616) Polyherbal formulation drug was procured from Care and Cure Herbs pvt Ltd, Chennai, India, in the form of capsule. Each capsule has the following composition in form of powder: Phyllanthus amarus (whole plant) 100 mg, Eclipta alba (leaves) 50 mg, Tephrosia purpurea (leaves) 30 mg, Curcuma longa (Rhizome) 30 mg, Picrrohiza kurooa (root) 20 mg, Withania somnifera (root) 100 mg, Pinius succinifera (Amber) 37.50 mg, Pistacia lentiscus (Resinous exudates) 25 mg, Orchis mascula (seed) 25 mg and Cycas circinalis (flower) 62.50 mg.
Liv 52 introduced in 1955, is a herbal tablet for liver protection from Himalayan Drug Company (India) and is recognized worldwide by health professionals. In India it is a standard drug prescribed by the medical fraternity to treat any liver related diseases. Each tablet contains Capparis spinosa 32 mg, Cichorium intybus 32 mg, Mandur bhasma 32 mg, Solanum nigrum 32mg, Terminalia arjuna 32 mg , Cassia occidentalis 16 mg, Achillea millefolium 16 mg and Tamarix gallica 16 mg.
Sprague Dawely rats, weighing 150 to 200 g obtained from Sri Ramachandra Medical College, Chennai, India, were used for this study, after the approval from Institutional Animal Ethics Committee, New Delhi, India, (IACE/ XXII/SRU/168/2011). Animals were housed individually in well ventilated polypropylene cages. A 12-h light/12-h dark artificial photoperiod, 22±3° room temperature and 50–70% relative humidity were maintained in the room. Animals had free access to pelleted feed (Nutrilab rodent, Tetragon Chemie Pvt Ltd., India) and reverse osmosis purified water (Rios, USA).
Acute oral toxicity
Acute oral toxicity study was performed according to the OECD test guideline 423- Acute toxic class method. Young healthy adult Sprague Dawley female rats weighing between 160-180 g body weights were divided into two groups of 3 animals each. Group I rats were treated with 0.5% carboxy methyl cellulose (CMC) and group II rats administrated with heptoplus (HP). Both CMC and HP were administrated orally once daily via gastric intubation at a dose level of 2000 mg/kg b.wt. Lethality and abnormal clinical signs if any were observed on the day of dosing and thereafter for 13 days. Body weight was recorded before dosing and thereafter once in a week till completion of the experiment.
Experimental study protocol
Animals used in the present study were divided in to six groups of 6 rats each. Daily once drugs were administered orally for a period of 30 days. Group I: Rats receiving CMC (vehicle), served as control; Group II: Rats treated with 50 mg/kg of each RIF and INH (intoxicated control) [13]; Group III: Rats supplemented with 100 mg/kg of Liv 52 along with 50 mg/kg each of RIF and INH; Group IV: Rats receiving 50 mg/kg of heptoplus (HP low dose) and 50 mg/kg each of RIF and INH; Group V: Rats treated with 100 mg/kg of heptoplus (HP high dose) along with 50 mg/kg each of RIF and INH; Group VI: Rats treated with heptoplus (HP control) alone 100 mg/kg b.w.
Collection of blood sample
Blood samples were collected in microcentrifuge tubes before sacrificing the animal on 15th and 30th day of experiment from retro-orbital venous plexus of rats, without adding any anticoagulant for separation of serum. Then serum samples were stored for various biochemical analysis.
Serum biochemical Assay for liver function
Serum biochemical parameters such as alanine transaminases (ALT), asparate transaminases (AST), total protein (TP), albumin (ALB), total bilirubin (TB), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), gamma glutamyltransferase (GGT) and C reactive protein (CRP) were assayed using biochemical kits supplied by Merck India Limited, Mumbai, India.
Antioxidant assay
At the end of study before sacrificing, serum samples were prepared to analyze the following antioxidant profile: Catalase (CAT) [14], malonaldehyde (MDA) [15], superoxide dismutase (SOD) [16], glutathione peroxidase (GPX) [17] and glutathione (GSH) [18].
Liver lysosomal enzyme studies
10% liver homogenate was prepared with 10% Trichloroacetic acid (TCA). The homogenate was filtered and centrifuged at 3000 rpm for 10 min. The supernatant was stored and the pellet obtained was again homogenized and centrifuged as above. The supernatant collected from each step was pooled and centrifuged at 12 000 g for 20 min. The pellet obtained was dispersed in 1 ml of 1.15% potassium chloride (KCl) and used for the estimation of lysosomal enzymes.
Cathepsin D
To 0.5 ml of the sample, 2 ml of buffered substrate was added, mixed well and incubated at 45° for 2 h. The reaction was arrested by adding 750 μl of 10% TCA. The tubes were incubated at room temperature for 1 h. The reaction mixture was centrifuged at 3500 rpm for 15 min. To 2 ml supernatant, 3 ml 0.5 N NaOH followed by 1.5 ml 1:2 Folins phenol reagent were added. The absorbance was read at 620 nm against a blank. A standard graph of tyrosine was plotted, from which the liberated tyrosine level was estimated.
β-Galactosidase
β-galactosidase was estimated by adding 0.1 ml of the lysosomal extract to the test tubes containing 0.2 ml sodium acetate buffer and 0.1 ml of 0.66 M substrate, p-nitrophenyl β-galactopyranoside. The reaction mixture was set to incubation at 37o for an hour. Immediately after the incubation period the reaction was arrested using 0.2 ml of 5% TCA. To the control tubes, lysosomal extract was added correspondingly after the incubation. All the tubes were again incubated for 15 min at 37o. Following incubation, 3 ml of 0.1 N Sodium hydroxide was added to all the tubes and centrifuged for 10 min at 1000 rpm. The optical density of the reaction mixture was measured at 400 nm using Thermo Scientific Multiskan Spectrophotometer, USA.
Histopathology analysis
Livers from rats were fixed in 10% neutral buffered formalin solution, dehydrated in graded alcohol and embedded in paraffin. Paraffin sections of 3-4 micron thickness were obtained, mounted on glass slides and counter-stained with hematoxylin and eosin for light microscopic analyses.
Statistical analysis
All the data were expressed as Mean±SEM. Statistical significance of data were analyzed by one-way analysis of variance (ANOVA) followed by Turkey–Kramer multiple comparisons using the Graph pad prism software package for windows (Version 5). P values <0.05 are considered as significant.
Results
Acute oral toxicity assay for HP
There were no treatment related mortality, abnormal clinical signs, remarkable body weight changes fig. 1 or gross pathological changes observed in any of the experimental animals. LD50 of heptoplus was found to be greater than 2000 mg/kg. Hence, the test drug falls in the “category-5” or “unclassified” in accordance to the globally harmonised system of classification of chemicals. Based on acute oral toxicity results, 100 mg/kg and 50 mg/kg of body weight of HP were taken as high dose and low dose supplementary agents for INH and RIF induced hepatotoxicity.
Serum biochemical markers for liver function
Test data obtained for serum biochemical markers for liver function on 15th and 30th day of experimental period, are presented in Tables 1-4. Significant elevation of (P≤0.05) serum AST, ALT, ALP and LDH was observed, in rats treated with INH and RIF. Supplementation of Liv 52 and HP (100 mg/kg) to the rats brought down the increase in serum transaminases, ALP and LDH to near normal levels. Increased levels of TB and CRP (P≤0.05) were observed in group II rats. However rats in Group III and V showed significant reduction of serum TB and CRP (P≤0.05) levels. TP and ALB are used to assay liver function. Significant decreases of serum TP (P≤0.05) and ALB (P≤0.05), is noticed in the rats in group II. Concomitant application of Liv 52 and HP (100 mg/kg) enhanced the concentration of proteins and ALB. Serum GGT is one of the highly sensitive markers for liver function. Significant increase of serum GGT (P≤0.05) is observed in group II rats. Co administration of and Liv 52 and HP on group III and V rats showed significant decrease of serum GGT.
| Groups | AST | ALT | GGT | ALP | LDH |
|---|---|---|---|---|---|
| I | 37.74±3.89 | 31.48±3.06 | 4.94±0.73 | 260.60±13.15 | 122.50±9.25 |
| II | 67.46±5.39* | 50.86±4.56* | 6.52±0.45 | 410.3±22.95* | 245.90±24.02* |
| III | 47.71±1.89# | 39.26±2.88 | 5.48±0.25 | 323.10±19.69# | 152.0±11.82# |
| IV | 51.12±2.33# | 39.50±2.87 | 6.15±0.52 | 353.0±11.39* | 164.0±14.5# |
| V | 49.32±4.57# | 36.38±1.18# | 5.62±0.65 | 323.90±5.73# | 140.7±8.95# |
| VI | 40.90±2.95# | 35.07±1.25# | 5.26±0.46 | 276.70±22.83# | 138.0±3.37# |
All values expressed as U/L, in form of mean±SEM, where n=6. If *P≤0.05 significantly different when compared to control group I, If #P≤0.05 statistically significant when compared to group II. AST: aspartate transaminases, ALT: alanine transaminases, GGT: gamma glutamyltransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, SEM: standard error of mean
Table 1: Serum enzymatic biochemical markers for liver function on day 15.
| Groups | TP | ALB | TB | CRP |
|---|---|---|---|---|
| I | 6.13±1.88 | 2.52±0.17 | 0.35±0.03 | 3.89±0.30 |
| II | 5.16±0.23 | 2.12±0.32 | 0.59±0.10 | 6.480±0.42* |
| III | 6.16±0.26# | 2.53±0.17 | 0.42±0.09 | 5.04+0.14*,# |
| IV | 5.56±0.20 | 2.22±0.14 | 0.43±0.04 | 5.37±0.08*,# |
| V | 5.79±0.27 | 2.45±0.17 | 0.30±0.07 | 4.69±0.22# |
| VI | 5.91±0.22 | 2.60±0.09 | 0.34±0.01 | 3.52±0.33# |
All values expressed as mg/dl, in form of mean±SEM, where n=6. If *P≤0.05 significantly different when compared to control group I, If #P≤0.05 statistically significant when compared to group II. TP: total protein, ALB: albumin, TB: total bilirubin, CRP: C‑reactive protein, SEM: standard error of mean
Table 2: Serum nonenzymatic biochemical markers for liver function on day 15
| Groups | AST | ALT | GGT | ALP | LDH |
|---|---|---|---|---|---|
| I | 66.51±3.72 | 41.39±2.81 | 4.89±0.46 | 322.40±15.08 | 166.9±7.80 |
| II | 145.0±2.64* | 77.85±4.8* | 7.00±0.35* | 569.30±32.55* | 331.1±28.34* |
| III | 81.19±5.26# | 46.32±1.93# | 5.42±0.30# | 391.30±26.34# | 182.3±15.43# |
| IV | 103.0±11.55# | 48.66±0.13# | 5.45±0.22# | 437.4±32.04*,# | 233.8±12.77# |
| V | 77.88±10.94# | 45.67±1.52# | 5.19±0.21# | 369.90±18.37# | 170.9±15.50# |
| VI | 68.48±5.18# | 38.76±2.52# | 5.08±0.33# | 294.70±20.74# | 178.0±16.19# |
All values expressed as U/l, in form of mean±SEM, where n=6. If *P≤0.05 significantly different when compared to control group I, If #P≤0.05 statistically significant when compared to group II. AST: Asparate transaminases, ALT: alanine transaminases, GGT: gamma glutamyltransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, SEM: standard error of mean
Table 3: Serum enzymatic biochemical markers for liver function on day 30
| Groups | TP | ALB | TB | CRP |
|---|---|---|---|---|
| I | 6.21±0.22 | 2.48±0.13 | 0.36±0.082 | 5.12±0.68 |
| II | 4.48±0.42* | 1.75±0.18* | 0.85±0.10* | 16.44±0.42* |
| III | 6.24±0.06# | 2.40±0.11# | 0.40±0.04# | 7.52±0.47*,# |
| IV | 5.29±0.16 | 2.38±0.19# | 0.46±0.07# | 7.82±0.52*,# |
| V | 6.36±0.23# | 2.57±0.08# | 0.40±0.06# | 5.71±4.97# |
| VI | 6.24±0.17# | 2.48±0.10# | 0.31±0.06# | 5.04±0.33# |
All values expressed as mg/dl, in form of mean±SEM, where n=6. If *P≤0.05 significantly different when compared to control group I, If #P≤0.05 statistically significant when compared to group II. TP: Total protein, ALB: albumin, TB: total bilirubin, CRP: C‑reactive protein, SEM: standard error of mean
Table 4: Serum nonenzymatic biochemical markers for liver function on day 30
Effect of HP on serum antioxidant profile
The effect of HP and Liv 52 on serum antioxidant profile on INH and RIF induced oxidative stress in rats is shown in Table 5. Significant decrease of serum GSH (P≤0.05), CAT (P≤0.05) and SOD (P≤0.05) is observed in group II rats. Concurrent application of Liv 52 and HP on group III and V rats completely brings back the normal levels of these serum antioxidants. A slight reduction in the activity of serum GPX was inferred in group II rats, which was restored with treatment of Liv 52 and HP. MDA formation is a marker of oxidative insult which is significantly increased (P≤0.01) in rats (group II). Treatment of HP and Liv 52 significantly reduce the serum MDA formation.
| Groups | GSH (µmol/dl) | GPX (mol/dl) | MDA (µmol/dl) | CAT (mmol/dl) | SOD (mmol/dl) |
|---|---|---|---|---|---|
| I | 5.42±0.31# | 3.06±0.19 | 2.95±0.10 | 18.52±0.85# | 7.93±0.2 |
| II | 3.81±0.15* | 2.36±0.17 | 3.68±0.14* | 15.37±0.59* | 5.98±0.23* |
| III | 5.34±0.35# | 2.87±0.14 | 2.82±0.08# | 17.91±0.06 | 7.41±0.21# |
| IV | 4.32±0.22 | 2.69±0.17 | 3.35±0.18 | 15.81±0.06 | 6.47±0.25* |
| V | 5.33±0.33# | 3.05±0.19 | 2.99±0.15# | 17.42±0.84 | 7.26±0.34# |
| VI | 5.37±0.32# | 3.01±0.18 | 2.86±0.06# | 18.37±0.82# | 7.77±0.28# |
Table 5: Effects of heptoplus on serum antioxidant profile
Effect of HP and Liv 52 on liver lysosomal enzymes
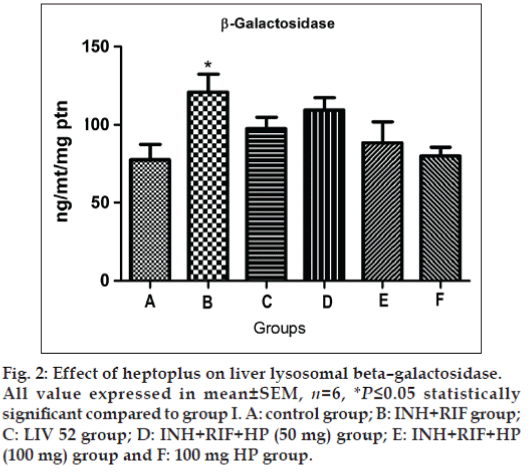
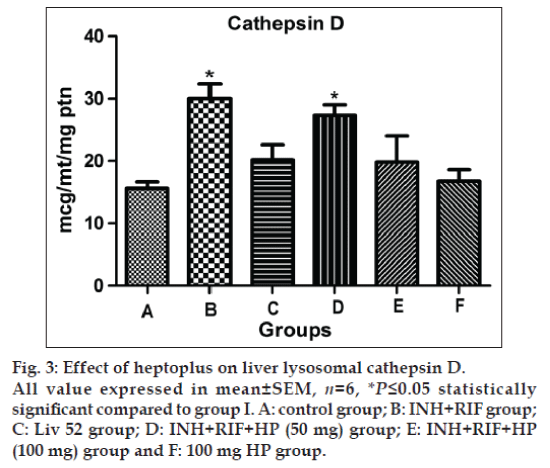
Significantly elevated activity of β -galactosidase (P≤0.05) and cathepsin D (P≤0.05) are observed in group II rats, as shown in figs. 2 and 3. The concurrent application of HP brings back the normal activity of both of these two enzymes in a dose dependent manner. Liv 52 treated rats in group III show normal lysosomal enzymatic activity.
Histopathology analysis
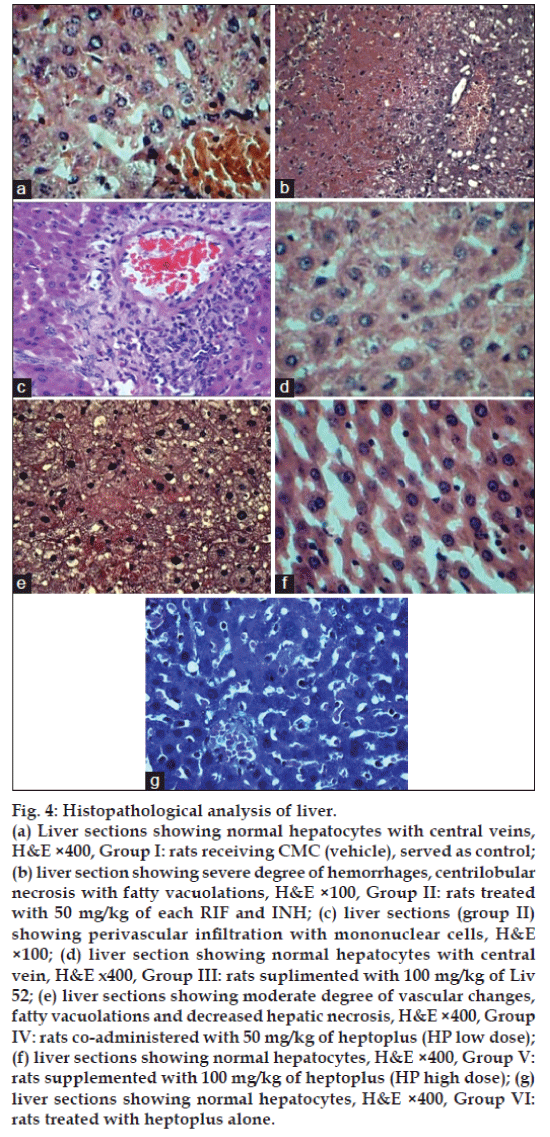
Histopathological analysis of rat liver is shown in fig. 4. Liver section in group II shows severe degree of vascular changes, lobular necrosis and moderate degree of fatty vacuolations with perivascular infiltration of mononuclear cells. Liv 52 and HP (100 mg/kg) treated rats liver section apparently revealed normal architecture similar to the control (Group I). HP control (Group VI) liver also revealed normal architecture.
Discussion
The present study is to analyze heptoplus as an oral supplement to protect the liver from oxidative damage induced by INH and RIF treatment in rats. Oxidative stress mediated liver injury is the main ascription mechanism behind antituberculosis drugs (ATD) induced hepatotoxicity [19]. Membrane integrity of hepatocytes is lost due to the generation of lipid peroxides, as a result of oxidative insult in the cells [20]. Anomalous increase of AST, ALT and LDH in the systemic circulation is a sign of hepatocelluar damage and a marker for necrosis [21]. Elevated levels of serum AST, ALT, and LDH indicate the presence of hepatocelluar damage and necrosis. HP supplement decreases these enzymes in the systemic circulation by curtailing the generation of lipid peroxides and maintaining the heptocytes integrity. Normally ALP is excreted in to the bile, during damage to the liver cells, it is unable to excrete ALP into bile, and hence ALP gets piled up in the serum [22]. Increased level of ALP in the systemic circulation is a caution of hepatocyte damage. HP supplement restores the normal ALP level in the systemic circulation may be by enhancing the ALP excretion in the bile and induce the regeneration of liver cells. Total bilirubin is an indicator for the assessment of liver function. Increased bilirubin level in the serum, may be due to the decreased hepatic clearance, endorsing jaundice condition [23]. Aberrant level of serum bilirubin is an indication of impaired liver function and prevalence of jaundice. HP treatment aids the hepoacytes to function properly, by boosting the hepatic clearance of bilirubin.
Estimation of total protein and albumin levels are used to assess the liver function. During hepatoxicity, liver cells are unable to synthesize proteins properly as a result of hepatocelluar damage [24]. Sharp decline of TP and ALB, due to improper function of hepatocytes was observed. HP treatment protected the hepatocytes from experimental injury and retained the function of the liver cells to synthesise plasma proteins. GGT is an enzyme found in the liver and is a sensitive marker of liver diseases [25]. Elevation of GGT in systemic circulation is a characteristic feature of liver damage. HP secured the liver cells from INH and RIF by maintaining the normal GGT level. C reactive protein is a systemic marker for inflammation from tissue damage [26]. Cumulative level of CRP is an index of liver inflammation. HP supplement reduced the inflammation in the liver and curtailed the CRP level in the serum. Lysosomal enzyme secretion is the one of the key factors for the determination of type of cell death. During oxidative stress free radicals react with lipid bilayer of various organelles including lysosomes and rupturing the membrane, as a result destabilization occurs [27]. Increased activity of liver lysosomal β-galactosidase and cathepsin D is witnessed in rats intoxicated with INH and RIF. HP administration protected the liver lysosomes from free radical induced rupturing and maintains normal lysosomal enzyme activity. GSH is required for the detoxification of toxic metabolites [28]. HYZ principle metabolite of INH, deplete GSH by reacting with their sulfahydral groups and initiate oxidative stress [29]. Low GSH content might be the effect of maximum utilization for detoxification. However HP supplement restores the GSH content by minimizing its utilization. GPX activity is reduced in accordance with GSH content [30]. GPX reduction is subsequent of GSH reduction. Supplement of HP conserves GSH content, there by retaining GPX activity. Elevation of MDA (malonaldehyde) is an indicator of excessive formation free radical and activation of lipid peroxidation (LPO) [31]. CAT and SOD are mutually supporting front line antioxidant enzymes, which are inactivated by the presence of lipid peroxides [32]. In the current study higher level of LPO along with reduced levels of CAT and SOD, indicate the presence of oxidative insult. Co-administration of HP maintains antioxidant status by inhibiting the formation of free radicals and curtails LPO. In authentication of above biochemical findings, toxicity induced rat liver section show severe degree of hemorrhage, centrilobular necrosis with fatty vacuolations and perivascular infiltration of mononuclear cells. Adjuvant application of HP protects hepatocytes from oxidative damage, which maintains normal architecture of liver with portal triad.
Heptoplus effectively protected the liver cells, from free radical induced oxidative damage by INH and RIF, may be due to the presence of active antioxidant ingredients like phyllanthine, hypophyllanthine, withaferin and curcumin, which are present in the polyherbal formulation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ravi V, Patel SS, Verma NK, Dutta D, Saleem TS. Hepatotoxicity activity of BombaxceibaLinn against isoniazid and rifampicin induced toxicity in experimental rats. Int J ApplSci Res Nat Prod 2010;3:19-26.
- Adnan J, Nagi AH, Shahzad M, Azamzia. The hepato-protective effect of Cassia fistula leaves in isoniazid and rifampicin induced hepatotoxicity in rodents. Biomedica 2010;26:25-9.
- Zeinab YA. Biochemical evaluation of some natural products against toxicity induced by antitubercular in rats. N Y Sci J 2012;5:69-80.
- Tayal V, Kalra BS, Agarwal S, Khurana N, Gupta U. Hepatoprotectiveeffect of tocopherol against isoniazid and rifampicin induced hepatotoxicity in albino rabbits. Indian J ExpBiol 2007;45:1031-6.
- Jiang Y, Renxiu P, Jie C, Yinghui L, Guicheng D. Effect of rifampin on CYP2E1-dependent hepatotoxicity of isoniazid in rats. Pharmacol Res 2009;59:112-9.
- Singh M, Sasi P, Gupta VH, Rai G, Amarapurkar DN, Wangikar PP. Protective effect of curcumin, silymarin and N-acetylcysteine on antitubercular drug-induced hepatotoxicity assessed in an in vitro model. Hum ExpToxicol 2012;31:788-97.
- Makhlouf HA, Helmy A, Fawzy E, El-Attar M, Rashed HA. A prospective study of antituberculous drug-induced hepatotoxicity in an area endemic for liver diseases. HepatolInt 2008;2:353-60.
- Yue J, Peng RX, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Pharmacol Sin 2004;25:699-704.
- Tostmann A, Boeree MJ, Peters WH, Roelofs HM, Aarnoutse RE, van der Ven AJ, et al. Isoniazid and its toxic metabolite hydrazine induce in vitro pyrazinamide toxicity. Int J Antimicrob Agents 2008;31:577-80.
- Sarich TC, Adams SP, Petricca G, Wright JM. Inhibition of isoniazid-induced hepatotoxicity in rabbits by pretreatment with an amidase inhibitor. J PharmacolExpTher 1999;289:695-702.
- Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 2003;37:924-30.
- Chen X, Xu J, Zhang C, Yu T, Wang H, Zhao M, et al.The protectiveeffects of ursodeoxycholic acid on isoniazid plus rifampicin induced liver injury in mice. Eur J Pharmacol 2011;659:53-60.
- Rana SV, Pal R, Vaiphie K, Singh K. Effect of different oral doses of isoniazid-rifampicin in rats. Mol Cell Biochem. 2006;289:39-47.
- Sinha AK. Colorimetric assay of catalase enzyme. Anal Biochem1972;47:389-94.
- Draper H, Hadley M. Malondialdehyde. Methods Enzymol 1990;186:421-31.
- Kakkor P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J BiochemBiophys 1984;21:131-3.
- Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 1976;74:214-26.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-8.
- Kale BP, Kothekar MA, Tayade HP, Jaju JB. Effect of aqueous extract of Azadirachtaindica leaves on hepatotoxicity by antituberculosis drugs in rats. Indian J Pharmacol 2003;35:177-80.
- Jadhav VB, Thakare VN, Suralkar AA, Deshpande AD, Naik SR. Hepatoprotective activity of Luffa acutangula against CCl4 and rifampicin induced liver toxicity in rats: A biochemical and histopathological evaluation. Indian J ExpBiol 2010;48:822-9.
- Anita S, Tej KB, Om PS. Clinical biochemistry of hepatotoxicity. J ClinToxicol 2011;S4:1-9.
- Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food ChemToxicol 2007;45:1551-7.
- Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: Hepatotoxicity of antituberculosis therapy. Am J RespirCrit Care Med 2006;174:935-52.
- Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr 2007;74:663-71.
- Daniel SP, Marshall MK. Evaluation of the liver. Laboratory tests. Schiff’s Diseases of the Liver. 8th ed. USA: JB Lippincott Publications; 1999. p. 205-39.
- Akinloye OA, Abioye OA, Olaojoyetan OE, Awosika OT Akinloye DI. Dose-dependent effects of paraquat on C-reactive protein, some lipid profile parameters and histology of tissues in male albino rats. BiochemPhysiol 2013;2:1-5.
- Ramadori G, Moriconi F, Malik I, Dudas J. Physiology and pathophysiology of liver inflammation, damage and repair. J PhysiolPharmacol 2008;59 Suppl 1:107-17.
- ViswanathaSwamy AH, Kulkarni RV, Thippeswamy AH, KotiBC, Gore A. Evaluation of hepatoprotective activity of Cissusquadrangularisstem extract against isoniazid-induced liver damage inrats. Indian J Pharmacol 2010;42:397-400.
- Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol 2006;45:117-26.
- Sodhi CP, Rana SV, Mehta SK, Vaiphei K, Attari S, Mehta S. Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug ChemToxicol 1997;20:255-69.
- Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des 2011;17:3460-73.
- Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci 2000;7:444-58.