- *Corresponding Author:
- Mangathayaru Kalachaveedu
Faculty of Pharmacy, Sri Ramachandra University, Porur, Chennai-600 116, India
E-mail: manga.kv@sriramachandra.edu.in
| Date of Submission | 02 December 2016 |
| Date of Revision | 28 February 2017 |
| Date of Acceptance | 13 August 2017 |
| Indian J Pharm Sci 2017;79(5):829-834 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Proper validation of traditional medical knowledge using current scientific techniques and reasoning is vital before complementary and alternative medicinal products are integrated into conventional clinical practice. The present study was designed to standardize a proprietary Ayurvedic polyherbal preparation, Madhuram. Concocted as a mixture of six popular antidiabetic herbs as per Ayurvedic therapeutics, this is being prescribed as a health supplement in prediabetes. Capsule contents were processed for HPTLC analysis based on markers phyllanthin, berberine, ellagic acid, gallic acid, gymnemic acid and sennoside B through sample enrichment and were quantified against reference standards. The retention factors were phyllanthin 0.35±0.03, berberine 0.32±0.02, gallic acid 0.41±0.02, ellagic acid 0.29±0.04, gymnemic acid 0.36±0.01 and sennoside B 0.41±0.01 in their respective solvent systems. The peaks for phyllanthin, berberine, gallic acid, ellagic acid, gymnemic acid in their respective chromatograms of the formulation authenticated the raw material. Sennoside B was not detected in its enriched fraction of sample. Quantitation by densiometric scanning followed by peak integration demonstrated the presence of 3.17 µg of phyllanthin, 4.92 µg of berberine, 72 µg of gallic acid, 66 µg of ellagic acid, 410 µg of gymnemic acid. The evaluation of antidiabetic poly herbal established the identity of label claim herbs. Quantification of markers characteristic of each herb has laid the groundwork for batch to batch standardization to check its content uniformity.
Keywords
Antidiabetic, HPTLC, phyto chemical marker, poly herbal formulation, standardization
Rising global herbal drug trade and rampant availability of herbal drug combinations are a cause for concern with regards to its safety and efficacy in view of their indiscriminate use. Thus, their quality control is now a mandatory regulatory requirement both for over the counter sale and towards integrating popularly prescribed herbals-both classical and proprietary into modern clinical practice. Despite being a major hub for medicinal plants with clinically proven efficacy in traditional medical practice, India is yet to exploit its potential in keeping with the growing international market for plant based products. Lack of standardization protocols and thus regulatory hurdles are major constraints for even popularly prescribed Indian herbals from reaching global markets. Development of analytical profiles is thus an essential prerequisite in herbal drug standardization and methods developed for single molecule drugs are not largely adaptable to multi constituent plant drugs.
In this scenario, high-performance thin-layer chromatography (HPTLC) is emerging as a preferred analytical tool for standardizing single herbs and polyherbal formulations [1] with better resolution and accuracy [2]. The WHO stresses on the importance of the quantitative and qualitative methods of characterizing samples, quantification of biomarkers and/or chemical markers and development of fingerprint profiles. There are several challenges to be overcome in the HPTLC characterization of herbal products. The chemical profile of the constituent herbs in herbal medicinal products (HMPs) may vary due to several factors, the important ones being the plant identity and seasonal variation, the ecotypic, genotypic and chemotypic variations, processing and other factors. As for HMPs, establishment of specific quality control method is very difficult because of their complex nature [3].
Identification of individual phytochemical markers for each plant in most cases is neither simple nor feasible and the causes are multifactorial. In such cases, a satisfactorily measurable molecule not necessarily the active constituent is often chosen as the marker. Herbal formulations pose an additional challenge since identified marker compounds of component plant material may not be detected in the formulation due to transformations/deterioration during storage. Thus identification of unique phytochemicals as markers in the formulation and development of an appropriate analysis method for their quantification are crucial steps involved in marker based standardization of polyherbals [4]. HPTLC finger print analysis being an invaluable aid in the standardization of Ayurvedic products, current work focuses on the design and HPTLC standardization of a polyherbal, Madhuram indicated as a herbal supplement in holistic management of diabetes. It consisted of dried whole plant of Phyllanthus amarus Schum. and Thonn. (Euphorbiaceae), leaves and stems of Tinospora cordifolia (Willd.) Miers ex Hook. F. and Thoms. (guduchi- Menispermaceae), whole fruit of Eugenia jambolina Lam (jamun fruits- Myrtaceae) and Emblica officinalis Gaertn. (amla fruits- Euphorbiaceae), leaves of Gymnema sylvestre R. Br. Ex (gurmar- Apocyanace), flowers of Cassia auriculata Linn (Tanner’s Cassia- Caesalpiniaceae). These are the essential herbs in a number of traditional antidiabetic formulations and their antidiabetic activity is well reported.
Phyllanthin is selected as a marker for Phyllanthus amarus, antidiabetic drug of repute in Ayurveda [5]. T. cordifolia is a well-known Indian bitter prescribed in diabetes and has been subjected to extensive phytochemical, pharmacological and clinical investigations. Berberine, characteristic of Menispermaceae, an isoquinoline alkaloid is chosen as marker for this herb [6]. Gallic acid and ellagic acid have been selected as reference markers for the detection of E. officinalis and E. jambolana, respectively [7]. Gymnemic acid, a triterpenoid saponin in the leaves of G. sylvestre has been selected as a reference marker [8]. Sennoside-B, characteristic anthraquinone glycoside of genus Cassia [9] has been taken up for C. auriculata flowers reportedly having potent hypoglycemic activity in alloxan-induced diabetic rats [10].
Madhuram capsules (3 different batches) were procured from local pharmacy shop and each lot was subjected to weight variation (2.22%W/W) analysis. Average weight of each capsule is 550 mg with 2.22% W/W weight variation. The variation of weight among the capsules was least, which showed a good ratio of excipients in the formulation. Capsule contents of twenty capsules from each batch were collected and stored in amber coloured container at room temperature.
Phytochemical standards phyllanthin, berberine chloride dihydate, gallic acid, ellagic acid, gymnemic acid and sennoside B (≥95.0% HPLC) were purchased from M/S Natural Remedies, Bangalore, India. All the solvents and chemicals in experiments were of analytical grade (Merck, Ltd., Mumbai). Silica gel 60 F254 HPTLC pre-coated plates were procured from (Merck, Darmstadt, Germany).
Enrichment of the polyherbal mixture (PHM) to exclude the interference of other phytoconstituents from the marker compound is a necessary preliminary step in HPTLC analysis. Pooled capsule contents of the poly herbal capsule were thus processed for such enrichment with respect to each marker from the herb and the process was optimized for marker compound extraction efficiency to achieve good fingerprinting using validated protocols.
Capsule contents were processed for phyllanthin enrichment as per Krishnamurti et al. [11]. PHM (5 g) was cold macerated with chloroform (3×50 ml), filtered through Whatmann No. 42 filter paper and concentrated on a water bath at 50°. The concentrated residue was dissolved in minimum quantity of warm methanol and extracted with 2×20 ml petroleum ether (40-60°). Petroleum ether layer containing phyllanthin and chloroform pigments was separated and enriched for phyllanthin by crystallization with ice cooled methanol (yield 60 mg). It was dissolved in methanol (60 mg/ml) and this solution (8, 16 μl) was applied on TLC plate for quantification.
Capsule contents were processed for berberine enrichment as per Mimansha et al. [12]. PHM (5 g) was extracted with 100 ml of ethanol by cold maceration for 24 h. The filtrate was concentrated to syrupy mass on a hot water bath (60°). Extraction with (2×20 ml) hot water, filtration and addition of HCl drop wise yielded a crystalline residue upon cooling in an ice bath for 30 min. Suction filtration and air drying gave the berberine enriched sample (20 mg). It was dissolved in methanol (10 mg/ml) and applied (8, 10 μl) on TLC plate for analysis.
PHM was processed for gallic acid and ellagic acid enrichment as per Laxman et al. [13]. PHM (5 g) was extracted with (2×50 ml) methanol for 24 h. Filtrate was concentrated on a hot water bath (60°) giving a brownish residue (yield=516 mg). Aliquot (50 mg/ml) was dissolved in methanol and applied on TLC plate for gallic acid (4, 8 μl) and ellagic acid (4, 6 μl) estimation.
PHM was processed for gymnemic acid detection as per Farzana et al. [14], for which 5 g was macerated with 50 ml methanol for 24 h and filtrate was concentrated on a hot water bath (60°). The concentrated extract was washed with (3×20 ml) petroleum ether to remove steroids and interfering pigments yielding the gymnemic acid rich sample (280 mg). It was dissolved in methanol (50 mg/ml) and used (4 μl) for gymnemic acid quantification.
Capsule contents were processed for sennoside enrichment as per Jean et al. [15]. PHM (5 g) was extracted with (50 ml) ordinary water and the aqueous solution is acidified with HCl to pH 2.5 followed by fractional separation with (15 ml) ethyl acetate to remove aglycones. The aqueous layer containing glycosides is collected and extracted with (3×55 ml) butanol, concentrated to 10 ml (50°) in vacuum evaporator and left to stand for overnight. Crystals that settled at the bottom were collected by suction filtration on a Buchner funnel (105 mg), dissolved in methanol (50 mg/ml) and applied on precoated TLC plate (4 μl) for sennoside quantification.
Stock solutions (1 mg/ml) of phyllanthin, berberine, gallic acid, ellagic acid, gymnemic acid and sennoside were prepared. From this, working solutions of phyllanthin (50 μg/ml), berberine (10 μg/ml), gallic acid (100 μg/ml), ellagic acid (100 μg/ml) and sennoside (100 μg/ml) working solutions were prepared and different volumes were applied on the precoated TLC plates to obtain linear plots. The applied concentrations were mentioned in Table 1.
| Parameter | Mobile phase | Saturation time | Derivatisation | Scanning wavelength |
|---|---|---|---|---|
| Phyllanthin | Toluene:ethylacetate:methanol:dichloromethane (8.5:1.5:0.3:0.1) | 15 min | 10% Sulphuric acid in methanol | 203 nm |
| Berberine | Butanol:ethylacetate:acetic acid:water (2.5:5:1.5:1) | 45 min | No derivatisation | 349 nm |
| Gallic acid | Toluene:ethylacetate:formicacid:methanol (3:3:0.4:0.1) | 15 min | No derivatisation | 280 nm |
| Ellagic acid | Toluene:ethylacetate:formicacid:methanol (3:3:0.8:0.2) | 15 min | No derivatisation | 280 nm |
| Gymnemic acid | n-butanol:ethanol:water (10:1:1) |
60 min | 10% Sulphuric acid in methanol | 200 nm |
| Sennoside | n-propanol:ethylacetate:water:glacial acetic acid (8:8:5.8:0.2) | 60 min | No derivatisation | 365 nm |
Table 1: Chromatographic parameters for individual marker compounds
The sample solutions were spotted in the form of bands with a Hamilton 100 μl syringe on pre-coated silica gel G 60 F254 (10×10 cm with 250 μm thickness, E. Merck) plate using a Camag Linomat V applicator (automated spray-on applicator equipped with a 100 μl syringe and operated with the settings distance from the plate side edge 15 mm, and distance from the bottom of the plate 10 mm). The slit dimension was kept 6.00×0.45 mm. Linear ascending development was carried out in 10×10 cm, Camag twin trough glass chamber saturated with the mobile phase at room temperature. After air drying the plates, densitometric scanning was performed using Camag TLC Scanner- III in absorbance mode. Mobile phase for phyllanthin was optimized based on its physicochemical properties whereas for berberine [12], gallic acid and ellagic acid, gymnemic acid and sennoside the solvent systems were adopted from literature [13-15]. Chromatography conditions, mobile phase, chamber saturation time and scanning wavelengths for individual markers are mentioned in Table 1. Photo documentation was performed using Camag Reprostar 3. The data of the peak areas were plotted against the corresponding concentrations. The obtained values were treated by linear regression analysis.
In this study content of the poly herbal capsules were processed for such enrichment with respect to marker compound from each ingredient and the process was optimized for marker compound extraction efficiency towards achieving a good fingerprinting.
Linear relationship was observed by plotting marker concentration against peak areas. The results were found to be linear over a range of 100-500 ng/band for phyllanthin, 20-100 ng/band for berberine, 200-1000 ng/band for gallic acid, ellagic acid, sennoside and 1000-5000 ng/band for gymnemic acid (Table 2).
| Parameters | Phyllanthin | Berberine | Gallic acid | Ellagic acid | Gymnemic acid | Sennoside |
|---|---|---|---|---|---|---|
| Linearity | 200-600 ng/band | 20-100 ng/band | 200-1000 ng/band | 100-500 ng/band | 1000-5000 ng/band | 200-1000 ng/band |
| R2 | 0.999 | 0.998 | 0.998 | 0.991 | 0.991 | 0.995 |
| Slope | 9.387 | 43.815 | 8.887 | 32.289 | 801.077 | 4.373 |
| Intercept | 2815.11 | 813.811 | 175.580 | 313.193 | -221.588 | 1080.13 |
| Sy.x | 1.16 | 3.08 | 3.58 | 7.92 | 9.04 | 4.30 |
| LOD (3.3×Sy.x/S) |
0.4 ng/µl | 0.23 ng/µl | 1.32 ng/µl | 1.01 ng/µl | 30 ng/µl | 3.2 ng/µl |
| LOQ (10×Sy.x/S) |
1.23 ng/µl | 0.7 ng/µl | 4.02 ng/µl | 3.07 ng/µl | 110 ng/µl | 9.8 ng/µl |
R: Regression value; LOD: loss on detection; LOQ: loss of quantification; Sy.x is standard deviation; S is slope of the calibration curve
Table 2: Calibration curve parameters for individual marker compounds
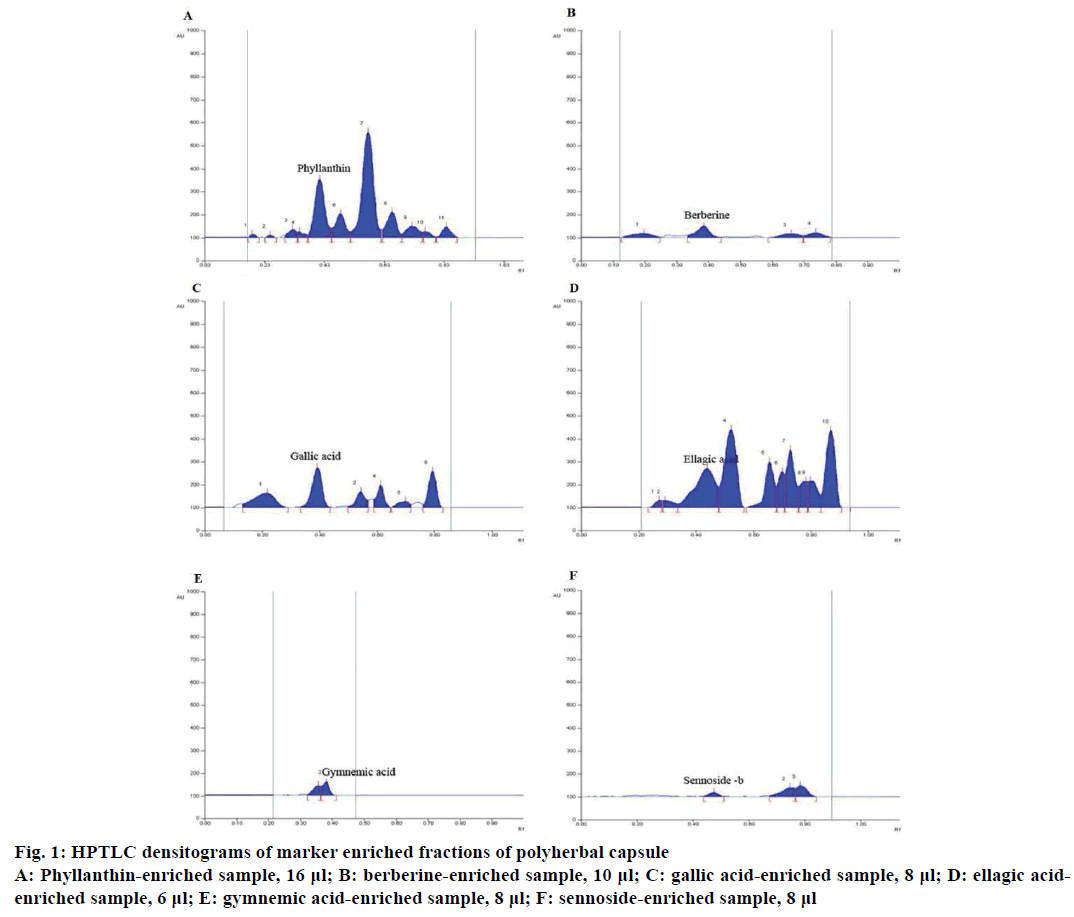
The chromatograms of phyllanthin, berberine, gallic acid, ellagic acid, gymnemic acid and sennoside B enriched fractions of the formulation are shown in Figure 1. Photo documentation of the TLC plates is depicted in Figure 2. The retention factors were 0.35±0.03 for phyllanthin, 0.32±0.02 for berberine, 0.41±0.02 for gallic acid, 0.29±0.04 for ellagic acid, 0.36±0.01 for gymnemic acid in their respective solvent systems. Quantitation by densiometric scanning followed by peak integration demonstrated the presence of 3.17 μg of phyllanthin, 4.92 μg of berberine, 72 μg of gallic acid, 66 μg of ellagic acid and 410 μg of gymnemic acid in each capsule. It is to be noted that sennoside B was not detected in the enriched fraction of the sample (Figures 1F and 2F). Omur et al. [16] have reported on the uncharacteristic microscopic features of Cassia species such as C. auriculata that is confounded by the nondetection of anthraquinoids in such species. In the absence of sennoside and with no specific marker for this species, its marker based quantification was not feasible.
Figure 1: HPTLC densitograms of marker enriched fractions of polyherbal capsule
A: Phyllanthin-enriched sample, 16 μl; B: berberine-enriched sample, 10 μl; C: gallic acid-enriched sample, 8 μl; D: ellagic acidenriched
sample, 6 μl; E: gymnemic acid-enriched sample, 8 μl; F: sennoside-enriched sample, 8 μl
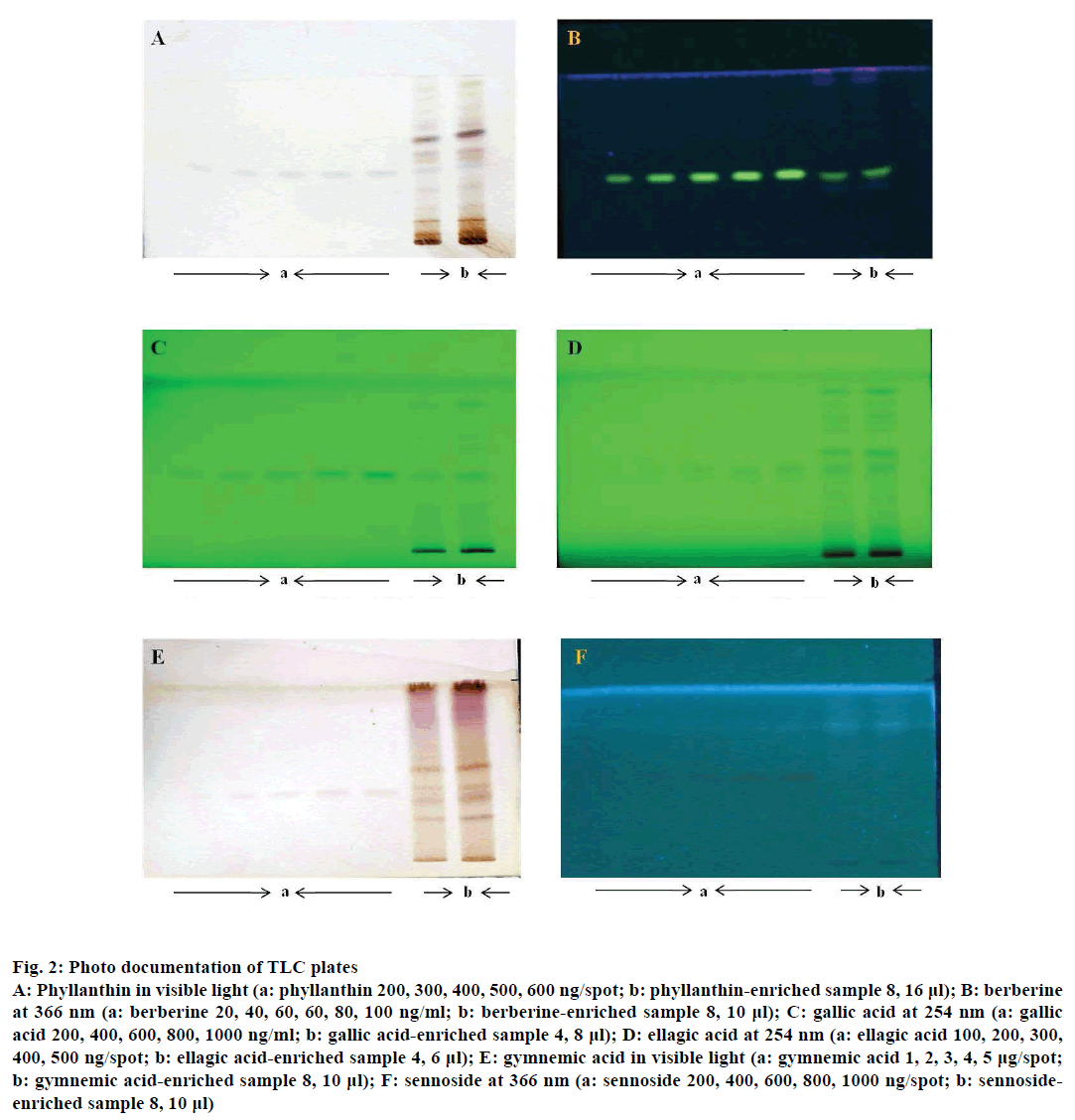
Figure 2: Photo documentation of TLC plates
A: Phyllanthin in visible light (a: phyllanthin 200, 300, 400, 500, 600 ng/spot; b: phyllanthin-enriched sample 8, 16 μl); B: berberine
at 366 nm (a: berberine 20, 40, 60, 60, 80, 100 ng/ml; b: berberine-enriched sample 8, 10 μl); C: gallic acid at 254 nm (a: gallic
acid 200, 400, 600, 800, 1000 ng/ml; b: gallic acid-enriched sample 4, 8 μl); D: ellagic acid at 254 nm (a: ellagic acid 100, 200, 300,
400, 500 ng/spot; b: ellagic acid-enriched sample 4, 6 μl); E: gymnemic acid in visible light (a: gymnemic acid 1, 2, 3, 4, 5 μg/spot;
b: gymnemic acid-enriched sample 8, 10 μl); F: sennoside at 366 nm (a: sennoside 200, 400, 600, 800, 1000 ng/spot; b: sennosideenriched
sample 8, 10 μl)
Quantification of plant specific markers for all the constituent herbs in Madhuram has first and foremost established their identity that is demonstrative of authenticity of the source herbs as indicated in the label claim. Quantification of source herbs contingent to their marker content was not possible due to nonavailability of quantitative data of markers in specific plant parts used for e.g., berberine content was reported in T. cordifolia stems that cannot be extrapolated to both stems and leaves present in Madhuram. To summarize this work has successfully developed the standardization protocol for the chosen poly herbal.
A six-herb combination antidiabetic poly herbal has been submitted to HPTLC analysis using markers identified for each herb. Capsule contents sampled from 3 different batches of the poly herbal have been individually processed for enrichment of each of the 6 selected markers for the respective herbs. These were then taken up for HPTLC run along with reference standard markers in the appropriate solvent systems based on trial and error optimization of the same. Linear regression analysis generated standard graphs for each marker that was the basis for the quantification of the chosen marker in the enriched fractions and thus capsule contents. Present study has thus succeeded in quantifying the phytochemical markers with reference to each plant in the antidiabetic poly herbal formulation using validated HPTLC methods. This work lays the blue print for the quality control of each batch of the formulation and for batch to batch content variation assay paving way for the development of this formulation as an ethically standardised herbal drug.
Financial assistance
Nill.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhakti JL, Vijay MM, Urmi DK, Rina HG. Marker based standardization of poly herbal formulation (SJT-DI-02) by high performance thin layer chromatography method. J Pharm Bioallied Sci 2014;6:213-19.
- Parimala M, Shoba FG. In vitro antimicrobial activity and HPTLC analysis of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. BMC Complement Altern Med 2014;14:361.
- Liang YZ, Xie PS, Chan K. Quality control of herbal medicines. Chromatogr B2004;812:53-70.
- Wani MS. Herbal medicine and its standardization. Pharm Rev 2007;5:1-6.
- Jay Ram P, Priyanka T, Vikas S, Nagendra SC, Vinod KD. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J Ethnopharmacol 2011;138:286-313.
- Sharma P, Upadhyay P, Mishra S, Sadhu A, Purohit S. Berberine a Potent substance for researcher: a review. World J Pharm Pharm Sci 2015;4:547-73.
- Muniappan A, Pandurangan SB. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2012;2:240-6.
- Thakur GS, Rohit S, Bhagwan SS, Mukeshwar P, Prasad GBKS, Prakash SB. Gymnema sylvestre: an alternative therapeutic agent for management of diabetes. J App Pharm Sci 2012;2:1-6.
- Hemen D, Lalitha L. A review on anthraquinones isolated from Cassia species and their applications. Indian J Nat Prod Resour 2012;3(3):291-319.
- Surana SJ, Gokhale SB, Jadhav RB, Sawant RL, Jyoti BW. Antihyperglycemic activity of various fractions of Cassia auriculata Linn. in alloxan diabetic rats. Indian J Pharm Sci 2008;70(2):227-29.
- Krishnamurti, GV, Seshadri TR. The Bitter principle of Phyllanthus niruri. Proc Indian Acad Sci 1946;24(4):357-64.
- Mimansha CP. Isolation of berberine from Berberis aristata by an acid dye method and optimization of parameters. Int J Pharm Sci Rev Res 2013;20(2):187-89
- Laxman S, Nancy P, Bala P. Determination of gallic acid in Phyllanthus emblica Linn. dried fruit powder by HPTLC. J Pharm Bioallied Sci 2010;2(2):105-08.
- Farzana C, Muhammad H. Isolation and characterization of gymnemic acid from indigenous Gymnema sylvestre. J App Pharm 2010;3(2):60-5.
- Jean P. Gabriel, Marcel Dumont, Robert Guillé. Extraction of sennosides. US Patent No: US 4256875 A, 1981.
- Omur DL, Neslihan K, Ebru U, Kuruuzum-Uz1 A, Guvenalp Z, Cavit K. HPLC fingerprinting of sennosides in laxative drugs with isolation of standard substances from some senna leaves. Rec Nat Prod 2011;5(4):261-70.