- *Corresponding Author:
- J. O. Adebayo
Department of Biochemistry, Faculty of Life Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria
E-mail: topebayo2002@yhaoo.com
| Date of Submission | 23 November 2016 |
| Date of Revision | 12 April 2017 |
| Date of Acceptance | 21 November 2017 |
| Indian J Pharm Sci 2018;80(1):99-107 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Pathogenesis of several diseases has been attributed to free radical modification of biomolecules within the body, which can be prevented or treated with antioxidants, especially those of natural origin. Some plant peptides have been reported to be effective antioxidants. Cysteine-stabilised peptide content of Morinda lucida leaf was extracted and purified using solvent extraction, column chromatography, reverse-phase high performance liquid chromatography and the type of cysteine-stabilised peptide present was determined using matrix-assisted laser desorption ionisation time of flight mass spectrometry analysis and ninhydrin staining. Antioxidant activities of the partially purified peptide fraction were evaluated using in vitro models. The presence of linear cysteine-stabilised peptide with masses ranging from 3.601 to 3.678 kDa and having three disulphide bonds was confirmed. Partially purified peptide fraction exhibited 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity and ferric reducing antioxidant power, which is statistically compared with that of butylated hydroxytoluene but displayed statistically lower total antioxidant capacity compared to glutathione. Nitric oxide scavenging activity of partially purified peptide fraction was statistically lower compared to ascorbic acid. Hydroxyl radical scavenging activity of partially purified peptide fraction was significantly higher (p<0.05) compared with that of glutathione at higher concentrations. Cysteine-stabilised peptide, identified for the first time in M. lucida leaf, possesses antioxidant properties and they may be effective in the management of reactive oxygen species-mediated pathological conditions but not reactive nitrogen species-mediated pathological conditions.
Keywords
Cysteine-stabilised peptide, Morinda lucida, leaf, antioxidant
Oxidation is essential in biological processes, such as metabolism of biomolecules. During oxidation, free radicals are generated. Though these free radicals are involved in important roles, such as signalling and defence against infection in the biological system [1], their over-production can lead to modifications of biomolecules [2], which have been implicated in the pathogenesis of several diseases such as cancer, diabetes and arteriosclerosis [3]. Thus, diets rich in antioxidants, which scavenge free radicals, may play a prominent role in preventing and alleviating such diseases [4]. This has led to an upsurge in studies on antioxidants of natural sources.
Recently, attention has been on peptides from protein hydrolysates of both plant and animal sources and their diverse biological activities, including antioxidant activity [5]. Leaves and seeds of plants belonging to the families Apocynaceae (dogbane), Rubiaceae (coffee), Violaceae (violet), Cucurbitaceae (cucurbit), Fabaceae (bean) and Solanaceae (potato family) have been reported to be rich in cysteine-stabilised peptides (CSP) [6]. One such plant, Morinda lucida Benth. (Rubiaceae), a rainforest tree, also called brimstone tree, is among the medicinal plants commonly used in Nigeria and West Africa for the treatment of diseases such as malaria and diabetes. Various studies have reported the antimalarial, antibacterial, uterotonic, antioxidant, and antidiabetic properties of M. lucida extract [7].
Though several secondary compounds have been isolated from various parts of M. lucida [8], a review of available literature on the plant revealed that no peptide has been isolated from it thus far, let alone evaluating such for their bioactivities. This study was, therefore, carried out to investigate if M. lucida leaf expresses CSP, extract such peptides and evaluate them for antioxidant activity.
Materials and Methods
Dichloromethane, methanol, acetonitrile, ninhydrin, glacial acetic acid, n-butanol, ethanol, butylated hydroxytoluene (BHT), ascorbic acid, sodium nitroprusside (SNP), sulphanilamide, naphthylethylenediamine dihydrochloride, phosphoric acid, sodium trioxophosphate (III), ammonium molybdate, potassium ferricyanide, trichloroacetic acid (TCA), thiobarbituric acid, iron (III) chloride and tetraoxosulphate (VI) acid were purchased from Merck, Germany, while de-oxy-2-D-ribose, reduced glutathione (GSH), trifluoroacetic acid (TFA) and 2,2-diphenyl-1-picrylhydrazyl (DPPH), endoproteinase Glu-C, formic acid and iodoacetamide were purchased from Sigma-Aldrich, St. Louis, MO, USA.
Plant material
Leaves were obtained from M. Lucida tree (Figure 1) on the campus of University of Ibadan, Nigeria, on 22/09/2014. The material was identified and authenticated at the Forest Research Institute of Nigeria, Ibadan, where a sample was deposited and assigned a voucher number FHI: 110187.
Ethical approval
This study was approved by the University Ethical Review Committee of the University of Ilorin, Ilorin, Nigeria (UERC Approval Number: UERC/ ASN/2015/067).
Extraction of peptides from M. Lucida leaf
Peptide fraction was obtained as described by Koehbach et al. [9]. Briefly, M. lucida leaves were air-dried and pulverised into powder. About 500 g of powdered leaves was percolated in 6 l of dichloromethane-methanol mixture (1:1, v/v) and left overnight at room temperature. The mixture was filtered to remove plant debris. Equal volume of distilled water was added to the filtrate; the aqueous layer was collected and concentrated using rotary evaporator.
A preloaded C18 solid phase extraction column was preconditioned with methanol, activated with solvent B (acetonitrile:distilled water; 9:1, v/v) and equilibrated with buffer A (distilled water-TFA; 100:0.05, v/v). The concentrated aqueous layer was loaded onto C18 column (Phenomenex, Aschaffenburg, Germany) and various fractions eluted with increasing concentrations (20, 80 and 100 %) of solvent B. The 80 % solvent B fraction, hereafter referred to as peptide extract (semipure extract or partially purified peptide fraction, PPP) was concentrated on a rotary evaporator, freeze-dried (Gallenkamp, UK) and kept at –20° for further use.
Preliminary matrix-assisted laser desorption ionisation time of flight mass spectrometry (MALDITOF/ TOF) analysis
Preliminary identification of peptides in eluted fraction was done using 0.5 μl each of peptide extract. This was mixed well with 3 μl matrix, alpha cyano- 4-hydroxycinnamic acid (CHCA, Sigma Aldrich, Germany) and 0.5 μl of the mixture was spotted on MALDI plate and allowed to dry. The dried plate was loaded onto the MALDI-TOF/TOF 4700 analyser (AB Sciex, Canada) operated in reflector positive ion mode acquiring 2000-4000 total shots per spectrum with a laser intensity of 3700 [9]. The result from the MALDI TOF was analysed based on peptide-like peaks and masses which fall within 2.5-4 kDa, which is one of the criteria for detecting the presence of a class of cysteinerich peptide (i.e. cyclotide) in a sample.
Reverse-phase high performance liquid chromatography (RP-HPLC) fractionation of peptide extract
The lyophilized peptide extract was reconstituted with a small volume of buffer A. The solution was further passed through a solid phase filter (Sartorius) before purification using semi-preparative RP-HPLC on Dionex Ultimate 3000 HPLC unit (Dionex, Amsterdam, Netherlands). HPLC specification used includes 250×10 mm; 5 μm, 100 A, Kromasil column, linear gradient of 0.1-1 % min-1 solvent B at a flow rate of 3 ml×min-1. Collected fractions were again analysed on the MALDI TOF/TOF analyser as earlier described. Fraction of interest was freeze dried and redissolved in solvent A for analytical HPLC (flow rate of 1 ml×min-1) for the determination of hydrophobicity of the peptides [10].
Reduction/alkylation of peptides and MALDI-MS analysis
Disulphide linkages within the peptide were reduced with 2 μl of dithiothreitol after reconstituting lyophilized peptide extract in 20 μl of 0.1 M ammonium bicarbonate (pH 8.2), and incubating at 65° for 10 min. Reduced sample was further alkylated with 4 μl of iodoacetamide. About 1 μl of TFA was used to stop the reaction. MALDI-TOF/TOF analyser was then used to investigate the reduced and alkylated sample. Kalata B1 was used as positive control [10].
Enzymatic digestion of reduced/alkylated peptides and MALDI TOF/TOF analysis
Reduced and alkylated peptides were enzymatically digested with endoproteinase Glu-C (Sigma-Aldrich, Austria). The previous reduction and alkylation reaction was stopped by adding 1 μl of TFA and incubated for 10 min at 37°. This was followed by the addition of 2 μl of the enzyme to the reaction mixture and further incubation for 3-14 h under mild agitation. The reaction was quenched with 1 μl of formic acid. 0.5 μl of the sample was mixed with 3 μl of matrix and spotted on the MALDI target plate for MALDI TOF MS analysis. All acquired spectra were processed with the Explorer 4800 software. Digested samples were kept at –20° for further analysis [9].
Ninhydrin staining of peptide extract
A test using ninhydrin stain was carried out to ascertain the linearity of the peptide configuration [11]. The extract was subjected to thin layer chromatography, using precoated plate (silica gel 60F254; Merck) with n-butanol:acetic acid:water (3:1:1 v/v) as the mobile phase and GSH as standard. The developed plates were sprayed with 0.2 % ninhydrin (10 mg ninhydrin in 5 ml absolute ethanol) and left at room temperature for 10 h for colour development.
DPPH radical scavenging assay (DRSA)
The scavenging activity of the peptide extract against DPPH radical was determined according to the method of McCune and Johns [12] with slight modifications for a 96-well clear flat-bottom plate. About 100 μl of 0.3 M methanolic DPPH was added to 100 μl of different concentrations of the PPP fraction and BHT and incubated at room temperature in the dark for 10 min. The absorbance of the blank (Ab) and samples (As) was measured at 517 nm using a 96-well microplate reader (Biorad Benchmark, Series 1.15). DRSA was calculated as [(Ab-As)]/Ab×100.
Nitric oxide (NO) scavenging assay
NO scavenging activity was measured according to the method of Fiorentino et al. [13]. NO was generated from SNP and measured as nitrite by Griess reaction. The assay mixture contained 2 ml of 5 mM SNP (in 0.1 M phosphate pH 7.4) and 0.5 ml of different concentrations of PPP fraction or ascorbic acid. The assay mixtures were incubated at 37° for 2 h. Thereafter, 0.1 ml of each reaction mixture was transferred into a 96-well microtitre plate followed by addition of 0.1 ml of Griess reagent (1 % sulphanilamide, 0.1 % naphthylethylenediamine dihydrochloride in 5 % phosphoric acid). This was kept in the dark for 10 min at room temperature, after which the absorbance was read at 530 nm. The NO scavenging activity was expressed as percentage relative to absorbance of the blank at 530 nm.
Determination of ferric reducing antioxidant power (FRAP)
The FRAP of the fraction was determined according to the method reported by Girgih et al. [14]. To 250 μl of varying concentrations of PPP fraction or BHT was added 250 μl of 0.2 M phosphate buffer (pH 6.6) and 250 μl of 1 % potassium ferricyanide solution. The mixtures were incubated at 50° for 20 min. After incubation, 250 μl of 10 % aqueous TCA was added. Then, to 250 μl of each PPP/TCA mixture was added 50 μl of 1.0 % of FeCl3 and 200 μl distilled water and allowed to stand at room temperature for 10 min. The mixtures were then centrifuged at 1000×g for 20 min. Thereafter, 200 μl of each supernatant was transferred to a 96-well plate and absorbance measured at 700 nm.
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of the extract was determined using the method of Kunchandy and Rao [15]. The reaction mixture consist of 100 μl of 2-deoxy-D-ribose (28 mM in 20 mM KH2PO4- KOH buffer, pH 7.4), 500 μl of PPP, 200μl of EDTA (1.04 mM) and 100 μl FeCl3 (200 mM), 100 μl H2O2 (1.0 mM) and 100 μl ascorbic acid (1.0 mM), which was incubated at 37° for 1 h. TBA (1 %, 1.0 ml) and 1.0 ml of TCA (2.8 %) were added and the mixtures incubated at 100° for 20 min. After cooling, an aliquot of 200 μl of each reaction mixture was transferred to a 96-well plate and absorbance measured at 532 nm.
Evaluation of total antioxidant capacity
The total antioxidant capacity was evaluated using the phosphomolybdenum assay [16]. Briefly, 0.1 ml of varying concentrations of PPP was combined with 1 ml reagent solution (0.6 M tetraoxosulphate (VI) acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The mixtures were incubated at 95° for 90 min. After cooling to room temperature, 200 μl of each mixture was transferred to a 96-well plate and absorbance measured at 695 nm.
Statistical analysis
The assays were conducted in triplicates and data expressed as mean±SEM. Data were analysed using Student t-test using SPSS 16.0 computer software package (SPSS Inc. Chicago, IL, USA). Differences at p<0.05 was considered significant.
Results and Discussion
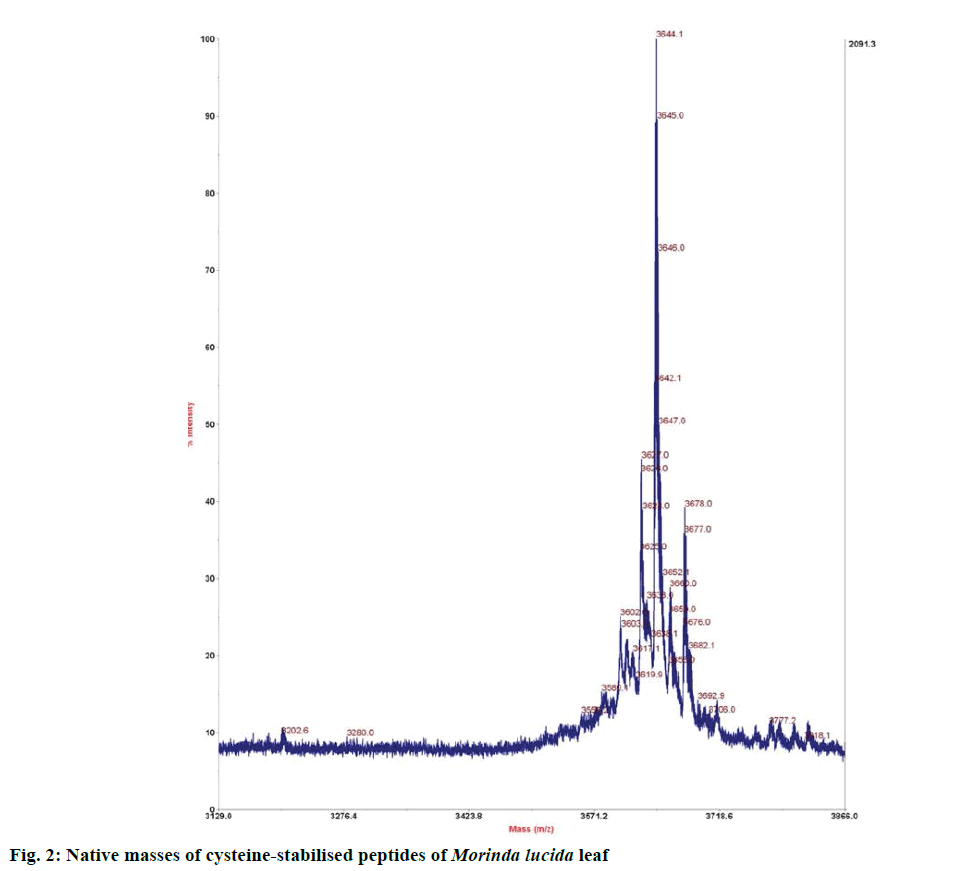
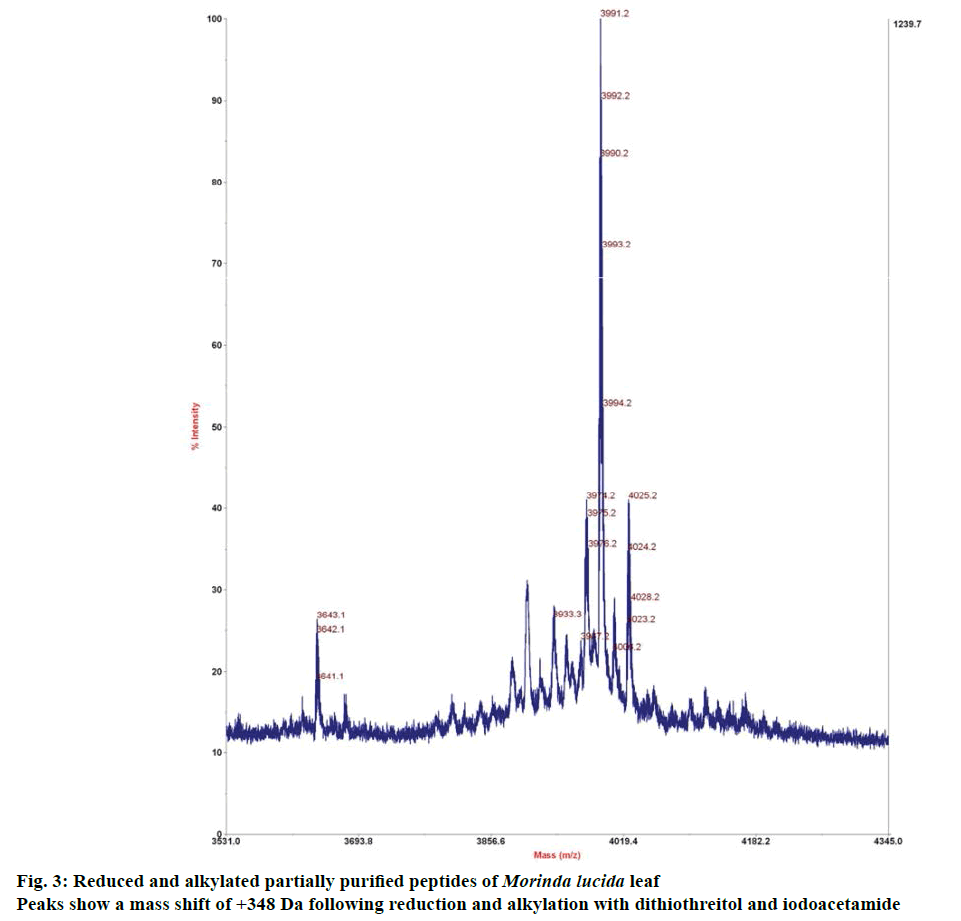
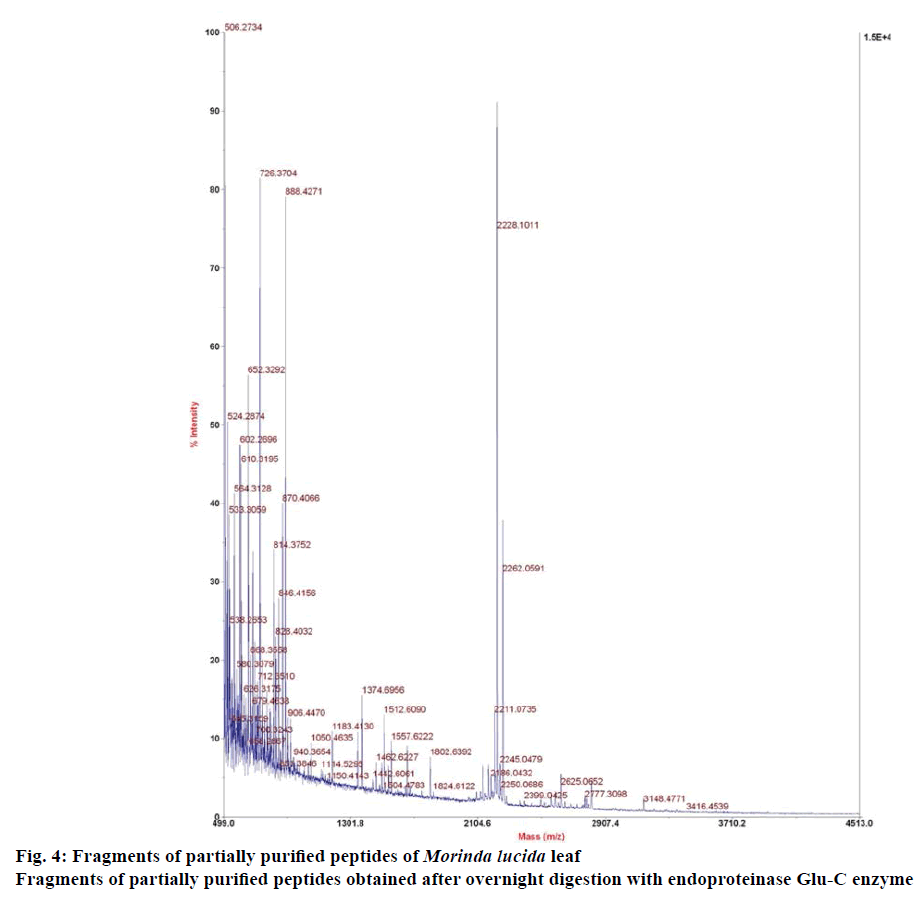
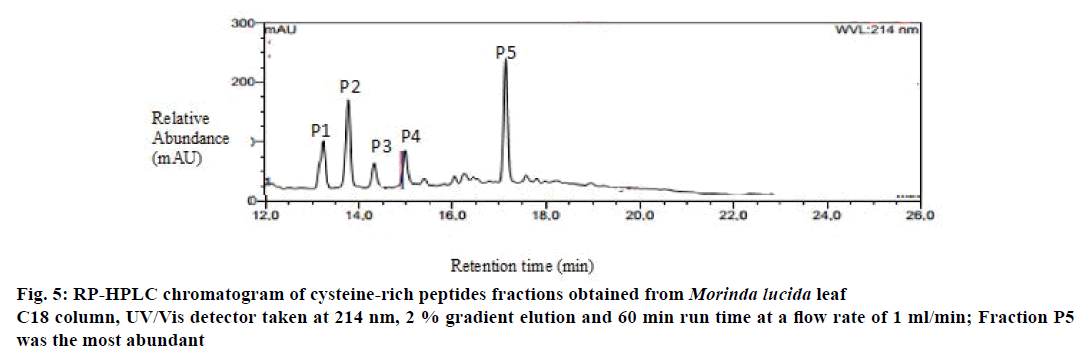
Preliminary chemical screening of PPP fraction of M. lucida leaf revealed the presence of peptides with MALDI masses ranging from 3.601 to 3.678 kDa in the peptide extract (Figure 2). Reduction and alkylation of M. lucida peptides resulted in a mass shift of +348 (Figure 3). Enzymatic digestion of M. lucida peptides with endoproteinase Glu-C (Figure 4) resulted in an irregular fragmentation of the peptides. M. lucida leaf peptides equally presented late elution characteristic on RPHPLC to drive home the hydrophobic behaviour of the peptides (Figure 5).
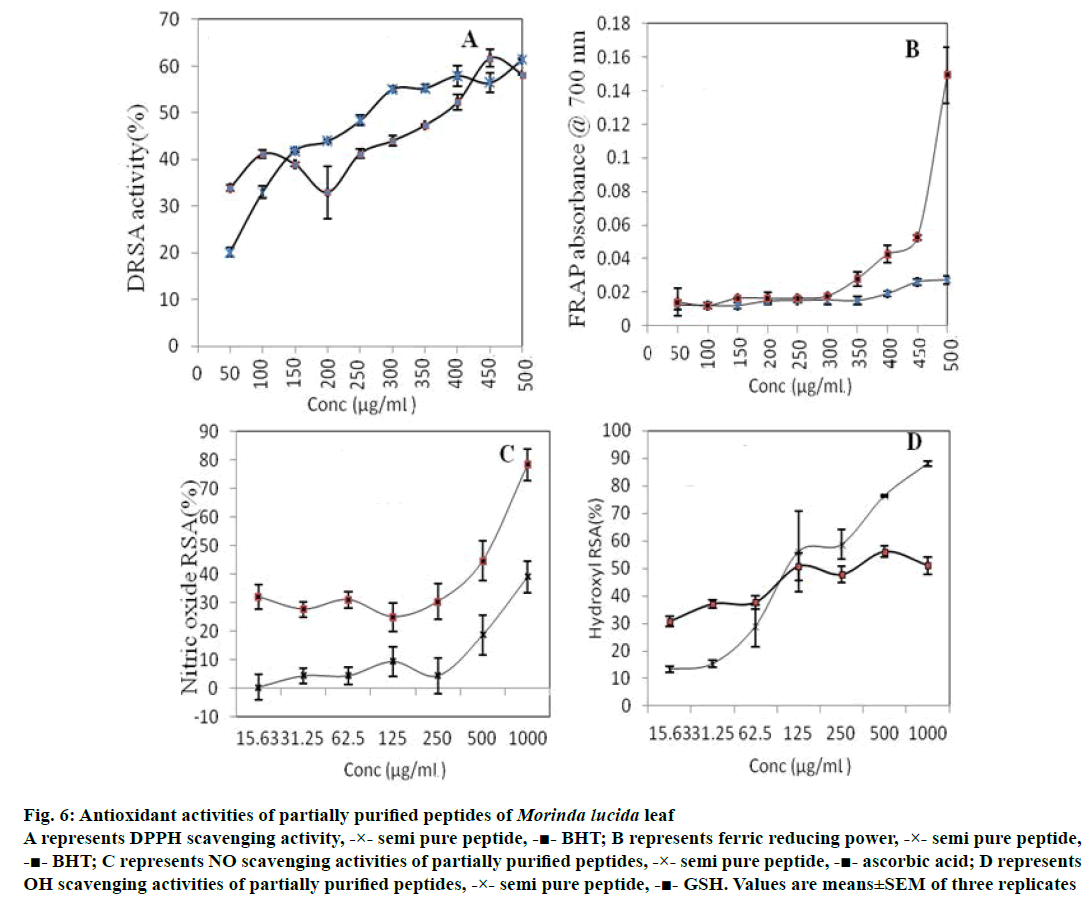
PPP displayed significantly lower (p<0.05) TAC compared to GSH at all concentrations (Table 1), but exhibited DPPH radical scavenging activity, which compared favourably with that of BHT (Figure 6A). The FRAP of PPP was not significantly different (p<0.05) from that of BHT at all concentrations studied (Figure 6B; especially at lower concentrations up to 300 μg/ml). The NO scavenging activity of PPP was significantly lower (p<0.05) than that of ascorbic acid at all concentrations studied (Figure 6C). OH scavenging activity of PPP was significantly higher (p<0.05) than that of GSH at higher concentrations (Figure 6D).
| Concentration (µg/ml) | Partially purified peptide (ascorbic acid equivalent, mg/ml) |
GSH (ascorbic acid equivalent, mg/ml) |
|---|---|---|
| 15.63 | 75.83±2.20 | 183.33±8.33* |
| 31.25 | 88.33±8.46 | 241.67±30.05* |
| 62.5 | 76.67±6.67 | 350.00±25.00* |
| 125 | 85.00±1.44 | 416.67±46.40* |
| 250 | 96.67±3.63 | 425.00±25.00* |
| 500 | 199.17±7.95 | 533.33±22.05* |
| 1000 | 261.67±16.73 | 658.33±36.32* |
Values are expressed as mean ± SEM of three determinations. *Values in each row are significantly different (p<0.05)
Table 1: Total antioxidant capacity of partially purified peptide of Morinda lucida leaf
Figure 6: Antioxidant activities of partially purified peptides of Morinda lucida leaf
A represents DPPH scavenging activity, -×- semi pure peptide, -■- BHT; B represents ferric reducing power, -×- semi pure peptide,
-■- BHT; C represents NO scavenging activities of partially purified peptides, -×- semi pure peptide, -■- ascorbic acid; D represents
OH scavenging activities of partially purified peptides, -×- semi pure peptide, -■- GSH. Values are means±SEM of three replicates
The results from mass spectrometry analysis confirmed six cysteine residues forming three disulphide bonds within the peptide molecules [9]. No ring opening (typical of cyclotides) was observed suggesting free N and C terminals or the presence of more than one glutamic acid residue. M. lucida peptides equally presented late elution characteristic on RP-HPLC to drive home the hydrophobic behaviour of the peptides. Various studies have implicated diverse biological activities of cysteine-rich peptides of plant origin, especially those of the families Apocynaceae, Rubiaceae, Violaceae, Cucurbitaceae, Fabaceae and Solanaceae. There is, however, no previous report on the presence of cysteine-rich peptides in M. lucida leaf. Here, we extracted stable cysteine-rich peptides from M. lucida leaf. The discovery criteria used for the cysteine rich peptides include peptide mass range of 2.5-4.0 kDa, late elution on RP-HPLC, mass shift of +348 following reduction and alkylation of native peptides and finally an addition of 18 Da after 3-14 h enzymatic digest using endoproteinase Glu-C to determine whether the peptides present are cyclic peptides or not. It has been reported that several cysteine-rich peptides have low molecular weight (2.5-4.0 kDa) [17]. Results of this study indicated that M. lucida leaf expresses non-cyclic low molecular weight peptides, which appear to be stable and rich in cysteine residues. M. lucida belongs to the family Rubiaceae and the present result corroborates earlier reports that seeds and leaves of plants belonging to this family possess pharmaceutically interesting cysteine-rich cyclotides [18]. As already described for cysteine-rich peptides, the native function of M. lucida leaf peptides may be related to host protection [18]. Further chemical treatment with ninhydrin for colour reaction produced purple colour, indicative of the presence of linear peptide.
M. lucida is popular in Nigerian ethnomedicine with several of its folkloric claims validated scientifically [7]. Majority of the remedies made from M. lucida target inflammation, which may result from free radicals.
Antioxidants have been known to scavenge free radicals in order to restore the integrity of the cellular environment [3]. Thus, the antioxidant properties of cysteine-rich peptides of M. lucida leaf were also evaluated in this study using in vitro models. DRSA has been widely used to determine the antioxidant activity of various plant extracts [19]. The observed DRSA of PPP is similar to those of cysteine-containing peptide fractions of hemp seed protein hydrolysate reported earlier [14]. It has been reported that cysteine can directly react with free radicals, though the antioxidant activity of single amino acids is far lower than that of the peptide containing the amino acid [20]. The observed high DRSA could be as a result of its cysteine content.
FRAP is commonly used to evaluate electron or hydrogen donating capacity of natural antioxidants [21]. The similar FRAP values of PPP to that of BHT suggests that it has high hydrogen donating capacity. Sulphur-containing amino acids have been reported to contribute positively to FRAP values [22], which may account for the observed FRAP of PPP.
The low NO scavenging values obtained for M. lucida PPP suggest that it may not be efficient in scavenging NO and thus, may not be effective in the management/ treatment of NO-mediated pathological conditions e.g. NO-induced hypertension. NO is associated with the generation of ONOO– that could cause protein structure modification and DNA fragmentation. Thus, NO scavengers could attenuate risk of excessive NO production. A similar study on flaxseed protein-derived peptide fractions, at 0.2 mg/ml, revealed a weak NO scavenging property [23].
Hydroxyl radical is said to be the most reactive free radical; removal of OH– is therefore crucial in the defence of living systems against various diseases [24]. In this study, M. lucida PPP displayed higher (p<0.05) OH- scavenging activity compared to GSH at higher concentrations, producing 80 % OH– scavenging activity at 1 mg/ml. OH– scavenging activities from other plant sources have been reported. For example, a purified chickpea peptide fraction had over 81 % OH– scavenging activity at 1.5 mg/ml [19], which is also similar to the result obtained for alfalfa leaf protein hydrolysate which had 80 % hydroxyl scavenging activity at 1.2 mg/ml [25]. This suggests that PPP may be a good hydroxyl scavenging agent that may effectively prevent lipid and protein oxidation. The poor concentration-dependent manner (Table 1 and Figure 6) as regards the antioxidant activity was observed at lower concentrations suggesting a threshold at which direct proportionality between the concentration of PPP and antioxidant activity takes effect.
The results of these study revealed that M. lucida leaf possesses non-cyclic cysteine-rich peptides, which possess high hydroxyl radical scavenging activity but low NO scavenging activity. Thus the cysteinerich peptides of M. lucida leaf may be effective in the treatment of ROS-mediated pathological conditions but not RNS-mediated pathological conditions.
Conflicts of interest
There are no conflicts of interest
Financial support and sponsorship
Nil.
References
- Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 2005;4:5.
- Lee J, Koo N, Min DB. Reactive oxygen species, aging and antioxidative nutraceuticals. Compr Rev Food Sci Food Saf 2004;3:21-33.
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 2007;35:1147-50.
- López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal Chim Acta 2013;763:1-10.
- Girgih AT, He R, Hasan FM, Udenigwe CC, Gill TA, Aluko RE. Evaluation of the in vitro antioxidant properties of a cod (Gadusmorhua) protein hydrolysate and peptide fractions. Food Chem 2015;173:652-9.
- Koehbach J, Attah AF, O’Brien M, Freissmuth M, Gruber CW. Peptidomics screening for the discovery of uterotonic plant peptides. BMC PharmacolToxicol 2012;13:A89.
- Lawal HO, Etatuvi SO, Fawehinmi AB. Ethnomedicinal and pharmacological properties of Morinda lucida. J Nat Prod 2012;5:93-9.
- Adewunmi CO, Adesogan EK. Anthraquinones and oruwacin from Morinda lucida as possible agents in fascioliasis and schistosomiasis control. Fitoterapia 1984;5:259-64.
- Koehbach J, Attah AF, Berger A, Hellinger R., Kutchan TM, Carpenter EJ, et al. Cyclotidediscovery in Gentianalesrevisited-identification and characterization of cyclic cystine-knot peptides and their phylogenetic distribution in Rubiaceae plants. Biopolymers 2013a;100:438-52.
- Gruber CW. Global cyclotide adventure: a journey dedicated to the discovery of circular peptides from flowering plants. Biopolymers 2010;94:565-72.
- Jork H Funk W, Fishcer W, Wimmer H. Thin-layer Chromatography: Reagents and Detection Methods. Physical and chemical detection methods: activation reactions, reagent sequences, reagents II. Vol. 1b. Weinheim, Germany: Wiley-VCH; 1994.
- McCune LM, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol 2002;82:197-205.
- Fiorentino A, Ricci A, D’Abrosca B, Pacifico S, Golino A, Letizia M, et al. Potential food additives from Carexdistachya roots: identification and in vitro antioxidant properties. J Agric Food Chem 2008;56:8218-25.
- Girgih AT, Udenigwe CC, Aluko RE. Reverse-phase HPLC separation of hemp seed (Cannabis sativa L.) protein hydrolysate produced peptide fractions with enhanced antioxidant capacity. Plant Foods Hum Nutr 2013;68:39-46.
- Kunchandy E, Rao MNA. Oxygen radical scavenging activity of curcumin. Int J Pharm 1990;58:237-40.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem1999;269:337-41.
- Poth AG, Colgrave ML, Philip R, Kerenga B, Daly NL, Anderson MA, et al. Discovery of cyclotides in the Fabaceae plant family provides new insights into the cyclization, evolution, and distribution of circular proteins. ACS ChemBiol 2011;6:345-55.
- Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol 1999;294:1327-36.
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). J Food Chem 2008;106:444-50.
- Li Y, Yu J. Research progress in structure-activity relationship of bioactive peptides. J Med Food 2014;18:1-10.
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterization of the antioxidant properties of deodorised aqueous extracts from selected Lamiaceaeherbs. Food Chem 2003;83:255-62.
- Udenigwe CC, Aluko RE, Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int J Mol Sci 2011;12:48-3161.
- Udenigwe CC, Lu YL, Han CH, Hou WC, Aluko RE. Flaxseed protein-derived peptide fractions: Antioxidant properties and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Food Chem 2009;116:277-84.
- Zhong S, Ma C, Lin YC, Luo Y. Antioxidant properties of peptide fractions from silver carp (Hypophthalmichthys molitrix) processing by-product protein hydrolysates evaluated by electron spin resonance spectrometry. Food Chem 2011;126:1636-42.
- Xie ZJ, Huang JR, Xu XM, Jin ZY. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem 2008;111:370-6.