- Corresponding Author:
- X. Ding
Key Laboratory of Southwest China Wildlife Resources Conservation, College of Life Sciences, China West Normal University, Nanchong, China
E-mail: dingxiang319@yahoo.cn
| Date of Submission | 08 October 2012 |
| Date of Revision | 20 March 2013 |
| Date of Acceptance | 15 April 2013 |
| Indian J Pharm Sci 2013;75(4):393-399 |
Abstract
More and more fungal polysaccharides have been reported to exhibit a variety of biological activities, including antitumor, antioxidant and immunostimulant activity. The non-starch polysaccharides have emerged as an important class of bioactive natural products. In this study, the immune activities of a novel polysaccharide (LDG-A) isolated from Lactarius deliciosus (L. ex Fr.) Gray were investigated at 20, 40 and 80 mg/kg dose levels. The inhibitory rate in mice treated with 80 mg/kg LDG-A can reach 68.422%, being the highest in the three doses, which may be comparable to mannatide. Histology of immune organs showed that the tissues were arranged in more regular and firm pattern, but the tumor tissue arranged looser in LDG-A group than those in control group. Meanwhile, there was no obvious damage to other organs, such as heart, lung, and kidney. The antitumor activity of the LDG-A was usually believed to be a consequence of the stimulation of the cell-mediated immune response because it can significantly promote the lymphocyte and macrophage cells in the dose range of 50-200 μg/ml and 100-400 μg/ml in vitro, respectively. The level of cytokines (IL-6, TNF-α, and NO) of macrophage cells induced by LDG-A treatment was similar to lipopolysaccharides at different concentrations. The expression of all these genes studied (TNF-α, IL-6, and iNOS mRNA) in the untreated macrophage was little, but increased dramatically in a dose-dependent manner in the LDG-A-treated cells. The results obtained in the present study indicated that the purified polysaccharide of L. deliciosus (L. ex Fr.) Gray is a potential source of natural immune-stimulating substances.More and more fungal polysaccharides have been reported to exhibit a variety of biological activities, including antitumor, antioxidant and immunostimulant activity. The non-starch polysaccharides have emerged as an important class of bioactive natural products. In this study, the immune activities of a novel polysaccharide (LDG-A) isolated from Lactarius deliciosus (L. ex Fr.) Gray were investigated at 20, 40 and 80 mg/kg dose levels. The inhibitory rate in mice treated with 80 mg/kg LDG-A can reach 68.422%, being the highest in the three doses, which may be comparable to mannatide. Histology of immune organs showed that the tissues were arranged in more regular and firm pattern, but the tumor tissue arranged looser in LDG-A group than those in control group. Meanwhile, there was no obvious damage to other organs, such as heart, lung, and kidney. The antitumor activity of the LDG-A was usually believed to be a consequence of the stimulation of the cell-mediated immune response because it can significantly promote the lymphocyte and macrophage cells in the dose range of 50-200 μg/ml and 100-400 μg/ml in vitro, respectively. The level of cytokines (IL-6, TNF-α, and NO) of macrophage cells induced by LDG-A treatment was similar to lipopolysaccharides at different concentrations. The expression of all these genes studied (TNF-α, IL-6, and iNOS mRNA) in the untreated macrophage was little, but increased dramatically in a dose-dependent manner in the LDG-A-treated cells. The results obtained in the present study indicated that the purified polysaccharide of L. deliciosus (L. ex Fr.) Gray is a potential source of natural immune-stimulating substances.
Keywords
Polysaccharide, immune activities, mechanisms, Lactarius deliciosus (L. ex Fr.) Gray
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. Fungal polysaccharide is a kind of active organic compounds that is found in the fruiting bodies, mycelium and fermentation broth of large edible and medicinal fungi [1]. Fungal polysaccharides are long carbohydrate molecules, of repeating units joined together by glycosidic bonds. They are often linear, but may also be highly branched [2]. In recent years, as more and more fungal polysaccharides have been reported to exhibit a variety of biological activities, including antitumor, antioxidant and immunostimulant activity. Non-starch polysaccharides have emerged as an important class of bioactive natural products [3-8]. Certain mushrooms enjoy usage as therapeutics in folk medicines, such as traditional Chinese medicine. Notable medicinal mushrooms with a well documented history of use include Agaricus subrufescens [9,10], Ganoderma lucidum [11], and Ophiocordyceps sinensis [12]. The shiitake mushroom is a source of lentinan, a clinical drug approved for use in cancer treatments in several countries, including Japan [13,14]. In Europe and Japan, polysaccharide-K (Krestin), a chemical derived from Trametes versicolor, is an approved adjuvant for cancer therapy [15].
Lactarius deliciosus (L. ex Fr.) Gray is a kind of fungi belonging to Lactarius which grows in Xiaojin country of Sichuan province in China at an elevation of 3750 m. In our previous work, one novel water-soluble polysaccharide was extracted and purified from the fruiting bodies of L. deliciosus using a DEAE cellulose column chromatography and a Sephadex G-100 column chromatography. Its chemical structures have been characterized [16]. The present objective is to investigate the immune activities and molecular mechanisms of the novel polysaccharide isolated from L. deliciosus.
Materials and Methods
S180 tumor cells were maintained in peritoneal cavities of Kunming strain male mice obtained from Institute of Biochemistry and Molecular Immunology of North Sichuan Medical College (NSMC). Nanchong, China. Male Kunming strain mice, weighed 25.0±1.0 g, purchased from NSMC, were housed six per plastic cages with wood chip bedding in an animal room with a 12 h light and 12 h dark cycle at room temperature (25±2º) and allowed free access to standard laboratory diet (purchased from the Institute of Biochemistry and Molecular Immunology of the NSMC). The animal experiments were conducted according to the ‘Guidelines for Animal Experimentation’ of the NSMC.
Isolation for polysaccharide
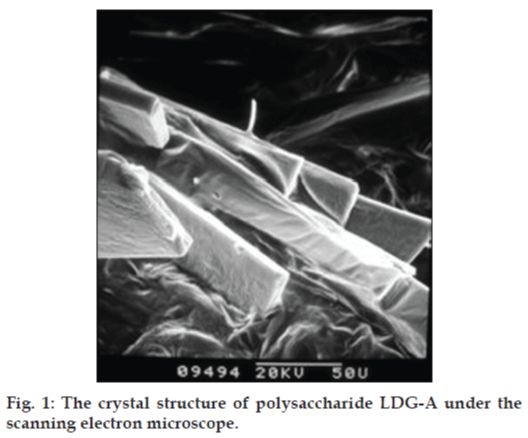
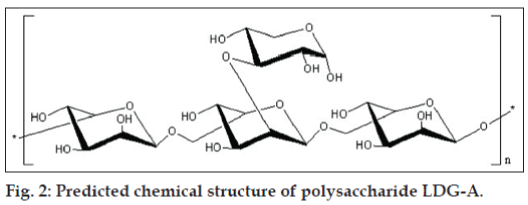
The fruiting bodies of L. deliciosus was collected in Xiaojing country of Sichuan province, China, and was authenticated at College of Life Sciences, Sichuan University, Chengdu, China and a voucher specimen has been preserved in Key Laboratory of Southwest China Wildlife Resources Conservation, College of Life Sciences, China West Normal University. After the fruiting bodies of L. deliciosus were soaked with 95% EtOH, the residue was dried and then extracted with boiling water for three times (5 h each). Subsequently, the filtrate was concentrated, dialyzed against distilled water through a semipermeable membrane with a cutoff threshold of 5000 D, and centrifuged, the supernatant was added with three volumes of 95% EtOH to precipitate crude polysaccharides. Sevag method [17] was used for the deproteination, crude polysaccharides named LDG (2 g) was subjected to a DEAE cellulose column and eluted stepwise with 0, 0.1, 0.2, 0.3, 0.4, 0.5, and 1.0 m NaCl. The eluent was monitored by the phenol–sulfuric acid method [18]. The 0.1 M NaCl elution was concentrated, lyophilized, and purified on a Sephadex G-100 column (2.6×60 cm). The purified L. deliciosus polysaccharide, named LDG-A, was obtained by the above processes and the yield rate of LDG-A was 0.215% (0.430 g) for the starting material. Structural features of L. deliciosus polysaccharide (LDG-A) were investigated by a combination of total hydrolysis, methylation analysis, gas chromatography–mass spectrometry (GC-MS), scanning electron microscope (SEM), infrared (IR) spectra, nuclear magnetic resonance (NMR) spectroscopy, and dynamical analysis of the atomic force microscope (AFM) studies. The results indicated that L. deliciosus polysaccharide (LDG-A) have a backbone of 1, 6-disubstituted-α-L-mannopyrano se which branches at O-2 and the branches were mainly composed of a (2 → 3)-α-D-xylopyranose residue (figs. 1 and 2).
Assay of antitumor activity in vivo
S180 tumor cells (3×106) were implanted subcutaneously into right hind groin of the mice. Mice were randomly divided into five groups (n=6). One day after the inoculation, LDG-A was dissolved in distilled water and administered intraperitoneally to the mice at the doses of 20, 40, and 80 mg/kg, respectively. Positive and negative controls were set for comparison. The positive control was given with 0.2 ml mannatide (20 mg/kg) and negative one with physiological saline instead of the test solution. Animals were sacrificed after 2 weeks. The body weights were measured. Tumors, spleens, and livers were excised and the tumor inhibitory ratio were calculated by following formula: Inhibition ratio (%)= [(A-B)/A]×100, where A and B were the average tumor weights of the negative control and treated groups, respectively. The organ index was calculated by following formula: Organ index=C/D, where C was the weight of the organ and D was the weight of the mice.
Histopathology and morphological observations
After treating the mice with LDG-A as described above, a portion of the tissues were cut into small pieces, fixed in Heidenhain’s Susa Fluid (HgCl2: 4.5 g; NaCl: 0.5 g; distilled water: 80.0 ml; formalin: 20.0 ml; acetic acid: 4.0 ml; trichloroaceticacid: 2.0 ml), and then stained with hematoxylin and eosin (HE), examined and photographed under an Olympus microscope.
Preparation of lymphocyte cells
The homogenized spleens were treated in aseptic condition. Single spleen cells suspension were prepared in pH 7.0 phosphate-buffered saline (0.15 mol/l of NaCl and 0.02 mol/l sodium phosphate)containing 0.1% w/v bovine serum albumin by forcing spleen fragments through a fine wire mesh. Samples were washed twice in phosphate buffered saline/0.1% w/v bovine serum albumin and in low speed centrifugation, 200 rpm, 5 min. The cells were resuspended to a concentration of 5×106 cells/ml in RPMI1640 completely cultivated liquid (HyClone, USA).
Assay of lymphocyte proliferation
One hundred microliters (5×106 cells/ml) single spleen cells sample was placed on a 96 well microplate and cultured for 24 h. Twenty microliters LDG-A of different concentrations (25, 50, 100, 200 μg/ml) was added to each test well as the experimental group, 20 μl ConA (50 μg/ml) was added as a positive control group, and 20 μl RPMI-1640 (Gibco, USA) cultivated liquid as a blank control group. And then 80 μl RPMI-1640 cultivated liquid was added to each well for the total volume of 200 μl. Eight repeated wells were used for each concentration. The cell samples were incubated in a 5% CO2 -air mixture at 37° for 68 h. MTT cellular viability assay was used for lymphocyte proliferation analysis. Calculation of lymphocyte proliferation was done: Rate of lymphocyte proliferation (%)= [(T−C)/C]×100, where T is optical density value of test well; C is that of control well.
Preparation of peritoneal macrophage cells
BALB/c mice of 6 to 8 week old were injected intraperitoneally with 1 ml of 3% thioglycollate (Sigma Chemical Co., St. Louis, MO, USA). Four days after injection, mice were euthanized and peritoneal exudate cells were collected by lavage with 5 ml of sterile cold D-Hank’s. The exudate cells were collected and cultured in 60-mm dishes with RPMI-1640 containing 10% heat-inactivated FBS, penicillin (100 IU/ml) and streptomycin (100 mg/ml) (RPMI-FBS). After 1-h incubation at 37º, the cultures were washed twice with RPMI-1640 to remove non-adherent cells and the adherent cells were collected by gently scraped. The viability of macrophages was assessed by trypan blue exclusion.
Assay of macrophage stimulation
Macrophages (1×106 cells/ml) were plated in a 96 well plate (Corning, NY). The cells were cultured in phenol red-free RPMI-FBS medium containing increasing concentrations of LDG-A from 50 to 400 μg/ml (20 μl per well) as the experimental group, and 20 μl LPS (50 μg/ml) was added as positive control group, respectively. And then 80 μl phenol red free RPMI-FBS cultivated liquid was added to each well for the total volume of 200 μl for each test. Eight repeated wells were used for each concentration. The cell samples were incubated in a 5% CO2 -air mixture at 37º for 4 h. Then 50 μl of neutral red solution was added to a final concentration of 0.72 mg/l and continue to culture for 30 min; aspirated the neutral red solution, washed three times and added 200 μl lysis buffer (glacial acetic acid:ethanol=1:1), standing overnight until complete cell lysis and recorded the optical density (OD).
Nitric oxide determination
The amount of stable nitrite and 100 ml of culture supernatants were mixed with an equal volume of the Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, and 2.5% H3PO4). This mixture was incubated at room temperature for 10 min. The absorbance at 540 nm was read on a Thermo multiskan ascent reader (NY, USA). The nitrite concentration was determined by extrapolation based on a standard sodium nitrite curve [19].
Cytokines determination
The amount of TNF-α and IL-6 of culture supernatants were determined by ELISA kit (R and D Systems China Co. Ltd., Shanghai, China) following the instruction of manufacturers.
Quantitative RT-PCR detection of related gene expression
The peritoneal macrophages were harvested after stimulated by various concentration of LDG-A for 4 h. The total cellular RNA was extracted using Trizol reagent (Invitrogen, USA) and reverse-transcribed into cDNA using oligo (dT)18 primers (Invitrogen). Amplification of each target cDNA was performed in the iCycler system (Bio-Rad, USA). PCR products were quantified using SYBR Green I. β-actin was used as an endogenous control to normalize expression levels among samples. A standard curve of each primer set was generated using LPS-induced macrophage cDNA. The PCR primers chosen were shown in Table 1. The relative expression abundance was calculated by the following formula: Relative expression abundance=mols of detected mRNA/mols of β-actin mRNA
| Gene | Antisense (5’-3’) | Sense (5’-3’) | Tm (ºC) | Product size (bp) |
|---|---|---|---|---|
| β-actin | GCTGTCCCTGTATGC CTCT | TTGATGTCACGCACG ATTT | 55.4 | 222 |
| IL-6 | GCCTTCTTGGGACTGATGCTGG | CTCTGGCTTTGTCTTTCTTGTT | 51.7 | 385 |
| TNF-α | GCCTATGTCTCAGCCTCT | GGTTGACTTTCTCCTGGTAT | 53.4 | 423 |
| iNOS | GAGCGAGTTGTGGATTGTC | GGGAGGAGCTGATGGAGT | 55.2 | 376 |
Table 1: Primer designs
Statistical analysis:
The data were expressed as mean±SD. The significance of difference was evaluated with one-way ANOVA, followed by Student’s t-test to statistically identify differences between the control and treated groups. Significant differences was set at P<0.05.
Results and Discussion
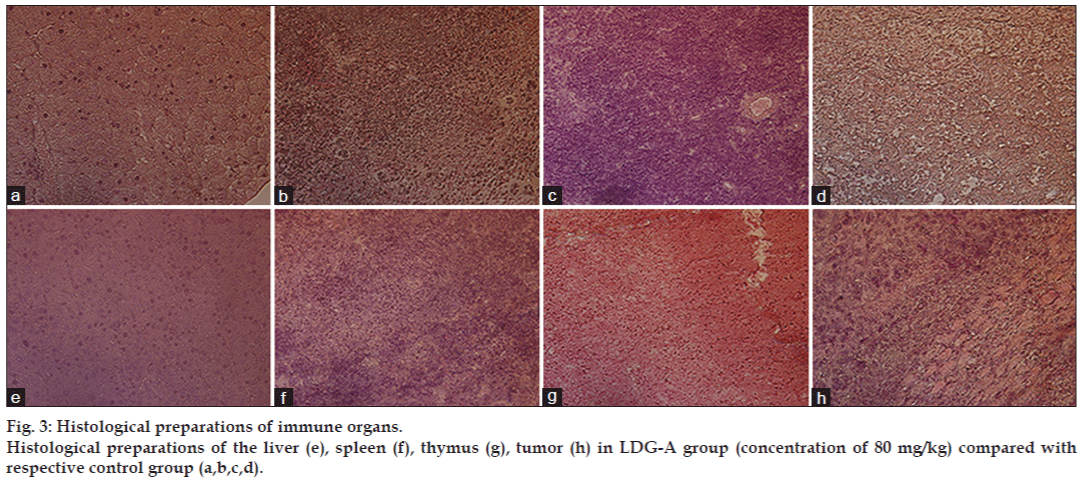
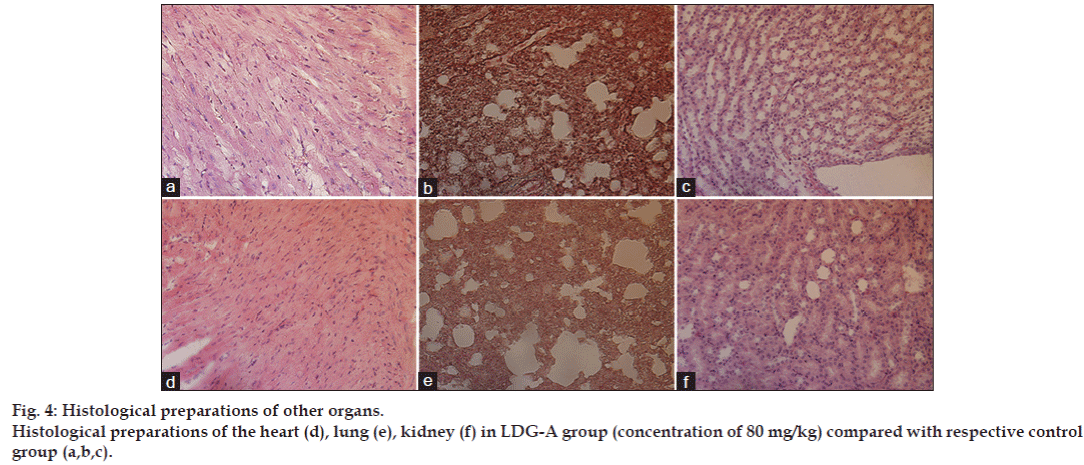
The antitumor activity of the polysaccharide was usually believed to be a consequence of the stimulation of the cell-mediated immune response [20]. To detect the antitumor activity of LDG-A in vivo, we used the mice transplanted S180 to evaluate the effects and the results were summarized in Table 2. The weight and the histological preparations of the vital organ in each female rat in the control group were compared to that in the treated group in order to measure the effect of the drug. LDG-A could inhibit the growth of the tumors (P<0.05) in a dose-dependent manner. The inhibitory rate in mice treated with 80 mg/kg LDG-A was 68.422%, being the highest in the three doses. Furthermore, during the experiments, the appetite, activity, and coat luster of every mice in LDG-A treated groups were better than the mice treated with mannatide. Histology of immune organs such as the liver, spleen, and thymus showed that tissues appear more regular and firmer, and the tumor tissues are more loosely arranged in LDG-A group than those in control group. But there is no obvious damage to other organs, such as heart, lung, and kidney (figs. 3 and 4). The results also showed little change in average liver weight in test groups, indicating that LDG-A did not cause serious liver damage. On the 14th day, the average tumor weight of negative control mice was 3.74 g, whereas the average tumor weight of mice in LDG-A group at dose of 80 mg/kg was 1.181g; tumor weights were also reduced in doses of 20 mg/kg and 40 mg/kg, to 2.081 g and 1.783 g, respectively (P<0.05). It is noteworthy that the average weights of the spleens and thymus in test groups were greater in doses of 40 mg/kg than that in the mannatide mice (P<0.05), and even that of the negative control mice, indicating that LDG-A could increase the weights of immune organs in moderate doses (Table 2). These results suggested that activating immune responses in the host might be one of the mechanisms of antitumor activity of LDG-A, as various reports are available for antitumor polysaccharides.
| Group | Spleen index (mg/g) | Liver index (mg/g) | Thymus index (mg/g) | Average tumor weight (g) | Inhibitory rate of tumor (%) |
|---|---|---|---|---|---|
| N | 5.784 ± 2.108 | 60.307 ± 9.395 | 1.429 ± 0.706 | 3.740 ± 0.423 | - |
| L1 | 7.619 ± 2.414 | 56.468 ± 4.509 | 4.741 ± 0.301 | 2.081 ± 0.740 | 44.358* |
| L2 | 7.807 ± 3.427 | 66.351 ± 8.802 | 7.790 ± 3.208 | 1.783 ± 0.430 | 52.326* |
| L3 | 5.202 ± 0.989 | 59.977 ± 4.786 | 0.903 ± 0.401 | 1.181 ± 0.241 | 68.422* |
| M | 7.0439 ± 3.105 | 58.263 ± 5.399 | 2.862 ± 1.727 | 1.581 ± 0.706 | 57.727* |
The values are expressed as mean ± SD for n=8. Significant differences from negative control group and positive control group were evaluated using Student’s t test: *P<0.05, N: negative control group; L1, L2, L3 indicating LDG-A groups of 20 mg/kg, 40 mg/kg, 80 mg/kg, respectively; Man: positive control group of mannatide
Table 2: Antitumor activities of ldg-a on s180 tumor
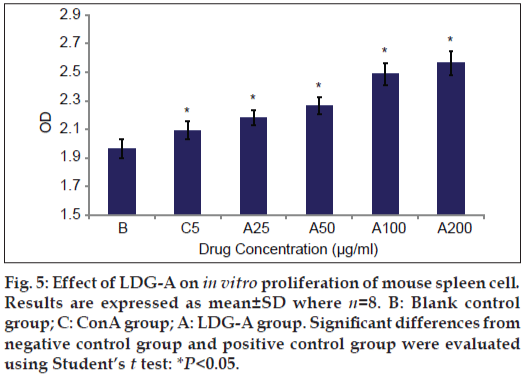
The antitumor activity of the polysaccharide was usually believed to be a consequence of the stimulation of the cell-mediated immune response. The present study showed the immune activity of the LDG-A by assay of lymphocyte proliferation and macrophage stimulation. Proliferation of splenocytes is an indicator of immune activation. The purified polysaccharide LDG-A was able to induce proliferation of splenocytes as shown in fig. 5. LDG-A can significantly promote the proliferation of spleen cells at the concentration of 5 μg/ml and 25 μg/ml dose (P<0.05), while it can promote the proliferation of spleen cells in the concentration of 50-200 μg/ml dose range compared with the control group (P<0.05).
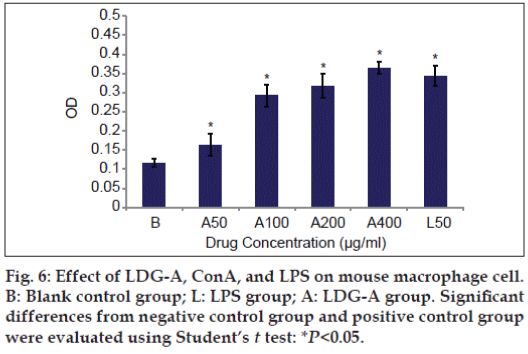
Polysaccharides are good stimulators of macrophage owing to presence of various receptors on the macrophage membrane. In this study, LDG-A also can significantly promote the phagocytosis of mouse peritoneal macrophages at the concentration 50 μg/ml dose (P<0.05), and can very significantly promote the phagocytosis of mouse peritoneal macrophages within the dose range of 100-400 μg/ml compared with the control group (P<0.05, fig. 6). The promoter capacity of both lymphocyte and macrophage cells and the concentration of the LDG-A was positively correlated.
Using the ELISA method, the level of IL-6 and TNF-α secreted by LDG-A-activated macrophages was compared with control. Obviously, the level of both cytokines secreted by LDG-A-stimulated macrophages was much higher than medium-treated macrophages. LPS of 50 mg/ml was the positive control. The level of cytokines induced by LDG-A treatment was similar to that induced by LPS at a different concentration. It is noted that the productions of IL-6 were stimulated at a high level when the concentration of LDG-A was only 50 mg/ml (Table 3).
| Group | N | A1 | A2 | A3 | L |
|---|---|---|---|---|---|
| TNF-α(pg/ml) | 121.3 ± 9.7 | 167.4 ± 6.3* | 177.5 ± 3.2* | 183.0 ± 2.4* | 181.3 ± 5.2* |
| NO (mm) | 2.3 ± 0.1 | 8.2 ± 0.3* | 11.4 ± 0.4* | 12.6 ± 0.7* | 9.1 ± 0.4* |
| IL-6(pg/ml) | 103.7 ± 8.6 | 155.7 ± 8.2* | 157.37 ± 5.2* | 161.4 ± 5.4* | 160.6 ± 4.5 |
The values are expressed as mean±SD for n=6. Significant differences from negative control group and positive control group were evaluated using Student’s t test: *P<0.05. N: Negative control group; A1, A2, A3 indicating LDG-A groups of 50 mg/ml, 200 mg/ml, 400 mg/ml, respectively; L: positive control group of LPS group of 50 mg/ml
Table 3: Production of no and cytokines in pm stimulated by ldg-a
Quantitative RT-PCR results showed a significant increase in the level of TNF-α, IL-6, and iNOS mRNA in LDG-A-treated peritoneal macrophages compared to those untreated. The positive control, LPS 50 mg/ml, also promoted the expression of these genes. The expression of the genes studied (TNF-α, IL-6, and iNOS) in the untreated macrophage were 0.01, 0.11 and 0.01, respectively, but increased dramatically to 0.49, 1.77, and 1.10 in a dose-dependent manner in the LDG-A-treated (400 mg/ml) cells, respectively (Table 4).
| Group | N | A1 | A2 | A3 | L |
|---|---|---|---|---|---|
| TNF-α | 0.01 ± 0.00 | 0.18 ± 0.01* | 0.32 ± 0.02* | 0.49 ± 0.04* | 0.12 ± 0.02* |
| NO | 0.11 ± 0.01 | 0.73 ± 0.04* | 1.49 ± 0.22* | 1.77 ± 0.18* | 1.34 ± 0.26* |
| IL-6 | 0.01 ± 0.00 | 0.22 ± 0.01* | 0.58 ± 0.03* | 1.10 ± 0.08* | 0.14 ± 0.01* |
The values are expressed as mean±SD for n=6. Significant differences from negative control group and positive control group were evaluated using Student’s t test: *P<0.05. N: Negative control group; A1, A2, A3 indicating LDG-A groups of 50 mg/ml, 200 mg/ml, 400 mg/ml, respectively; L: positive control group of LPS group of 50 mg/ml
Table 4: Expression of tnf-α, inos and il-6 mrna in pm stimulated by ldg-a
The role of activated macrophages in the defense against tumor cells has been investigated extensively over the last decades [21-23]. Accumulated evidence indicated that activated macrophages are able to recognize and lyse tumor cells including those that are resistant to cytostatic drugs. Therefore, macrophage activation can play a role in novel immunotherapeutic approaches to the treatment of cancer [23]. Macrophages can kill the tumor cells either by macrophage-mediated tumor cytotoxicity or antibody-dependent cellular cytotoxicity (ADCC) and both processes end up releasing cytotoxic mediators including TNF-α, NO and reactive oxygen intermediates or phagocytosis [24]. TNF-α is one of most important mediators involved in tumor cell killing by the induction of multiple intracellular pathways, such as the generation of reactive oxygen intermediates in mitochondria preceding plasma membrane permeabilization [25] and induction of iNOS expression. Ultimately, these processes can lead to cell death. LDG-A could increase the secretion of TNF-α from macrophage and the expression of TNF-α mRNA in vitro. The toxic effects of NO and its derivatives on target cells are due to several mechanisms [26]. Our results demonstrated that LDG-A increased NO release and induced expression of iNOS gene to several folds in vitro. It is rational to assume that the release of TNF-α and NO of macrophage which is activated by LDG-A may be the important mechanism of the antitumor effect of LDG-A. However, further evidence is required. It is well known that IL-6 is also considered as a major immune and inflammatory mediator in cancer. This research indicated that macrophages were induced to enhance the secretion and expression of the cytokines IL-6 by LDG-A. These results point that these cytokines may be involved in the antitumor effect of LDG-A.
Acknowledgements
This project was supported by Application Foundation Project of Sichuan Province (2011JY0135 and 2013JY0094), Foundation Project of Educational Committee of Sichuan Province (10ZC120), Youth Foundation Project of Educational Committee of Sichuan Province (09ZB088), National Natural Science Foundation of China (31200012), and Doctor Startup Foundation Project of China West Normal University (11B019) and (11B020). Authors thank Prof. Zhirong Yang, College of Life Sciences, Sichuan University, Chengdu, China for plant authentication.
References
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher level phylogenetic classification of the Fungi.Mycol Res 2007;111:509-47.
- Bertozzi CR, Kiessling LL. Chemical glycobiology. Science 2001;291:2357-64.
- Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. ApplMicrobiol Biotech 2002;60:258-74.
- Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors and immunity.ProcSocExpBiol Med 1999;221:281-93.
- Pauline MR, Time E, Peter C, Ian AW, Raymond AD. Glycosylation and the immune system. Science 2001;291:2370-6.
- Chen Y, Xie MY, Nie SP, Li C, Wang YX. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganodermaatrum. Food Chem 2008;107:231-41.
- Angeli JP, Ribeiro LR, Gonzaga ML, SoaresSde A, Ricardo MP, Tsuboy MS, et al. Protective effects of β-glucan extracted from Agaricusbrasiliensis against chemically indueed DNA damage inhuman lymPhoeytes. Cell BiolToxicol 2006;22:285-91.
- 8.Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT, Lo CK, et al. A polysaccharide isolated from Cordycepssinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci 2003;73:2503-13.
- Hetland G, Johnson E, Lyberg T, Bernardshaw S, Tryggestad AM, Grinde B. Effects of the medicinal mushroom AgaricusblazeiMurill on immunity, infection and cancer. Scand J Immunol 2008;68:363-70.
- Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom AgaricusblazeiMurrill: Review of literature and pharmaco-toxicological problems. Evid Based Complement Alternat Med 2008;5:3-15.
- Paterson RR. Ganoderma: A therapeutic fungal biofactory. Phytochemistry 2006;67:1985-2001.
- Paterson RR. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory. Phytochemistry 2008;69:1469-95.
- Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. PerspectBiol Med 2006;49:159-70.
- Halpern GM, Miller A. Medicinal Mushrooms: Ancient Remedies for Modern Ailments. New York: M. Evans and Co; 2002. p. 116.
- Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res 2002;22:1737-54.
- Xiang D, Yi-ling H, Wanru H. Structure feature and antitumor activity of a novel polysaccharide isolated from Lactariusdeliciosus Gray. CarbohydrPolym 2012;89:397-402.
- Staub AM. Removal of protein-Sevag method. Methods CarbohydrChem 1965;5:5-6.
- Dubois M, Gillis KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956;28:350-6.
- Keller R, Geiges M, Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res 1990;50:1421-5.
- Ooi VE, Liu F. Immunomodulation and anticancer activity of polysaccharide-protein complexes. Curr Med Chem 2000;7:715-29.
- Fidler IJ, Kleinerman ES.Therapy of cancer metastasis by systemic activation of macrophages; from bench to the clinic. Res Immunol 1993;144:274-6.
- Flick DA, Gifford GE. Comparison of in vitro cell cytotoxicity assays for tumor necrosis factor. J Immunol 1984;68:167-75.
- Klostergaard J. Macrophages tumoricial mechanism. Res Immunol 1993;87:581-6.
- Klimp AH, deVries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer.Crit Rev OncolHematol 2002;44:143-61.
- Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. ProcNatlAcadSci USA 1995;92:8115-21.
- Kroncke KD, Fehsel K, Kolb BV. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities.BiolChem Hoppe Seyler 1995;376:327-35.