- *Corresponding Author:
- U. S. Baghel
Department of Pharmaceutical Chemistry and Analysis, Khalsa College of Pharmacy, G. T. Road, Amritsar-143 002
E-mail: drusb1985@yahoo.com
| Date of Submission | 04 December 2015 |
| Date of Revision | 07 November 2016 |
| Date of Acceptance | 20 February 2017 |
| Indian J Pharm Sci 2017;79(2):197-203 |
Abstract
Fast, sensitive, precise and selective high performance liquid chromatography and high performance thin layer chromatography methods were developed for the simultaneous estimation of curcumin and quercetin in a polyherbal formulation. Validation of those methods was carried out with respect to linearity, range, precision, accuracy and robustness. Both methods were applied to a polyherbal formulation to find the amount of curcumin and quercetin and compare. Curcumin and quercetin were found to be 9.5 mg and 0.613 mg in the polyherbal formulation using the high-performance liquid chromatography method whereas with the high performance thin layer chromatography method, curcumin and quercetin were found to be 9.7 mg and 0.674 mg, respectively. Both methods identified and quantified curcumin and quercetin effectively.
Keywords
HPLC, HPTLC, curcumin, quercetin, polyherbal formulation
World Health Organization (WHO) has stressed on the need for scientific validity of herbal drugs and ensuring, devising and implementing sound science [1]. WHO has published guidelines for quality control of herbal medicines to ensure the identity, purity and content of herbal materials [2]. A number of techniques can be used for the analysis of herbal medicines such as high performance liquid chromatography (HPLC), gas chromatography-mass spectroscopy (GC-MS), HPLCnuclear magnetic resonance (HPLC-NMR), 13C-NMR, near infrared (NIR). These techniques can be used for the structure elucidation of herbal drugs. HPLC and high performance thin layer chromatography (HPTLC) are the techniques that can be used to analyse most of the herbal drugs [3]. HPLC and HPTLC have been employed for a number of times in analysing number of herbal drugs [4-12].
Curcumin (CMN) is yellow in colour and derived from the plant Curcuma longa Linn. CMN has a number of activities such as antioxidant, antiinflammatory, antiviral, antibacterial, antifungal and anticancer. CMN has potential in number of diseases such as arthritis, Alzheimer’s disease, diabetes and allergies [13-16]. Quercetin (QCN) is a flavonol, which is a subclass of flavonoids. QCN is found in number of fruits and vegetables such as onion (Allium cepa Linn.), apple (Malus pumila Miller), black tea (Camellia sinensis (L.) Kuntze) and black currant (Ribes nigrum Linn.). QCN has number of activities such as antioxidative, anticarcinogenic, antiinflammatory, antiaggregatory and vasodilating activity [17-19].
Estimation of QCN has been done by using HPTLC for a number of times individually and also in combination with other compounds [20-23]. Also a number of validated HPLC methods have been devised for the estimation of QCN both individually and in combination with other compounds [24-27]. Quantification of CMN has been done by HPTLC technique [28]. Another stability indicating HPTLC method has been developed to quantify CMN in pharmaceutical formulations [29]. A HPLC method for simultaneous determination of CMN and silymarin in various dosage forms has also been reported. Analysis of degradation products of CMN has been done using HPTLC [30]. Other methods are also available for estimation of curcumin [31,32].
Materials and Methods
HPLC grade water and methanol and analytical grade ethyl acetate, formic acid, glacial acetic acid, acetone, toluene, sodium acetate were purchased from Loba Chemie, Mumbai, India. HPLC grade acetonitrile was purchased from Fisher Scientific, Mumbai, India. Analytical grade ethanol was purchased from Changshu Yangyuan Chemicals, China. Marker compounds, CMN and QCN were purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India, and Divya madhunashini vati was purchased from Divya Pharmacy, Amritsar. All other reagents employed were of analytical grade.
Shimadzu UV-1700 and Lab India UV-3000 UV spectrophotometers were used for UV/Vis spectrophotometric analysis. UFLC Shimadzu with binary prominence LC-20 AD liquid pump, SPD-20 A prominence UV/Vis detector and a 77251 Rheodyne injector with 20 μl loop was used for HPLC analysis. Inertsil C18 column (250 mm×4.6 mm i.d., 5 μ) with gradient mixer SUS 20 A were used in HPLC analysis. Millipore filter of 0.45 μ pore size was used for filtering mobile phase for HPLC. Camag HPTLC was used for HPTLC analysis. Precoated TLC plates Silica gel 60F254 from Merck, Germany were used with layer thickness 0.2 mm. Samples were applied by means of pressurized nitrogen gas (150 kg/cm2) through Camag automatic TLC sampler 4 fitted with a 25 μl syringe. The bands were visualized in Camag UV cabinet at 254 nm.
The plant materials were collected from local area in Fatehgarh Taprian, Ropar, Punjab, India in the month of October. Then, plants were identified and authenticated as C. longa (Specimen number ACL/11/12) and Syzigium cuminii (Specimen number ASC/11/12) at NIPER, Mohali, Punjab, India. A herbarium sheet of the plants were submitted in the herbarium of the NIPER, Mohali, Punjab.
Literature survey revealed that several solvents like methanol [33], ethanol [34] and acetonitrile [35] were used for the extraction of CMN and QCN from plant and polyherbal formulation. The efficiency of extraction with methanol was greater than others so methanol was selected for standard and sample preparation. The CMN and QCN standards were also found to be stable in this solvent at room temperature and also during the entire duration of study design.
Preparation of standard solution for HPLC analysis
Depending on the polarity of both the drugs, solubility analysis for both the drugs was done in various solvents such as methanol, acetic acid, ethanol, 0.1 N NaOH, 0.1 N HCl. Methanol was selected as the solvent for study. Standard solutions of both CMN and QCN were prepared using HPLC grade methanol giving concentration of 125 μg/ml in each separately and also in combination.
Preparation of sample solution for HPLC analysis
Twenty tablets of Divya madhunashini vati were weighed and powdered. From this, a sample solution containing 10 mg/ml drug was prepared using HPLC grade methanol and filtered through 0.45 μ Millipore nylon filter. Final concentration of CMN was 10 μg/ml and for QCN was 50 μg/ml.
HPLC conditions
The column chromatography was performed on a HPLC system equipped with binary prominence LC-20 AD liquid pump, SPD-20 A prominence UV/Vis detector and a 77251 Rheodyne injector with 20 μl loop. Inertsil C18 column (250×4.6 mm i.d., 5 μ) with gradient mixer SUS 20 A were used in HPLC analysis. Millipore filter of 0.45 μ pore size was used for filtering mobile phase for HPLC. Wavelength was set at 397 nm. Operation, data acquisition and analysis were performed by using LC solution 1.22 version Single Channel software.
Chromatographic method
Conditions were optimized for individual CMN and QCN separately. Then, optimization was carried out for simultaneous estimation. Isosbestic point was found by using UV spectra at 397 nm. Methanol and water in ratio of 75:25 v/v isocratic elution mode with the flow rate of 1 ml/min were used for HPLC analysis. Each chromatographic run was completed within 10 min. Then, this HPLC method was applied to the sample solution of marketed polyherbal formulation and concentration of CMN and QCN was calculated using the Eqn. 1, (sample area/standard area)×(weight of standard/50)×(100/sample weight×purity of standard/100)×(100–W/100×average weight), where, W is the percent water content in standard drug.
Preparation of standard solution and HPTLC conditions
A working standard solution of CMN (10 μg/ml) was prepared using methanol. Similarly, for QCN, 50 μg/ ml working standard solution was prepared using methanol. The planar chromatography was performed on fresh aluminium plates (10×10 cm) precoated with silica gel 60 F254 of layer thickness 0.2 mm (Merck, Germany). Before use, the plates were developed in the mobile phase and were activated horizontally for 20 min at 120 ± 0.5° using an oven to remove elutable components. Samples were applied to the plates as bands 6 mm wide, 10 mm from the bottom, by means of a pressurized nitrogen gas (150 kg/cm2) through Camag automatic TLC sampler 4 fitted with a 25 μ syringe. The bands were visualized in Camag UV cabinet at 254 nm. Developing chamber was Camag glass twin through chamber (10×10 cm). Densitometer consisted of Camag TLC scanner 3 operated by WinCATS software. The scanning speed and data resolution were 20 mm/s and 100 μm/step, respectively.
HPTLC method for simultaneous estimation of CMN and QCN
Mobile phase consisting of toluene/ethyl acetate/ formic acid (4.5:4:0.1, v/v/v) was used. Sample was applied using automatic TLC sampler. Then, TLC plate was developed over a development distance of 8 cm (migration time of 15 min) in solvent. After this the plates were dried at 60 ± 0.5° and then densitometric analysis was done. Calibration curve was constructed using different volumes of standard solution consisting of mixture of CMN and QCN.
Preparation of sample solution for HPTLC
Sample solution was prepared using Divya madhunashini vati tablets. Twenty tablets were weighed and finely powdered. A quantity of powder equivalent to about 500 mg transferred to a 50 ml volumetric flask, dissolved in methanol, volume made up with methanol and filtered through a 0.45 μm Millipore nylon membrane filter under vacuum. Concentration of CMN and QCN was calculated using the Eqn. 1.
Validation of HPLC and HPTLC method
Linearity and range were established for both the methods. Precision was evaluated by measuring intraday and interday precision. Accuracy was established by performing recovery studies. Robustness was established by making deliberate minor variations in the wavelength and calculating the percent deviation from original method. System suitability testing was performed before starting the experiment. Methanol was used as the extraction solvent as efficiency of the extraction was more with methanol than with other solvents.
Results and Discussion
Overlay spectra of CMN and QCN in methanol were recorded on a UV/Vis spectrophotometer and an isosbestic point was chosen at 397 nm. The solvent type, solvent strength, detection wavelength and flow rate were varied to determine the chromatographic conditions giving the best separation. Other conditions such as time required for analysis, flow rate of mobile phase, symmetry of the eluted peaks, assay sensitivity and solvent noise during drug analysis were also considered during optimization. It was determined at 397 nm, both the drugs can be detected simultaneously with good separation, sensitivity, consistent baseline and no mobile phase interference.
Methanol and sodium acetate buffer in the ratio of 70:30 was used in the beginning but it showed tailing. Different ratios of methanol to sodium acetate buffer such as 75:25, 80:20 and 85:15 were tried but they all showed tailing except the ratio 75:25 showed good resolution. However, mobile phase consisting of methanol:water in the ratio of 70:30 offered better resolution but with tailing. Further changing the ratio of methanol:water to 75:25, gave the best resolution and minimum tailing. Any further changes in the ratio of methanol:water resulted in either poor resolution or poor tailing. Finally, methanol:water at the ratio of 75:25 was selected as the mobile phase. Chromatograms obtained using methanol:water mobile phase in the ratio of 75:25. The developed HPLC method was applied to the polyherbal formulation. CMN and QCN were found to be 9.5 and 0.613 mg, respectively in the marketed polyherbal formulation.
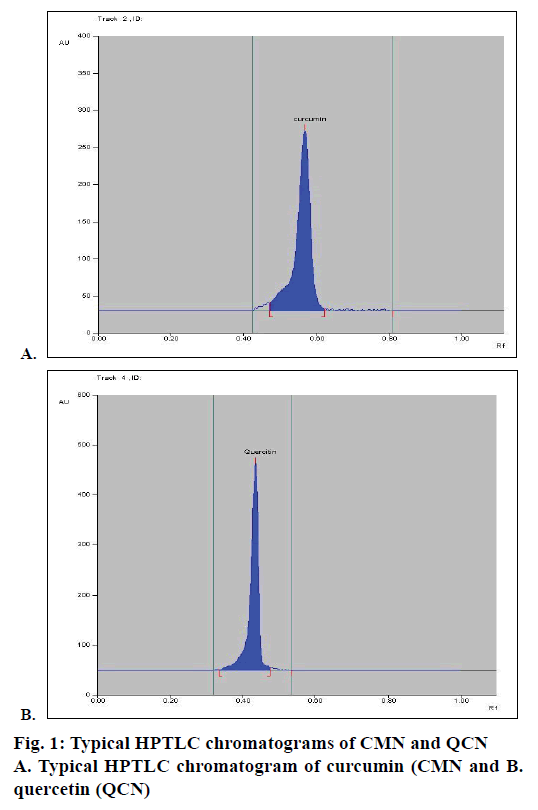
Mobile phase optimization for the HPTLC method was started by using a mobile phase consisting of toluene:ethyl acetate:glacial acetic acid:formic acid in the ratio of 4.5:3:1:0.2, but it gave poor resolution. Further modifying the ratio of this mobile phase and increasing the ratio of toluene to 5.5 did not improve results and also gave poor resolution. An improved Rf but poor resolution resulted in further modification of ratio of mobile phase to 5:3:05:0.2. Then, ethanol was added to the mobile phase and a ratio of 4.5:3:1:1:0.2 for toluene:ethyl acetate:glacial acetic acid:ethanol:formic acid was used to develop chromatogram, which also resulted in poor resolution. However, by removing ethanol and glacial acetic acid and using toluene:ethyl acetate:formic acid as mobile phase in the ratio of 4.5:4:0.2 gave better resolution. Further modifying this mobile phase ratio to 4.5:4:0.1 resulted in the best resolution and gave Rf value for CMN and QCN as 0.58 and 0.49, respectively. So, toluene:ethyl acetate:formic acid in ratio of 4.5:4:0.1 was finalized for use as the mobile phase for HPTLC. The optimized TLC plate containing CMN, QCN ran using toluene:ethyl acetate:formic acid in ratio of 4.5:4:0.1 as mobile phase. Typical chromatograms of CMN and QCN are shown in Figure 1A and B, respectively.
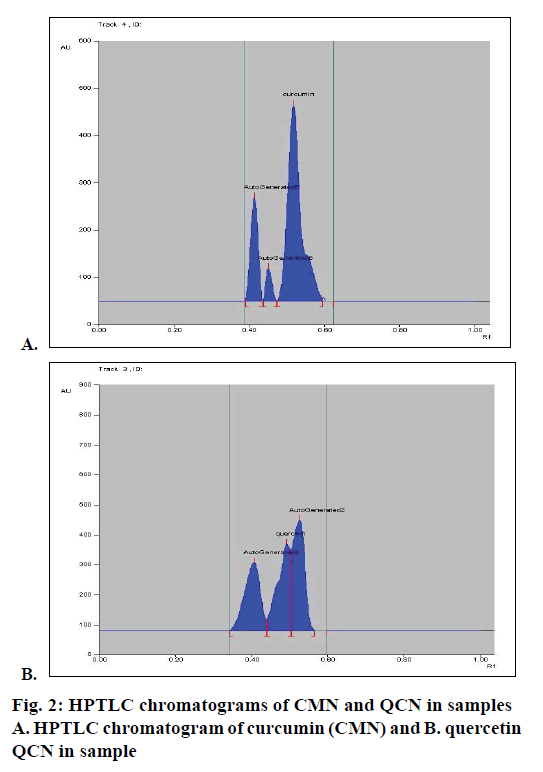
Calibration curve was constructed by plotting the peak area ratios of CMN and QCN versus the concentrations of the calibration standards. When the developed method was applied to the sample, HPTLC chromatograms were obtained for CMN and QCN, which were shown in Figure 2A and B, respectively. The CMN and QCN were found to be 9.7 and 0.674 mg, respectively in the marketed polyherbal formulation.
Linearity was established within concentration range 2-12 μg/ml (Table 1). Precision was determined by analysing three concentration and three replicates of each concentration. Data obtained for determining precision was given in Table 2. Accuracy was calculated as the percent recovery by analysing known added amounts of analyte to the sample. A total of nine determinations were made at three concentration levels (three concentrations and three replicates of each concentration), the data of which was presented in Table 3.
| Compound | Concentration (µg/ml) | Slope (n=2) | Intercept (n=2) | r2 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | RSD | SEM | Mean ± SD | RSD | SEM | |||
| CMN | 2-12 | 56320.6 ± 3.36 | 0.006 | 1.50 | 25602.6 ± 2.40 | 0.009 | 1.08 | 0.996 |
| QCN | 2-12 | 13969.2 ± 3.63 | 0.026 | 1.63 | 2661.2 ± 1.64 | -0.062 | 0.74 | 0.997 |
Table 1: Summary of linearity data of hplc method
| Concentration (µg/ml) | Inter-day precision (n=3) | Intra-day precision (n=3) | ||||
|---|---|---|---|---|---|---|
| Mean area ± SD | SEM | RSD | Mean area ± SD | SEM | RSD | |
| CMN | ||||||
| 6 | 385239.1 ± 558.9 | 395.22 | 0.1451 | 385194.8 ± 621.9 | 439.79 | 0.1615 |
| 8 | 471068 ± 706.90 | 499.80 | 0.1500 | 470996.4 ± 808.11 | 571.42 | 0.1716 |
| 10 | 594895.8 ± 913.9 | 646.29 | 0.1536 | 594967.3 ± 1015.13 | 717.81 | 0.1706 |
| QCN | ||||||
| 6 | 84729.5 ± 201.26 | 142.32 | 0.2375 | 84778.4 ± 132.11 | 93.42 | 0.15 |
| 8 | 105354.7 ± 238.6 | 168.73 | 0.23 | 105403.7 ± 169.31 | 119.72 | 0.16 |
| 10 | 134612.9 ± 287.0 | 202.95 | 0.2132 | 134678.9 ± 193.67 | 136.94 | 0.14 |
Table 2: Precision data of hplc method
| Amount added (n=3) | Content (mg/ml) | Amount found (mg/ml) ± SD | Recovery (%) | SEM | %RSD |
|---|---|---|---|---|---|
| CMN | |||||
| 80% | 9.0 | 9.003 ± 0.1517 | 100.04 | 0.0876 | 1.6856 |
| 100% | 10.0 | 10.17 ± 0.02 | 113 | 0.0115 | 0.1966 |
| 120% | 11.0 | 11.24 ± 0.1167 | 124.92 | 0.067 | 1.0385 |
| QCN | |||||
| 80% | 9.0 | 9.023 ± 0.1553 | 100.26 | 0.0897 | 1.7216 |
| 100% | 10.0 | 9.89 ± 0.04 | 109.88 | 0.0230 | 0.4045 |
| 120% | 11.0 | 10.86 ± 0.1686 | 120.70 | 0.0973 | 1.5522 |
Table 3: Summary of accuracy data of hplc method
Robustness was determined by making small deliberate changes in the wavelength. Analysis was performed thrice, first at the original wavelength of 397 nm, second at 395 nm and third at 399 nm. Data obtained was presented in Table 4. The solution of CMN and QCN mixture was applied on the TLC plate at a concentration ranging from 20 to 1200 ng/ band on nine different tracks with 10 mm distance among the tracks from x-axis and y-axis, respectively. Three replications of each standard were performed on different TLC plates. The calibration data was plotted as the peak areas of respective standard (y-axis) against the nominal concentration (x-axis). Linearity data was given in Table 5. For this repeatability studies were performed. Precision and accuracy studies were done by taking 12 determinations (4 concentrations/3 replicates each). Samples at four different concentration levels (20, 40, 60 and 80 ng/band for CMN and 100, 200, 300, 400 ng/band for QCN) were applied in hexplicate on the same day. Sample concentration was estimated from the calibration curve. The intraday and interday accuracy and precision of the assay were assessed by the average relative percentage deviation (DEV) from the nominal concentration and the relative standard deviation (RSD) values respectively, based on the reported guidelines [38,39]. Precision (RSD) and accuracy (DEV) were calculated by using the following Eqns., RSD = standard deviation/average calculated concentration×100; DEV = 1–(average calculated concentration/nominal concentration×100). Precision and accuracy data of HPTLC were given in Table 6.
| Method | Wavelength (nm) | Active drug % | Percent deviation | |||
|---|---|---|---|---|---|---|
| CMN | QCN | CMN | QCN | CMN | QCN | |
| Original Method | 397 | 397 | 100.83 | 100.15 | - | - |
| Changed Method | 395 | 395 | 100.53 | 99.87 | 0.30 | 0.28 |
| 399 | 399 | 101.15 | 100.46 | 0.32 | 0.31 | |
Table 4: Summary of robustness data of hplc method
| Parameter | CMN (n=5) | QCN (n=5) |
|---|---|---|
| Concentration range (mg/band) | 20-100 | 100-1200 |
| r2 | 0.996 | 0.997 |
| Equation of line | y=1139x+6336 | y=699.8x–519.9 |
| %RSD of slope | 0.20 | 0.14 |
Table 5: Summary of linearity data for hptlc
| Nominal amount (ng/band) CMN QCN |
CMN (n=3) | QCN (n=3) | |||||
|---|---|---|---|---|---|---|---|
| Calculated (ng/band) Mean ± SD | %RSD | %DEV | Calculated (ng/band) Mean ± SD | %RSD | %DEV | ||
| 20 | 100 | 19.81 ± 0.075 | 0.39 | 0.92 | 102.72 ± 1.86 | 1.81 | -2.27 |
| 40 | 200 | 39.11 ± 0.930 | 2.38 | 2.23 | 197.98 ± 1.55 | 0.78 | 1.008 |
| 60 | 300 | 58.99 ± 1.14 | 1.93 | 1.68 | 296.91 ± 2.40 | 0.81 | 1.03 |
| 80 | 400 | 81.39 ± 2.13 | 2.61 | -1.74 | 397.83 ± 1.59 | 0.39 | 0.54 |
Table 6: Summary of precision and accuracy by hptlc
Novel HPLC and HPTLC methods were developed for the estimation of CMN and QCN in the polyherbal formulation. Both the methods were found to be specific, sensitive, robust, accurate, precise and linear. Both the methods were able to differentiate CMN and QCN simultaneously and quantified CMN and QCN successfully in the polyherbal formulation. It appears that both the methods are suitable for use in quality control.
Acknowledgements
The authors wish to thank Dr. A. S. Sandhu, NIPER, Mohali, Punjab, India for identifying and authenticating the plant materials used in this investigation.
Conflict of interest
The authors declare no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Tilburt JC, Kaptchuk TJ. Herbal medicine research and global health: an ethical analysis. Bull World Health Organ 2008;86:594-9.
- http://apps.who.int/medicinedocs/documents/h1791e/h1791e.pdf.
- Liang YZ, Xie P, Chan K. Quality control of herbal medicines. J Chromatogr B AnalytTechnol Biomed Life Sci 2004;812:53-70.
- D’Mello PM, Joshi UJ, Shetgiri PP, Dasgupta TK, Darji KK. A simple HPLC method for quantitation of quercetin in herbal extracts. J AOAC Int 2011;94:100-5.
- Syed HK, Liew KB, Loh GOK, Peh KK. Stability indicating HPLC-UV method for detection of curcumin in Curcuma longaextract and emulsion formulation. Food Chem 2015;170:321-6.
- Wu DT, Cheong KL, Deng Y, Lin PC, Wei F, Lv XJ, et al. Characterization and comparison of polysaccharides from Lyciumbarbarumin China using saccharide mapping based on PACE and HPTLC. CarbohydrPolym 2015;134:12-9.
- Bala M, Pratap K, Verma PK, Singh B, Padwad Y. Validation of ethnomedicinal potential of Tinosporacordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J Ethnopharmacol 2015;175:131-7.
- Jyotshna, Srivastava P, Killadi B, Shanker K. Uni-dimensional double development HPTLC-densitometry method for simultaneous analysis of mangiferin and lupeolcontent in mango (Mangiferaindica) pulp and peel during storage. Food Chem 2015;176:91-8.
- Srinivasan S, Wankhar W, Rathinasamy S, Rajan R. Free radical scavenging potential and HPTLC analysis of Indigoferatinctorialinn (Fabaceae). J Pharm Anal 2016;6:125-31.
- Guzelmeric E, Vovk I, Yesilada E. Development and validation of an HPTLC method for apigenin 7-O-glucoside in chamomile flowers and its application for fingerprint discrimination of chamomile-like materials. J Pharm Biomed Anal 2015;107:108-18.
- Goswami D, Thakker JN, Dhandhukia PC. Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from l-tryptophan (Trp) using HPTLC. J Microbiol Methods 2015;110:7-14.
- Bazylko A, Boruc K, Borzym J, Kiss AK. Aqueous and ethanolic extracts of Galinsogaparvifloraand Galinsogaciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. PhytochemLett 2015;11:394-8.
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. AdvExp Med Biol 2007;595:1-75.
- Shakeri A, Sahebkar A. Optimized curcumin formulations for the treatment of Alzheimer’s disease: A patent evaluation. J Neurosci Res 2016;94:111-3.
- Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. NeurosciLett 2016;610:139-43.
- Boyanapalli SSS, Tony Kong AN. “Curcumin, the king of spices”: Epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr Pharmacol Rep 2015;1:129-39.
- Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res 2004;24:851-74.
- Meireles M, Moura E, Vieira-Coelho MA, Santos-Buelga C, Gonzalez-Manzano S, Dueñas M, et al.Flavonoids as dopaminergic neuromodulators. MolNutr Food Res 2016;60:495-501.
- Li Y, Zhou S, Li J, Sun Y, Hasimu H, Liu R, et al. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1-40-induced toxicity. Acta Pharm Sin B 2015;5:47-54.
- Jain R, Rajput S. Development of pharmacognostical parameters and estimation of quercetin using HPTLC in leaves of NelumbonuciferaGaertn. Pharmacogn J 2012;4:31-7.
- Amir M, Mujeeb M, Ahmad S, Akhtar M, Ashraf K. Design expert-supported development and validation of HPTLC method: An application in simultaneous estimation of quercetin and rutin in Punicagranatum, Tamarindusindicaand Prunusdomestica. Pharm Methods 2013;4:62-7.
- Hussain MS, Fareed S, Ali M, Rahman MA. Validation of the method for the simultaneous estimation of bioactive marker gallic acid and quercetin in Abutilon indicum by HPTLC. Asian Pacific J Trop Dis 2012;2:S76-S83.
- Hussain MS, Fareed S, Ali M. Hyphenated chromatographic analysis of bioactive gallic acid and quercetin in Hygrophilaauriculata(K. Schum) Heine growing wildly in marshy places in India by validated HPTLC method. Asian Pac J Trop Biomed 2012;2:S477-S83.
- Chen X, Xiao J. RP-HPLC-DAD determination of flavonoids: separation of quercetin, luteolin and apigenin in Marchantiaconvoluta. Iran J Pharm Res 2005;4:175-81.
- Savic IM, Nikolic VD, Savic IM, Nikolic LB, Stankovic MZ. Development and validation of a new RP-HPLC method for determination of quercetin in green tea. J Anal Chem 2013;68:906-11.
- Careri M, Corradini C, Elviri L, Nicoletti I, Zagnoni I. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking byproducts. J Agric Food Chem 2003;51:5226-31.
- Landim LP, Feitoza GS, Da Costa JGM. Development and validation of a HPLC method for the quantification of three flavonoids in a crude extract of Dimorphandragardneriana. Rev Bras Farmacogn 2013;23:58-64.
- Ashraf K, Mujeeb M, Ahmad A, Amir M, Mallick MN, Sharma D. Validated HPTLC analysis method for quantification of variability in content of curcumin in Curcuma longaL (turmeric) collected from different geographical region of India. Asian Pac J Trop Biomed 2012;2:S584-S8.
- Ansari MJ, Ahmad S, Kohli K, Ali J, Khar RK. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. J Pharm Biomed Anal 2005;39:132-8.
- Siddiqui NA. Evaluation of thermo sensitivity of curcumin and quantification of ferulic acid and vanillin as degradation products by a validated HPTLC method. Pak J Pharm Sci 2015;28:299-305.
- Chen Z, Zhu L, Song T, Chen J, Guo Z. A novel curcumin assay with the metal ion Cu (II) as a simple probe by resonance light scattering technique. SpectrochimActaMolBiomolSpectrosc 2009;72:518-22.
- Kadam PV, Bhingare CL, Nikam RY, Pawar SA. Development and validation of UV spectrophotometric method for the estimation of curcumin in cream formulation. Pharm Methods 2013;4:43-5.
- Meena KL, Yadav BL. Some traditional ethnomedicinal plants of southern Rajasthan. Indian J TraditKnowl2010;9:471-4.
- Jagetia GC, Baliga MS, Venkatesh P. Influence of seed extract of Syzygiumcumini(Jamun) on mice exposed to different doses of gamma-radiation. J Radiat Res 2005;46:59-65.
- Scotter MJ. Synthesis and chemical characterisation of curcuminoid colouring principles for their potential use as HPLC standards for the determination of curcumin colour in foods. LWT-Food SciTechnol 2009;42:1345-51.
- Reich E, Schibli A, DeBatt A. Validation of high-performance thin-layer chromatographic methods for the identification of botanicals in a cGMP environment. J AOAC Int 2008;91:13-20.