- *Corresponding Author:
- O. Ojurongbe
Departments of Medical Microbiology and Parasitology
E-mail: oojurongbe@lautech.edu.ng
| Date of Submission | 18 May 2014 |
| Date of Revision | 26 January 2015 |
| Date of Acceptance | 08 August 2015 |
| Indian J Pharm Sci 2015;77(4): 504-510 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The rising problem of resistance to most commonly used antimalarials remains a major challenge in the control of malaria suggesting the need for new antimalarial agents. This work explores the antiplasmodial potential of ethanol extract of Russelia equisetiformis in chloroquine Plasmodium berghei infected mice. Swiss albino mice were intraperitoneally infected with chloroquine-resistant P. berghei (ANKA). Experimental mice were treated for four days consecutively with graded doses of plant extracts and standard antimalarial drugs (artesunate and chloroquine) at a dose of 10 mg/kg body weight used as control. The extract showed a dose-dependent activity in the chemosuppression of P. berghei parasites by 31.6, 44.7, 48.4 and 86.5% at doses of 100, 200, 400 and 800 mg/kg, while chloroquine (10 mg/kg) and artesunate produced 59.4 and 68.4%, respectively. The extract showed a significant decrease in parasitaemia (P<0.05). The level of parasitemia and decrease in weight in all the treated groups was significantly lower (P<0.05) compared with the infected but untreated mice. The plant extract was devoid of toxicity at the highest dose tested (5000 mg/kg). The study concluded that the ethanol extract of R. equisetiformis possesses antimalarial effect, which supports the folk medicine claim of its use in the treatment of malaria.
Keywords
Antiplasmodial, plant extract, Russelia equisetiformis, Plasmodium berghei
Malaria is still among the most important parasitic diseases worldwide as up to three million deaths due to malaria are recorded around the world annually, with Africa bearing more than 90% of the burden [1]. In 2009, approximately 781 000 deaths among which 89% cases were found in the African region resulted from malaria [2]. This parasitic disease is directly responsible for one in five childhood deaths in Africa and directly contributes to illness and deaths from diarrheal disease, respiratory infections and malnutrition [3]. One of the reasons for continual increase in the global prevalence of malaria resulted from increasing resistance of the parasite to antimalarial drugs and this makes the search for new antimalarial drugs imperative [4].
There is an urgent need for the discovery and the development of new effective and safe drugs. Over the years, plants have been important sources of new drugs and several medicinal plants continue to provide easily accessible alternatives to widely used antimalarials [5]. It is also well known that most widely used curative antimalarial drugs such as quinine, obtained from cinchoma species and artemisinins obtained from artemesia species are plant products [6,7]. Also, plants remain the main source of many phytochemical compounds with antiplasmodial activity [8].
Russelia equisetiformis of the family Scrophulariaceae is a medicinal plant used by traditional healers to treat malaria, cancer and inflammatory diseases and it is also claimed to promote hair growth among the Yoruba tribe in Nigeria [9]. Personal communication with this tribe revealed that, the whole plant is used for the treatment of pain and inflammation [10]. Medicinally, the plant is used for the treatment of diabetes and leukemia in Southwestern, Nigeria [10]. The extract of the plant has been reported to possess antibacterial, analgesic and antiinflammatory activities [11]. Recently, two flavonoid compounds isolated from R. equisetiformis were reported to have potential analgesic activity [12]. Flavonoids, triterpenes saponins are known to either produce inhibitory, and/or stimulatory effects on convulsions [13,14]. In addition to its antiinflammatory activity, R. equisetiformis has also been reported to have membrane stabilizing properties of which phenyl ethanoid glycosides of this plant, russectinol and russeliaoside, were identified as its active constituents [11,15].
The molecular formula of russectinol was determined as C29H36O15 by El‑MS [12]. This El‑MS (negative‑ion mode) of russectinol displayed a pseudo‑molecular ion peak [M‑H]+ at m/z 623.IR vmax KBr (cm-1): 3424, 2937, 1825, 1594, 1363 and 1H-NMR spectrum exhibited the presence of eight olefinic protons, one methyl doublet signals, two anomeric proton signals (δHI 4.39 and δHI 5.20) confirmed by two anomeric carbons (δCI 103.20 and δCI 102.03) [12]. The molecular formula of russeliaoside was determined as C23H26O10 by EI‑MS (negative‑ion mode) of russeliaoside displayed a pseudo‑molecular ion peak [M‑H]+ at m/z 461 [12]. The 1H‑NMR spectrum exhibited the presence of eight olefinic protons, one methyl doublet signals, one anomeric proton signal (δH1 5.21) confirmed by one anomeric carbon (δC1 102.07) indicating that the compound contains one sugar moiety (rhamnose) [12].
Treatment of malaria by traditional methods could be a promising source of new anti‑malarial compounds. In Africa, more than 80% of people use traditional medicines and most families have recourse to this medicine based on plants extracts for the curative treatment of malaria [16]. The traditional medicine of this continent constitutes an important source for ethno pharmacological investigations. In this paper, we report for the first time the in vivo antiplasmodial activities of R. equisetiformis using the suppressive and prophylactic model.
Sample of chloroquine resistant P. berghei ANKA clone, was received from Malaria Research Laboratory, Institute for Medical Research and Training (IMRAT), University College Hospital Ibadan, Nigeria and was used for the study to evaluate the antimalarial activity of the plant material used in this study. Each mouse was infected intravenously in the tail vein with 1×107 parasitized erythrocytes from an infected donor mouse. The day of infection was defined as day zero (D0) and subsequent days D1, D2, D3, D4, D5.
The plant sample was collected in the month of October 2012 from Bodija in the South West of Nigeria. The plant was identified in the herbarium of the Forest Research Institute, Ibadan, Nigeria, where voucher specimen was deposited with voucher number 106998. The whole plant excluding the root was air dried at room temperature and powdered in a grinding machine and 300 g of the powdered plant was macerated with 3 l of 90% ethanol (1:10 w/v) for 48 h and then filtered. The filtrate was dried with a rotatory evaporator. The residue was collected and stored for onward use. Standard chloroquine sulphate (Batch No. 442; May and Baker Nigeria Plc., Lagos) and artesunate (Mekophar Chemical Pharmaceutical Joint‑Stock Company, Vietnam) were used as standard reference for the antimalarial screening in this study.
The assessment of minimum lethal dose was carried out in two phases as previously described [17]. In the phase 1 study, 3 groups of 3 mice each were used. The mice in the 3 groups were orally administered with doses of 10, 100 and 1000 mg/kg of the extract and monitored for 24 h for mortality. In the phase 2 study also 3 groups of 3 mice in each were used. The mice in the 3 groups were orally administered with 1600, 2900 and 5000 mg/kg of the extract and monitored for 24 h for mortality if any. The phase 2 study was carried out based on the result of the observation of mortality in phase 1 study. The lethal dose and the penultimate dose to the lethal dose would indicate the value of the LD50 [18].
Swiss albino mice (18‑25 g) obtained from the animal house of Biomedical Sciences, College of Health Sciences LAUTECH Osogbo, were acclimatized for 7 days before commencing the experiment. The mice were infected with 0.2 ml blood containing about 1×107 dose of P. berghei berghei (16.60%) from a donor mouse. Each mouse was inoculated on D0, (intraperitoneally) for the suppressive model and on the fifth day (D4) for the prophylactic model.
Peters’ 4‑day suppressive test against chloroquine‑resistant P. berghei infection in mice was used for the evaluation of schizontocidal activity on early infection [19,20]. The mice were divided into 7 groups of five mice each. The first 4 groups were administered 100, 200, 400 and 800 mg/kg/day doses of the extract for 4 consecutive days, while the groups 5 and 6 were administered chloroquine and artesunate 10 mg/kg/day and the group 7 was administered an equivalent volume (0.2 ml) of normal saline (control group) for 4 consecutive days (D1‑D4) using oral cannula. On the fifth day (D5), thin blood films were prepared from blood collected from the tail and stained with 10% Giemsa and viewed under the X100 objective (oil immersion) [21]. The numbers of infected erythrocytes were counted until 100 erythrocytes were achieved. The mean per cent suppression of parasitaemia was calculated in comparison to controls as, % suppression=((% parasitemia in negative control−% parasitemia in test group)/(% parasitemia in negative control))×100.
The prophylactic activity of the extract was performed as previously described [20]. In this procedure, the adult mice were randomized into 7 groups of 5 mice each. Group 1 was treated with normal saline, groups 2 to 5 were treated with 100, 200, 400 and 800 mg/kg of the extract orally, group 6 and 7 (positive control) were treated with 10 mg/kg of the standard drug chloroquine and artesunate. Treatment was initiated on D0 and continued till day 4 when the mice were all infected (intraperitoneally) with the parasite. Blood smears were then made from each mouse 72 h after inoculation on day 7. The rectal temperature of each mouse was also determined daily by using a thermaprobe thermometer. Blood samples were taken from the tail vein of each mouse for thin film microscopy and the number of the parasitized cells was determined and the percentage suppression evaluated [22]. The body weight of each mouse in all the groups was taken before infection (day 0) and then daily up to day 5.
Experimental procedures and protocols used in this study conform to the “Guide to the care and use of animals in research and teaching” (NIH publications number 85‑93 revised in 1985). One‑way analysis of variance (ANOVA), followed by Student Newman‑Keuls’ test was used to analyse and compare results at a 95% confidence interval. Statistical significance was set at P<0.05. The results were expressed as mean±SEM (standard error of mean).
The weight of the brown crude extract obtained from the extraction procedure was 11.10 g and the percentage yield was 4.6%. For the acute toxicity test, all the animals were still alive at the end of the experiment and the LD50 value was greater than 5 g/kg and is of no harm. Hence, the extract can be declared harmless [18].
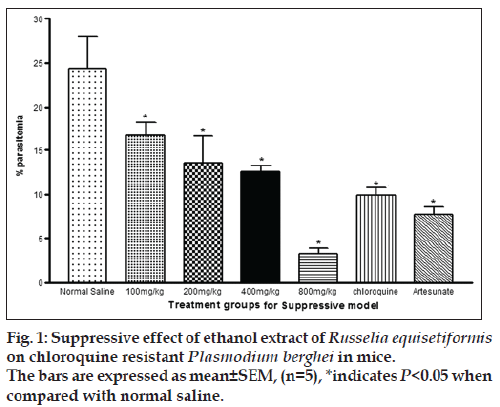
In the suppressive test, the mean parasitaemia on day 5 post infection were 16.7±1.5, 13.5±3.1, 12.6±0.6 and 3.3±0.6 for 100, 200, 400 and 800 mg/kg, respectively. The value for control was 24.4±3.7 while chloroquine and artesunate were 9.9±0.9 and 7.7±1 (P<0.05). The percentage chemosuppression of the extract were 31.6, 44.7, 48.4 and 86.5%, respectively while those of chloroquine and artesunate were 59.4 and 68.4%. The % parasitaemia was observed to be decreasing with increasing dose levels of the extract, an indication that the highest dose exhibited the optimal chemo‑suppression. These values were significant in comparison to control (P<0.05) while 800 mg/kg, chloroquine and artesunate were highly significant to control (P<0.05). Similarly chloroquine and artesunate exhibited significant increase in % chemosuppression compared to control (P<0.05). While the percentage parasitaemia in chloroquine and artesunate were much lower than in extract at the lowest dose (fig. 1), the percentage parasitemia of the highest dose of the extract was lower in comparison to chloroquine and artesunate but the difference was not statistically significant. The % chemosuppression observed in chloroquine and artesunate was slightly different but not statistically significant (P>0.05, fig. 1). Also it was observed that the weight of the animals decreased 24 h post infection, which was reversed on 72 h (day 3) for the treated animals. The untreated animal group experience further decrease in weight after 72 h (Table 1).
| Dose mg/kg | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|---|
| Control | 26.6 ± 0.7 | 25.6 ± 0.4 | 25.4 ± 0.9 | 25.3 ± 1.0 | 25.6 ± 0.7 | 25.4 ± 0.9 |
| 100 mg/kg R. equisetiformis | 25.2 ± 1.4 | 23.7 ± 1.4 | 25.2 ± 1.4 | 24.8 ± 1.5 | 25.5 ± 1.5 | 25.1 ± 1.4 |
| 200 mg/kg R. equisetiformis | 25.4 ± 1.5 | 24.3 ± 1.3 | 23.5 ± 1.5 | 23.7 ± 1.8 | 24.7 ± 2.1 | 24.3 ± 2.5 |
| 400 mg/kg R. equisetiformis | 19.8 ± 1.0 | 19.7 ± 1.1 | 20.0 ± 1.0 | 20.0 ± 1.1 | 20.7 ± 1.1 | 20.7 ± 1.1 |
| 800 mg/kg R. equisetiformis | 22.8 ± 0.9 | 21.5 ± 0.9 | 22.3 ± 0.6 | 22.2 ± 0.7 | 22.3 ± 0.8 | 21.8 ± 0.8 |
| Chloroquine | 26.5 ± 0.2 | 25.7 ± 0.2 | 26.1 ± 0.3 | 26.1 ± 0.2 | 26.1 ± 0.2 | 26.4 ± 0.3 |
Table 1: Effects Of Malaria On Bodyweight
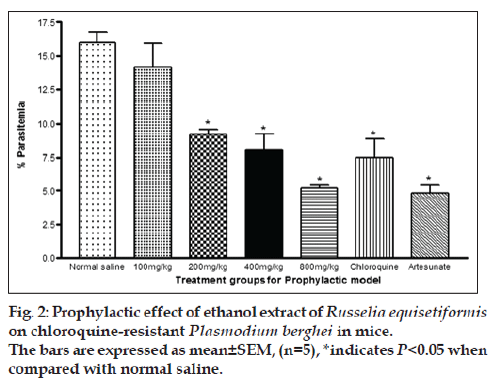
Percent parasitaemia for 100, 200, 400 and 800 mg/kg were 14.14±1.81, 9.2±0.40, 8.08±1.21 and 5.2±0.4, respectively. The value for control was 15.98±0.83 while the values for chloroquine and artesunate were 7.5±1.5 and 4.8±0.6, respectively. The % chemosuppression of the extract at 100, 200, 400 and 800 mg/kg were 11.51, 42.41, 49.44 and 67.46, respectively while that of chloroquine and artesunate were 53.07 and 70.0%. Similar to what was observed in the suppressive model, the % parasitaemia was observed to be increasing with increase in dose levels of the extract, indicating that optimal chemosuppression was also exhibited at the highest dose in this model. These values were highly significant in comparison to control (P<0.05). As observed in the suppressive model, chloroquine and Artesunate also exhibited a significant (P<0.05) decrease in % parasitaemia when compared to control. However, the % parasitaemia seen in chloroquine and Artesunate were significantly (P<0.05) lower than in extract at all dose levels except for the highest dose with chloroquine (fig. 2).
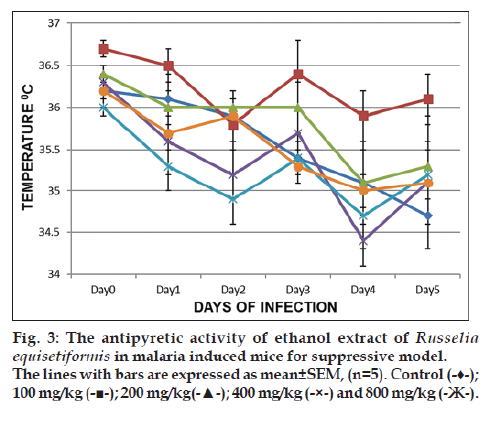
Fig. 3 summarises the results of the suppressive study of Russelia equisetformis for the antipyretic investigations in malaria‑induced mice. The temperature pattern from D0 to D5 showed that the decrease of temperature was prominent with extract (800, 400 and 100 mg/kg), chloroquine as compared to the control on day 1 which were all significant at day 2 as evidence of establishment of malaria. However, the temperatures of mice that received extract (100, 400 and 800 mg/kg) were increased compared to that of control. The reversal of temperature, which was prominent with 100 mg/kg is contrary to an optimal activity. Similar pattern was observed in the prophylactic model (result not shown).
The present study investigates the antiplasmodial activities of ethanol extracts of R. equisetiformis for the treatment of malaria infection using the 4‑day suppressive test and prophylactic model. The 4‑day suppressive test commonly used for antimalarial screening and the determination of percent inhibition of parasitemia is the most reliable parameter [19]. A mean group parasitemia level of less than or equal to 90% of mock‑treated control animals usually indicates that the test compound is active in standard screening [23]. The in vivo results of the extracts at different doses show that they are capable of reducing the level of parasites in circulation both in suppressive and prophylactic models. Their activities were observed to be dose dependent and significantly higher amounts of the crude extracts were required to elicit such activities when compared to artesunate and chloroquine used as standard drugs in the study. This significant suppression of parasitemia by the ethanol extract of R. equisetiformis in divided doses on day 4 is in agreement with previous studies with ethanol extract of Asperagus africanus [24] and Azadirachta indica [25]. The phytosteroids and flavonoids detected in R. equisetiformis could as well be responsible for the antimalarial effect as these metabolites have been proved to possess potential immunomodulatory effects in other plants [26]. In view of this, the extract can be considered to contain some antimalarial active ingredients that could serve as a template for the production of relatively inexpensive antimalarial drugs. It is clear from our results that the percentage parasitemia of P. berghei infected mice treated with extract of R. equisetiformis, changed significantly (P≤0.05) in comparison to the non‑treated infected mice. Moreover, the ethanol extract administered at a dose of 800 mg/kg per day for five days resulted in >50% reduction in per cent parasitemia, a performance that may likely be improved upon if the crude extract is purified to identify and isolate active substituents. The increase in activity observed in the extract as the dose increases in both the suppressive and prophylactic studies explains the fact that the crude extract exerted a pronounced activity against the malaria parasite at an optimal dose of 800 mg/kg.
The oral median lethal dose (LD50) of the crude extract was estimated to be ≥5 g/kg orally since there were no mortality at all dose levels used [17]. The absence of death following the oral administration of R. equisetiformis extract at 5000 mg/kg observed in mice implies that the extract is practically non‑toxic. It has previously been noted that any chemical that exhibited an LD50 more than 5000 mg/kg is practically non‑toxic based on Hodge and Sterner Toxicity Scale [27]. On the contrary, it was shown that the methanol extract of the plant has an oral LD50 of 3.6 g/kg when administered intraperitoneall [28]. The oral administration has been reported to be about 100 times less toxic than intraperitoneal [29]. Also, it has been previously noted that that acute toxicity test on the same extract or drug yielded different values from laboratory to laboratory [17]. Acute toxicity tests with the extracts have also demonstrated their safety because the highest dose used for the screening did not cause death. Interestingly, the highest dose used to treat parasite‑infected mice, which elicited antimalarial activity, was much lower than the highest acute dose.
There has been no previous report on the antipyretic activity of the ethanol extract of the plant and this has prompt the evaluation of the plant for its antipyretic activity. Malaria is known to induce high body temperature in humans [30], and low temperature in rodent mice [31]. Using the two models of malaria treatment, it was shown that the extract displayed antipyretic activity at all the models by raising the body temperature in the malaria‑infected mice. The reversed temperature displayed was optimum at 100 and 800 mg/kg in suppressive and prophylactic model respectively when compared to the control. This activity is on contrary with the optimum antimalarial activity by the same dose in malaria suppressive model, but correlates with optimum activity by the same dose in malaria prophylactic model, seeing that the extract remarkably reversed the body temperature that was lowered by malaria parasites in this study. In ethnomedicine, other works have shown that Dodonaea angustifolia seed and Azadirachta indica leaves as antimalarial agents were able to reverse the temperature induced by malaria parasites [32,33]. This comparison explains the fact that traditional plants are good remedies for malaria infection and fever as claimed in the folklore.
Our results suggest that the ethanol extract of R. equisetiformis show some intrinsic antimalaria activity by its percentage chemo suppression and prophylactic ability against chloroquine‑resistant P. berghi parasites. This performance can surely be improved upon in future studies if the crude extract is purified and the active substituents identified. The extracts have considerably low or no toxicities in experimental mice. This findings support the traditional use of this plant for the treatment of malaria.
Acknowledgements
The authors are grateful to Dr. G. O. Gbotosho, Malaria Research Laboratory, Institute for Medical Research and Training (IMRAT), University College Hospital Ibadan, Nigeria for providing the malaria parasite used for the study, also to Mrs Sakirat Braimah for technical assistance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: What’s new, what’s needed: A summary. Am J Trop Med Hyg 2004;71 2 Suppl: 1-15.
- World Health Organization. World Malaria Report 2010. Geneva: World Health Organization; 2010.
- Ehrhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S, Kubo M, et al. Malaria, anemia, and malnutrition in African children – Defining intervention priorities. J Infect Dis 2006;194:108-14.
- Ojurongbe O, Ogungbamigbe TO, Fagbenro-Beyioku AF, Fendel R, Kremsner PG, Kun JF. Rapid detection of Pfcrt and Pfmdr1 mutations in Plasmodium falciparum isolates by FRET and in vivo response to chloroquine among children from Osogbo, Nigeria. Malar J 2007;6:41.
- Appiah-Opong R, Nyarko AK, Dodoo D, Gyang FN, Koram KA, Ayisi NK. Antiplasmodial activity of extracts of Tridax procumbens and Phyllanthus amarus in in vitro Plasmodium falciparum culture systems. Ghana Med J 2011;45:143-50.
- Krishna S, Uhlemann AC, Haynes RK. Artemisinins: Mechanisms of action and potential for resistance. Drug Resist Updat 2004;7:233-44.
- Van Geldre E, Vergauwe A, Van den Eeckhout E. State of the art of the production of the antimalarial compound artemisinin in plants. Plant Mol Biol 1997;33:199-209.
- Musila MF, Dossaji SF, Nguta JM, Lukhoba CW, Munyao JM. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. J Ethnopharmacol 2013;146:557-61.
- Kolawole O, Kolawole O. Effects of Russelia equisetiformis methanol and aqueous extracts on hepatic function indices. Biol Med 2010;2:38-41.
- Awe EO, Adeloye A, Makinde JM. Antiinflammatory activity of Russelia equisetiformis schlecht and cham: Identification of its active constituent. J Intercult Ethnopharmacol 2012;1:25-9.
- Awe EO, Makinde JM, Olajide OA, Wakeel OK. Evaluation of the anti-inflammatory and analgesic properties of the extract of Russelia equisetiformis (schlecht & cham) Scrophulariacae. Inflammopharmacology 2004;12:399-405.
- Awe EO, Adeloye A, Idowu T, Olajide OA, Makinde J. Antinociceptive effect of Russelia equisetiformis leave extracts: Identification of its active constituents. Phytomedicine 2008;15:301-5.
- Majumder P, Bhattacharjee P. Investigation of phytochemicals and anti-convulsant activity of the plant Coleus amboinicus (lour.). Int J Green Pharm 2013;7:211.
- Chowdhury B, Bhattamisra SK, Das MC. Anti-convulsant action and amelioration of oxidative stress by Glycyrrhiza glabra root extract in pentylenetetrazole- induced seizure in albino rats. Indian J Pharmacol 2013;45:40-3.
- Awe E, Makinde J, Adeloye O, Banjoko S. Membrane stabilizing activity of Russelia equisetiformis, schlecht & chan. J Nat Prod 2009;2:3-9.
- Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J Ethnobiol Ethnomed 2013;9:31.
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol 1983;54:275-87.
- Corbett J, Wright K, Baillie A. The Biochemical Mode of Action of Pesticides. 2nd ed. London: Academic Press; 1984.
- Innocent E, Moshi MJ, Masimba PJ, Mbwambo ZH, Kapingu MC, Kamuhabwa A. Screening of traditionally used plants for in vivo antimalarial activity in mice. Afr J Tradit Complement Altern Med 2009;6:163-7.
- Peters W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 1975;69:155-71.
- Ljungström I, Perlmann H, Schlichtherle M, Scherf A, Wahlgren M. Method in Malaria Research. Manassas, Virginia: Malaria Research and Reference Reagent Resource/American Type Culture Collection; 2004.
- Su RB, Wei XL, Liu Y, Li J. Antimalarial effect of agmatine on Plasmodium berghei K173 strain. Acta Pharmacol Sin 2003;24:918-22.
- Shittu I, Emmanuel A, Nok AJ. Antimalaria effect of the ethanolic stem bark extracts of Ficus platyphylla Del. J Parasitol Res 2011;2011:618209.
- Oketch-Rabah HA, Dossaji SF, Christensen SB, Frydenvang K, Lemmich E, Cornett C, et al. Antiprotozoal compounds from Asparagus africanus. J Nat Prod 1997;60:1017-22.
- Priyanka J, Hingorani L, Nilima K. Pharmacodynamic evaluation for antiplasmodial activity of Holarrhena antidysentrica (Kutaja) and Azadirachta indica (Neemb) in Plasmodium berghei infected mice model. Asian Pac J Trop Med 2013;6:520-4.
- Aherne SA, Kerry JP, O’Brien NM. Effects of plant extracts on antioxidant status and oxidant-induced stress in Caco-2 cells. Br J Nutr 2007;97:321-8.
- Sandu RB, Tartau L, Miron A, Zagnat M, Ghiciuc CM, Lupusoru CE. Experimental researches on acute toxicity of a Bidens tripartita extract in mice – Preliminary investigations. Rev Med Chir Soc Med Nat Iasi 2012;116:1230-4.
- Kolawole O, Wakeel O. Preliminary study on the toxicity of methanol extract of Russelia equisetiformis. J Med Pharm Sci 2006;2:1-3.
- Jutamaad N, Aimmon S, Yodhtal T. Toxicological and antimalarial acticty of the eurycomalactone and Eurycoma longifolia jack extracts in mice. Thai J Phytopharmacy 1998;20:14-27.
- Perera MK, Herath NP, Pathirana SL, Phone-Kyaw M, Alles HK, Mendis KN, et al. Association of high plasma TNF-alpha levels and TNF-alpha/IL-10 ratios with TNF2 allele in severe P. falciparum malaria patients in Sri Lanka. Pathog Glob Health 2013;107:21-9.
- Curfs JH, van der Meide PH, Billiau A, Meuwissen JH, Eling WM. Plasmodium berghei: Recombinant interferon-gamma and the development of parasitemia and cerebral lesions in malaria-infected mice. Exp Parasitol 1993;77:212-23.
- Iwalewa EO, Mohammed K, Omotola OO. Contributory pharmacological effects of Azadirachta indica leaf in the treatment of malaria. Niger J Nat Prod Med 1999;3:42-6.
- Berhan M, Eyasu M, Kelbessa U. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop J Sci 2012;4:47-63.