- *Corresponding Author:
- B. Antony
R & D Laboratory, Arjuna Natural Extracts Ltd., P.B. No. 126, Bank Road, Alwaye-683 101, India
E-mail: benny@arjunanatural.com
| Date of Submission | 30 September 2004 |
| Date of Revision | 27 March 2006 |
| Date of Acceptance | 14 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 772-776 |
Abstract
The present study was conducted to evaluate the hepatoprotective effects of the Centella asiatica extract in carbon tetrachloride-induced liver injury in rats. Sprague Dawley rats were treated with alcohol extract of Centella asiatica orally in two doses (20 and 40 mg/kg/day) for 3 mo along with intraperitoneal injection of carbon tetrachloride (1 ml/kg). Biochemical parameters such as serum total protein, albumin and marker enzymes (aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) were estimated both before and after the experiment. Histopathological studies of liver were also carried out to confirm the biochemical changes. Carbon tetrachloride-induced hepatotoxic effects were evident by a significant (p < 0.05) increase in the serum marker enzymes and a decrease in the total serum protein and albumin. Administration of extract of Centella asiatica effectively inhibited these changes in a dose-dependent manner; maximum effect was with 40 mg/kg. Histopathological examination of liver tissue corroborated well with the biochemical changes. Hepatic steatosis, hydropic degeneration and necrosis were observed in carbon tetrachloride-treated group, while these were completely absent in the treatment group. Centella asiatica extract exhibited hepatoprotective action against carbon tetrachloride-induced liver injury. This effect is attributed to the presence of asiaticoside (14.5%) in the extract.
Centella asiatica (Linn) Urban, Synonym Hydrocotyle asiatica, belongs to the family Umbelliferae. It is a weed found throughout India, Sri Lanka and Madagascar. In Ayurveda it has been used in the treatment of skin diseases, local wounds and also for improving general mental ability, jaundice and hepatitis [1]. A study conducted in rats found that the administration of Centella asiatica leaf powder prevented mortality rate due to gross protein deficiency, increased blood protein nitrogen and prevented fatty infiltration of liver [2]. The therapeutic activity of a triterpenoid extract of Centella asiatica was observed in chronic hepatic disorders [3]. It was observed that in male Wistar rats, the administration of asiaticoside derivatives of Centella asiatica caused the restoration of 40% of the damaged liver cells to normal state [4]. Chemical studies on Centella asiatica leaves showed that one of the major components is asiaticoside [5]. The present study reports the protective effects of a water-dispersable extract of Centella asiatica leaves in CCl4-induced liver injury in rats.
Materials and Methods
All chemicals used for the experiment were of analytical grade. Methanol, hexane and acetonitrile were purchased from Merck India Ltd.
Preparation of the extract of Centella asiatica
Centella asiatica plants were collected from local suppliers during the months of August/September and pharmacognostically identified with the help of herbarium sample of the species kept at R&D Laboratory of Arjuna Natural Extracts, Aluva, Kerala. Dried leaves of Centella asiatica (300 g) were refluxed with 2 litres of 80% methanol for 3-4 h. After refluxing, the contents were cooled and filtered. The filtrate was collected and the residue was refluxed again with 1 litre of 80% methanol for 2 h. The process was repeated; the filtrates were pooled and concentrated up to 70% total dissolved solid (TDS) level. The crude extract thus obtained was refluxed with hexane for about 1 h at 80°. The residue obtained after hexane extraction was then kept for crystallization in a refrigerator for 4-5 d. The crystals formed were separated and dried under vacuum. The formed were separated and dried under vacuum. The dried material was then powdered and kept as Centella asiatica extract. The yield was 28.5 g (9.5%). The asiaticoside was estimated using HPLC (Shimadzu-class VP system) and found to be 14.5%.
Male Sprague Dawley rats weighing 250-280 g were used for the experiment. Animals were purchased from Small Animal Breeding Station, Veterinary College, Mannuthy, Thrissur, Kerala. They were housed in a temperature-controlled room (28 ± 1°) in clean polypropylene cages with 12 h light and 12 h dark cycles and fed with normal rat chow (Amrut Animal Feed, Maharashtra) and water ad libitum.
The study was conducted at Little Flower Medical Research Centre, Angamaly, after obtaining clearance from Institutional Animal Ethics Committee. After an acclimatization period, animals were divided into fivegroups of six each, comprising normal control, vehicle control, toxic control and treatment groups. Normal control group was given distilled water, while vehicle control group was administered coconut oil. CCl4 was given to toxic control group, while treatment groups wereadministered Centella asiatica extract along with CCl4. Liver injury was induced by an intraperitoneal injection (1 ml/kg) of CCl4 in coconut oil (1:1 ratio). Injection was given twice a week for 3 mo [6]. Oral feeding of Centella asiatica extract was started 3 d prior to intraperitoneal injection of CCl4 and continued for 3 mo.
Estimation of biochemical parameters
Blood samples were collected from the caudal vein before starting the experiment under light ether anaesthesia and by direct cardiac puncture after the completion of the study. Serum was separated for estimation of biochemical parameters. Total protein and albumin were estimated by Biuret and bromo cresol green method [7] respectively. The marker enzymes (AST, ALT and ALP) were studied by Bergmeyer method [8] and King and Amstrong method [9] respectively.
Histopathological studies
Anatomy of the liver was studied immediately after sacrificing all the animals. A small portion was fixed in 10% neutral buffered formalin as described by Luna [10]. Thin sections of 4-5 μm were taken, stained with Haematoxylin and Eosin and histology was studied.
Statistical analysis
Statistical analyses were carried out using ANOVA. Results were expressed as means ± SE. One-way ANOVA with repeated measures was used to analyse the variance over a period of time and for intergroup comparisons. Paired ‘t’ test was used to compare thebiochemical parameters both before and after experiment. Level of significance was set at p < 0.05.
Results and Discussion
Results of the present study show that serum total protein and albumin levels remained the same in groups A and B (controls) both before and after the treatment period. The serum protein level significantly (p < 0.05) decreased in group C (toxic control). In rats treated with Centella asiatica extract, the protein level reversed partially (Table 1). The serum albumin level was significantly decreased in toxic control group, which was reversed partially in group E (40 mg/kg), but in group D (20 mg/kg) there was no significant effect (Table 1).
| Group No | Total protein (g/dl)# | Albumin (g/dl)# | AST (U/L)## | ALT (U/L)## | ALP (KA)** | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 month | 3 month | 0 month | 3 month | 0 month | 3 month | 0 month | 3 month | 0 month | 3 month | |
| Control (A) | 6.8+0.5 | 6.7+0.5 | 4.3+0.3 | 3.9+0.3 | 32.7+2.1 | 32.8+2.8 | 33.5+2.2 | 32.8+2.8 | 31.8+3.6 | 29.8+3.6 |
| V. control (B) | 6.4+0.4 | 6.4+0.4 | 3.8+0.1 | 3.7+0.1 | 32.7+1.5 | 31+2.2 | 32.5+2.0 | 28+2.0 | 31.2+3.4 | 34.2+1.0 |
| CCl4 alone (C) | 7.0+0.1 | *3.1+0.1 | 3.5+0.1 | *1.1+0.1 | 32.5+2.0 | *65.5+0.5 | 33.2+0.2 | *65+2.0 | 34.5+2.0 | *78.6+6.9 |
| (56%) ↓ | (68%)↓ | (102%)↑ | (96%)↑ | (128%)↑ | ||||||
| CCl4+CA | ||||||||||
| (20 mg/kg) | 7.7+0.3 | *5.1+0.2 | 3.4+0.2 | *1.4+0.1 | 35.7+1.8 | *50.5+2.4 | 32+1.6 | *43+1.3 | 30.1+2.8 | *64.5+5.4 |
| (D) | (34%)↓ | (60%)↓ | (42%)↑ | (34%)↑ | (115%)↑ | |||||
| CCl4+CA | ||||||||||
| (40 mg/kg) | 7.3+0.2 | 6.2+0.4 | 2.5+0.4 | 1.9+0.4 | 35.2+0.2 | 44.8+2.0 | 32.7+2.3 | 38.2+1.0 | 30.6+3.0 | *59.8+6.9 |
| (E) (E) | (15%)↓ | (24%)↓ | (28%)↑ | (17%)↑ | (86%)↑ | |||||
Table 1: Effect of centella asiatica on biochemical parameters in ccl4-induced liver injury in rats
The serum marker enzyme such as ALP, AST and ALT levels remained normal in groups A and B both before and after experiment, but they were increased in all the other groups during the experimental period. In the CCl4-treated rats, there was a significant (p < 0.05) increase in ALT levels, which was decreased when treated with a dose of 20 mg/kg extract and back to almost normal level with 40 mg/kg dose of extract.
The ALP level increased (p < 0.05) in all three experimental groups. It was observed that in the group treated with 40 mg/kg of Centella asiatica extract, ALP level was partially inhibited to a significant (p < 0.05) level. The enzyme AST increased (p < 0.05) in all experimental groups after 3 mo. The percentage of increase observed was lower (p < 0.05) in the extract-treated groups when compared to toxic control group (C) (Table 1).
Macroscopic examination of liver in groups A and B showed normal architecture. In toxic control group (group C), liver was pale in colour with micro- and macro-nodules on the liver surface. In group D (20 mg/kg of extract), pale colour was not so prominent, but the surface of liver was rough with micro- and macro-nodules. In group E (40 mg extract/kg body weight), liver of 50% of animals became almost normal.
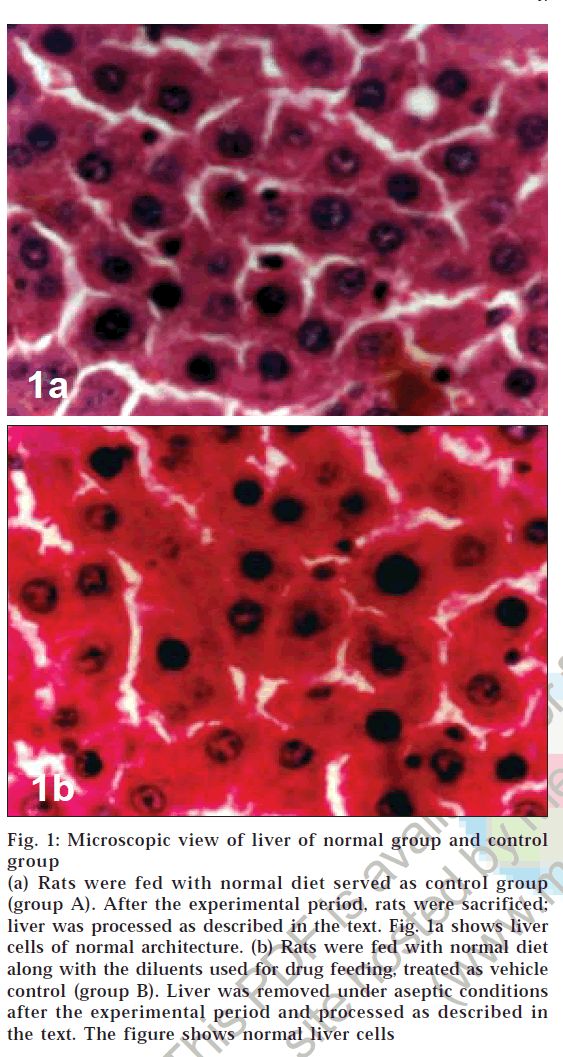
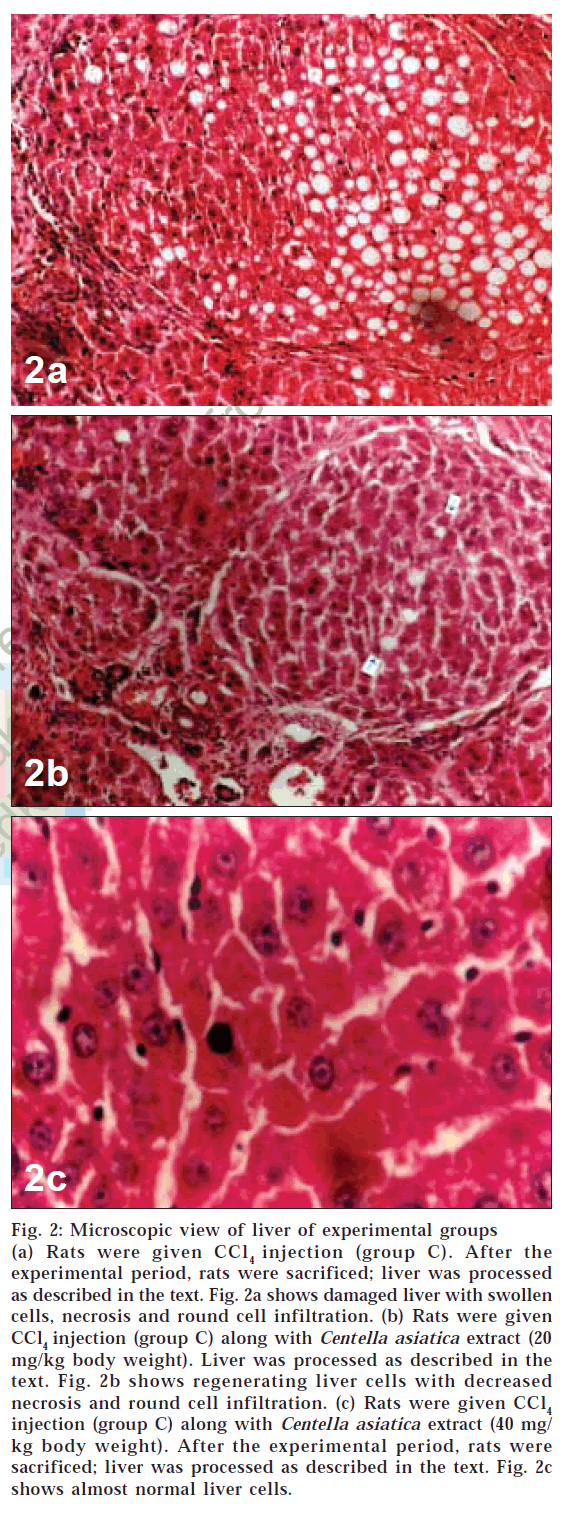
Microscopic examination of liver of control group animals showed normal hepatocytes (figs. 1a and 1b). Cross sections of liver in group C animals treated with CCl4 showed marked fatty changes. There was hepatic steatosis with hydropic degeneration. The cells appeared swollen with fat, giving a Swiss-cheese effect, with focal areas of necrosis and round cell infiltrate (chronic inflammatory cells) in portal areas and interlinked septa (fig. 2a). In group D animals, fatty change or necrosis was very negligible, and observed a decrease in round cell infiltrate was observed (fig. 2b), while in group E the liver showed more or less normal hepatocytes (fig. 2c). There was very little fatty change and round cell infiltration compared to that seen in animals of group D.
Figure 1: Microscopic view of liver of normal group and control
(a) Rats were fed with normal diet served as control group (group A). After the experimental period, rats were sacrificed; liver was processed as described in the text. Fig. 1a shows liver cells of normal architecture. (b) Rats were fed with normal diet along with the diluents used for drug feeding, treated as vehicle control (group B). Liver was removed under aseptic conditions after the experimental period and processed as described in the text. The figure shows normal liver cells
Figure 2: Microscopic view of liver of experimental groups (a) Rats were given CCl4 injection (group C). After the experimental period, rats were sacrificed; liver was processed as described in the text. Fig. 2a shows damaged liver with swollen cells, necrosis and round cell infiltration. (b) Rats were given CCl4 injection (group C) along with Centella asiatica extract (20 mg/kg body weight). Liver was processed as described in the text. Fig. 2b shows regenerating liver cells with decreased necrosis and round cell infiltration. (c) Rats were given CCl4 injection (group C) along with Centella asiatica extract (40 mg/ kg body weight). After the experimental period, rats were sacrificed; liver was processed as described in the text. Fig. 2c shows almost normal liver cells.
In the present study, CCl4 was used to produce liver injury in rats. Carbon tetrachloride is metabolized in the liver to toxic-free radical CCl3, which affects cellular permeability of hepatocytes, leading to elevated levels of serum marker enzymes like AST, ALT and ALP. It causes massive histopathological changes like necrosis, congested vessels, multifocal areas of fatty changes, nuclear disintegration, sinusoidal dilation, Kupffer cell hyperplasia, etc. The prevention of this phenomenon can be considered as hepatoprotective activity [11].
Serum albumin is a marker of synthetic function of the liver and is a valuable guide to the severity of chronic disease. Albumin is significantly decreased in the CCl 4-alone-treated group, while the decrease observed in the extract-treated groups was lesser than the former group. Rats treated with Centella asiatica extract have increased levels of total protein and albumin in serum, which indicates its hepatoprotective activity. Stimulation of protein synthesis has been advanced as a contributory mechanism, which accelerates the regeneration and production of liver cells. Aminotransferases are present in hepatocytes and leak into the blood with liver cell damage. High levels of these enzymes are seen in hepatic necrosis, myocardial infarction and muscle injury. In the present study, all the marker enzymes increased (p < 0.05) in the toxic control group, while there was a significant (p < 0.05) decrease in its activity in the treatment groups, indicating the hepatoprotective effect of Centella asiatica extract.
The surface of the liver was rough and showed nodules in the CCl4-treated group, while the intensity of damage was not so high in the treatment group. A dose-dependent hepatoprotection was observed in the present study. Rats treated with 40 mg of the extract had a higher degree of protection than those treated with 20 mg extract.
Centella asiatica extract provides hepatoprotective action against CCl4-induced liver injury in rats. This was evidenced from the present study by the inhibition of decrease in serum albumin and protein level and elevation in the serum marker enzymes - AST, ALT and ALP. Administration of the extract of Centella asiatica effectively inhibited fatty changes and round cell infiltrate in hepatocytes in a dose-dependent manner. The results of the present study are comparable with studies conducted with silymarin [12] and curcumin [13]
Previous studies showed that administration of asiaticoside, an isolated constituent of Centella asiatica, significantly increased the levels of antioxidant enzymes like super oxide dismutase, catalase, glutathione peroxidase in excision-type cutaneous wounds in rats [14].Antioxidants such as ellagic acid [15] and curcumin had been reported to protect liver injury and fibrosis induced by hepatotoxins [16]. Hence the hepatoprotective effects of Centella asiatica in the present study might be due to the potent antioxidant action of asiaticoside present (14.5%) in the extract.
Acknowledgements
We are grateful to Dr. K. J. Baby, M.D., Pathologist, Little Flower Hospital and Research Centre, Angamaly, for conducting histopathological studies.
References
- Arora,D.,Kumar,M.andDubey,S.D.,J.Natur.Remed.,2002, 2,143.
- Patil,J.S.,IndianDrugs,1998,35,711.
- Danese, P., Carnevali, C. and Bertazzoni, M.G., Contact dermatitis,1994,31,201.
- Lee,E-S.,Park,H.,Jew,S.,Ryu,J.H.,Kim,Y.C.,Lee,M.K.,Choi,J., Kim,T-H.,Zhao,L.X.andJahng,In;YurngdongBookofAbstracts, 219thACSNationalmeeting,SanFranciscoCA.March26-30, MEDI–137,AmericanChemicalSociety.WashingtonD.C.2000.
- Bonte,F.,Dumas,M.,ChaudagneC.andMeybeck,A.,PlantaMedica,1994,60,133.
- Abe,H.,Sakaguchi,M.,Odashima,S.andArichi,S.,NaunynSchmiedebergsArch. Pharma., 1982, 320,266.
- Gowenlock, A.H., McMurray, J.R., McLauchlan, D.M., Plasma proteins In; Varley’s Practical Clinical Biochemistry, 6th Edn., CBS Publishers, New Delhi, 1988, 401.
- Bergmeyer,H.,Bernt,E.,1950,In;VarleyH.,Gowenlock,A.H.,Bell, M.,PracticalClinicalBiochemistry,5thEdn.,WilliamHeinmannThressiamma, K.C. and Kuttan, R., Indian J. Physiol.AndMedicalBooksLtd,London,1980,741.
- King,E.J.,Amstrong,A.R.,MethodofKingandAmstrongIn;Varley,H.,Gowenlock,A.H.,Bell,M.,PracticalClinicalBiochemistry,5thEdn., William Heinmann Medical Books Ltd, London, 1980,897.
- Luna,L.G.,Manualofhistology,StainingmethodsofArmedForces Institute of Pathology. 3rdEdn., New York, McGraw Hill,1968.
- Jayasekhar,P.andMohanan,P.,IndianJ.Pharmacol.,1997,29,426.
- Mourelle,M.,Muriel,P.,Favari,L.andFranco,T.,Fund.Clin. Pharmacol.,1989,3,183.
- Srinivas,L.andShalini,V.K.,FreeRadicalBiol.Med.,1991,11, 277.
- Shukla,A.,Rasik,A.M.andDhawan,B.N.,Phyto.Research,1999,13,50.
- Thressiamma, K.C. and Kuttan, R., Indian J. Physiol.andPharmacol., 1996, 40. 363.
- Nishiqaki,I.,Kuttan,R.,Oku,H.,Ashoori,F.andYagiK.,J.Clin.Biochem.Nutr.1992,13,23.