- *Corresponding Author:
- P. M. Dandagi
Department of Pharmaceutics, K. L. E. S’s College of Pharmacy, J. N. M. C. Campus, Nehru Nagar, Belgaum - 590 010, India
E-mail: pmdandagi@yahoo.com
| Date of Submission | 7 April 2006 |
| Date of Revision | 26 February 2007 |
| Date of Acceptance | 24 May 2007 |
| Indian J Pharm Sci, 2007, 69 (3): 402-407 |
Abstract
Gelatin A microspheres of propranolol hydrochloride for intranasal systemic delivery were developed with the aim to avoid first pass metabolism, to improve the patient compliance, to use an alternative therapy to conventional dosage form, to achieve controlled blood level profiles, and to improve the therapeutic efficacy of propranolol hydrochloride in the treatment of various cardiovascular disorders and as a prophylactic for migraine. Gelatin A microspheres were prepared by emulsion crosslinking method using glutaradehyde as a crosslinking agent. Gelatin and chitosan were used as polymer and co polymer respectively. All the prepared microspheres were evaluated for physical characteristics, such as particle size, incorporation efficiency, swelling index, in vitro bioadhesion using rat jejunum and in vitro drug release in pH 6.6 phosphate buffer. Average particle size of microspheres was found to be in the size range 1-50 mm. Increase in drug and polymer concentration in the formulation increased incorporation efficiency. All the microsphers showed good bioadhesive properties and swelling indices and good sustained release of drug. The data indicates that propranolol hydrochloride release followed Higuchi's matrix and Peppa's model. Stability studies showed stability of formulation at all the conditions to which they were subjected.
Keywords
Gelatin A, microspheres, propranolol HCl and chitosan
Propranolol hydrochloride is drug of choice in many cardiovascular disorders and as a prophylactic in migraine [1]. Unfortunately, propranolol undergoes first pass metabolism. The bioavailability of propranolol after oral administration is approximately 20% [2]. Several approaches have been tried to develop nonoral formulations in addition to injections [3]. Among the non-invasive routes nasal administration has promising potential and a viable alternative for systemic medication of drug [4]. Nasal administration of propranolol hydrochloride in the form of solutions and organogels has already been reported but rapid nasal mucociliary clearance limits its sustained bioavailability [5]. Bioadhesive microspheres give more residence time to facilitate absorption through nasal mucosa against nasal mucociliary clearance [6]. Gelatin A is an acid hydrolytic product of collagen [7]. It is bioadhesive and biodegradable polymer that can be used for controlled drug delivery [8]. These observations promoted us to develop microspheres based on gelatin A as a promising formulation of propranolol hydrochloride for intranasal systemic administration as an alternative route to injections to increase the bioavailability, bypass first pass metabolism by the liver and to improve the release profile of the drug.
Materials and Methods
Propranolol hydrochloride and chitosan were gift samples obtained from Cipla R&D Mumbai and CIFT Cochi, India, respectively. Gelatin A, glutaraldehyde, liquid paraffin, acetone, isopropanol, dihydrogen potassium orthophosphate and sodium hydroxide were purchased from S. D. Fine Chemicals Mumbai. Tween 80 was purchased from Himedia Lab Pvt. Ltd., Mumbai. Glycine was purchased from Loba Chemie, Mumbai.
Preparation of microspheres [9]
Gelatin A microspheres were prepared by emulsion cross-linking method. The drug was dissolved in an aqueous gelatin solution (10% w/v), which was preheated at 40° for 1 h. The solution was added drop wise to liquid paraffin while stirring the mixture at 1500 rpm at 35° for 10 m. This gives water in oil (W/O) emulsion. Stirring was continued for further 10 m at 15° and the microspheres were washed three times with acetone and isopropyl alcohol, respectively. The washed microspheres were air dried and then dispersed in 5 ml of aqueous glutaraldehyde-saturated toluene solution (25% v/v) at room temperature for 3 h to allow cross linking. The microspheres were washed with toluene and treated with 100 ml of 10 mM glycine solution containing 0.1% w/v Tween 80 at 37° for 10 m to block unreacted gluteraldehyde [10]. The resultant microspheres were finally freezedried. The chitosan-gelatin based microspheres were prepared by exactly the same method as mentioned above except that the chitosan solution prepared in 1% W/V glacial acetic acid was first mixed with the gelatin A solution. The drug was dissolved into it and then emulsification was followed as above. Different amount of drug and polymer were used to obtain the microspheres of optimum properties and characteristics. Percentage composition of gelatin A and chitosan is given in Table 1.
| Formulation code | Gelatin A% w/v | Chitosan % w/v | Drug (% w/v) | Percentage % yield |
|---|---|---|---|---|
| MS1 | 10 | - | 40 | 78.00 |
| MS2 | 10 | - | 60 | 82.52 |
| MS3 | 10 | - | 80 | 79.20 |
| MS4 | 8 | 2 | 80 | 81.82 |
| MS5 | 6 | 4 | 80 | 85.12 |
Table 1: Formulae For Different Batches of Gelatin A Microspheres of Propranolol Hydrochloride And Percentage Yield
Particle size, shape and surface morphology analysis [11-13]
All the microspheres were evaluated with respect to their size and shape using optical microscope fitted with an ocular micrometer and a stage micrometer. The particle diameters of more than 100 microspheres were measured randomly by optical microscope. The average particle size was determined by using the Edmondson’s equation Dmean = Σnd/Σn, where n= number of microspheres observed and d= mean size range. The shape and surface morphology of the microspheres was studied by using a Jeol JSM-T330A scanning electron microscope.
To determine the incorporation efficiency, 25 mg of propranolol loaded microspheres were washed with 10 ml of phosphate buffer (pH 6.6) containing 0.1% (v/v) Tween 80 to remove the surface associated drug. The microspheres were then digested in 10 ml of 0.1 M HCl for 12 h at room temperature (25±20) to release the entrapped drug. Drug content was determined spectrophotometrically at 219 nm. Percent of total entrapment efficiency was determined by the formula; Total percentage entrapment efficiency = Percentage surface associated drug + percentage entrapped drug.
Swelling index was determined by measuring the extent of swelling of microspheres in phosphate buffer pH 6.6. To ensure the complete equilibrium, exactly weighed 100 mg of microspheres were allowed to swell in pH buffer 6.6 for 34 h. The excess surface adhered liquid drops were removed by blotting and the swollen microspheres were weighed by using microbalance. The hydrogel microspheres then dried in an oven at 60° for 5 h until there was no change in the dried mass of sample. The swelling index of the microsphere was calculated by using the formula swelling index= (mass of swollen microspheres–mass of dry microspheres/mass of dried microspheres)×100.
In vitro bioadhesion [17]
Bio-adhesive properties of propranolol-loaded microspheres were evaluated using everted sac technique. The animal study protocols have been approved by the Institutional Animal Ethical Committee’s (IAEC meeting proposal No: 39 Dated, 07/07/2005). Unfasted male Sprague dawley rats which were similarly nourished and grown in normal laboratory conditions were sacrificed and intestinal tissue was excised and flushed with 10 ml ice-cold isotonic phosphate buffer pH 7.2 containing 2 mg/ml glucose. Segment (6 cm) of jejunum was everted using a glass tube with a conical end of the tube. Through the opposite end of the tube 1.0-1.5 ml of isotonic phosphate buffer was poured until the sac was filled; thereafter the segment end was tightly tied. The intestinal tissue was maintained at 4° prior to incubation. The sacs were introduced into a 15 ml glass tube containing 60 mg of microspheres and 5 ml of phosphate buffer 7.2 incubated at 37° and shaken end over end after 30 m the sacs were removed, then the not attached microspheres were removed by centrifugation and dried The percentage of the attached microspheres was calculated by the difference between the initial amount of microspheres and amount of not attached microspheres before and after incubation.
To carry out the in vitro drug release, accurately weighed 50 mg of propranolol-loaded microspheres were dispersed in 400 ml of phosphate buffer (pH 6.6) in a beaker and maintained at 37±2° under continuous stirring at 100 rpm. At selected time intervals 5 ml samples were withdrawn through a hypodermic syringe fitted with a 0.4 μm Millipore filter and replaced with the same volume of pre-warmed fresh buffer solution to maintain a constant volume of the receptor compartment. The samples were analyzed spectrophotometrically at 219 nm. The released drug content was determined from the standard calibration curve of propranolol.
In vitro diffusion studies
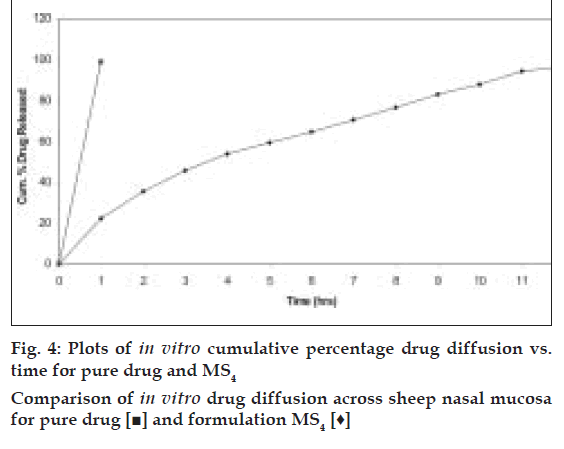
The in vitro diffusion study was performed using in vitro nasal diffusion cell [5]. The receptor chamber was filled with 60 ml of pH 6.8, phosphate buffer maintained at 37±2°. Accurately weighed microspheres equivalent to 10 mg propranolol were spread on sheep nasal mucosa. At selected time intervals 0.5 ml of diffusion samples were withdrawn through a hypodermic syringe and replaced with the same volume of pre-warmed fresh buffer solution to maintain a constant volume of the receptor compartment. The samples were analyzed spectrophotometrically at 219 nm
Stability studies of microspheres [18,19]
All the five batches of propranolol hydrochloride microspheres were tested for stability. The preparations were divided into 3 sets and were stored at 4° (refrigerator), room temperature and 40° (thermostatic oven). After 15, 30 and 60 d drug content of all the formulations was determined by the method discussed previously in entrapment efficiency section. In vitro release study was also carried out of the best formulation.
Results and Discussion
Gelatin A microspheres were prepared by using gelatin A alone and with different concentrations of chitosan with an intention to increase the mucoadhesion. Chitosan solutions of strengths 2 to 8% were tried. It was found that formulation of microspheres with more than 4% chitosan is not possible due to drastic increase in the viscosity followed by saturation of chitosan in 0.1% (v/ v) glacial acetic acid solution. The prepared microspheres were treated with Glycine solution to block unreacted glutaraldehyde. The maximum yield was found to be 85.12% for MS5 (Table 1).
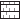
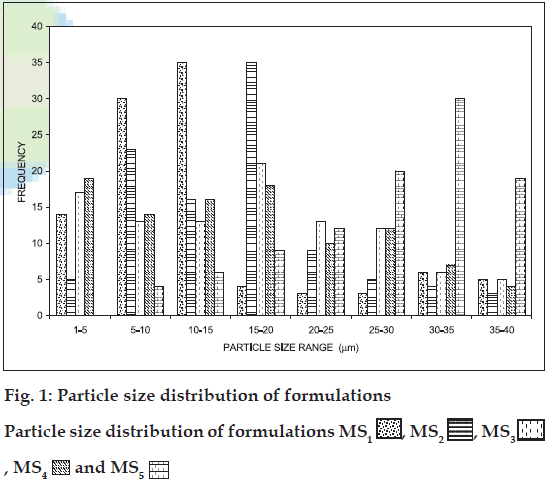
The mean size range of the all five batches of microspheres was estimated between 1-50 μm (fig. 1 and Table 2), which are suitable for intranasal administration. It was observed that as the amount of drug increased in the microspheres, the particle size also increased proportionally. Incorporation of chitosan yielded larger microspheres (fig. 2, D and E) this might be due to the more viscous nature of chitosan than gelatin, which brings about poor emulsification leading to the formation of larger globules of dispersed phase. The shape of the all five batches of microspheres was found to be spherical.
| Formulation code | Average particle size (μm)* | Total entrapment efficiency (%) | Averages welling Index# | Average bioadhesion(%)@ |
|---|---|---|---|---|
| MS1 | 13.00±1.25 | 48 | 0.992±0.32 | 78.86±1.25 |
| MS2 | 15.80±0.87 | 47 | 0.985±0.15 | 76.67±2.19 |
| MS3 | 21.75±1.76 | 45 | 0.883±0.08 | 75.83±1.98 |
| MS4 | 21.00±1.96 | 61 | 0.873±0.26 | 89.29±0.85 |
| MS5 | 32.43±2.19 | 58 | 0.847±0.18 | 91.33±0.93 |
*Values expressed as Mean±SD, n=100; #Values expressed as Mean±SD, n=3 and @Values expressed as Mean±SD, n=3
Table 2: Physical Charecteristics of Prepared Microspheres of Propranolol Hydrochloride
Scanning electron microscopic (SEM) analysis of the samples (fig. 2) revealed that all microspheres prepared were spherical in shape. Formulation MS1 (A), MS2 (B) and MS3 (C) were found to be slightly rough surfaced and formulation MS4 (D) and MS5 (E) were relatively smooth and uniform which is suitable for intranasal administration.
It was observed that increase in the concentration of the drug increases the entrapment efficiency and addition of chitosan in the formulation improved the entrapment efficiency (Table 2).
The swelling indices of microspheres prepared by using gelatin A along with the chitosan were found to be less than that of microspheres prepared by gelatin A alone (Table 2) which may be one of the reasons behind the extended release of drug from the microspheres prepared by using gelatin with chitosan
The results of in vitro bioadhesion carried out by everted sac technique showed that all the prepared microspheres have good mucoadhesive property (Table 2). Addition of chitosan to the formulation produced further increase in the mucoadhesion. This may be due to formation of secondary chemical bonds such as hydrogen bond or ionic bond or ionic interactions between the positively charged amino groups of chitosan and the negatively charged Sialic acid residue of mucus glycoproteins or mucins. Sialic acid carries a net negative charge and providing strong electrostatic interaction between mucin and chitosan. Formulation MS5 showed maximum mucoadhesion.
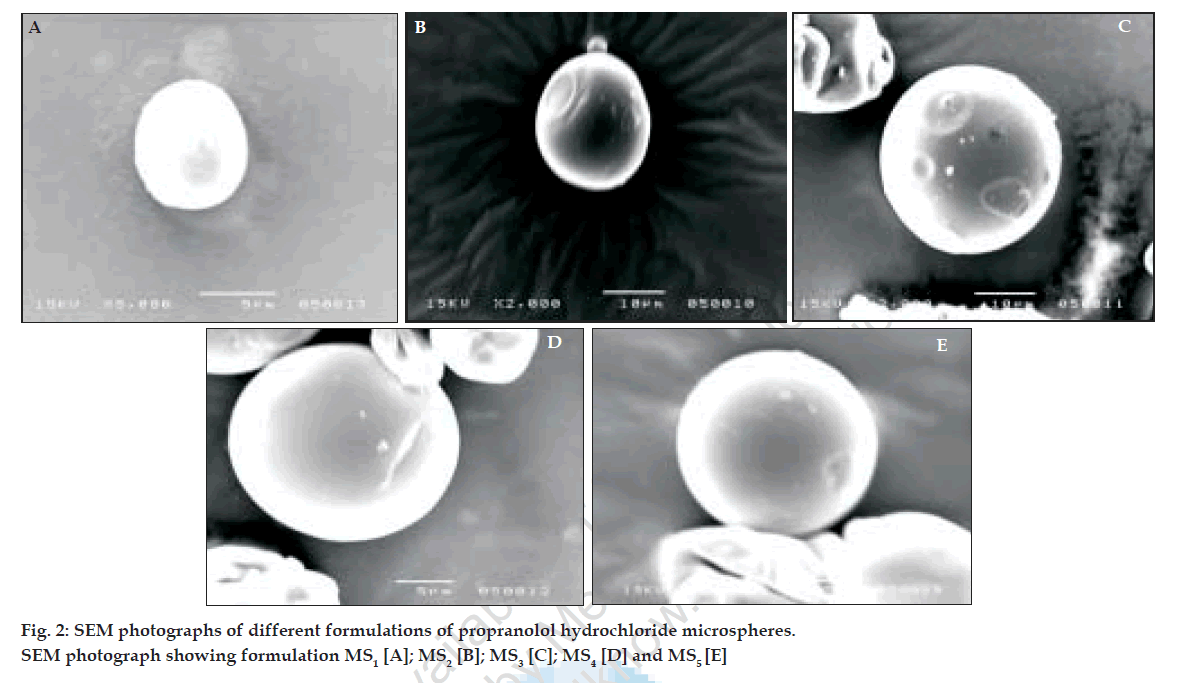
The release pattern of all the formulations appears to be slow release with negligible burst effect. The burst effect corresponds to the release of the drug located on or near surface of the microspheres or release of poorly entrapped drug. The slow release period may be due to the medium being diffused in to the polymer matrix and the drug diffusing out of the microspheres. Cumulative percent drug release after 12 h is shown in fig. 3. Kinetic values obtained for different formulations are indicated in Table 3.
| Formulation code | Kinetic models | ||||||
|---|---|---|---|---|---|---|---|
| Zero order | First order | Higuchi matrix | Peppa’s | Hixson Crowell | ‘n’ values | Best fit model | |
| MS1 | 0.9474 | 0.8827 | 0.9895 | 0.9993 | 0.9739 | 0.6237 | Peppa’s |
| MS2 | 0.9417 | 0.9455 | 0.9912 | 0.9985 | 0.9892 | 0.6011 | Peppa’s |
| MS3 | 0.9425 | 0.9327 | 0.9915 | 0.9989 | 0.9835 | 0.5990 | Peppa’s |
| MS4 | 0.8845 | 0.9935 | 0.9970 | 0.9967 | 0.9859 | 0.5500 | Higuchi |
| MS5 | 0.9487 | 0.9387 | 0.9893 | 0.9991 | 0.9870 | 0.6180 | Peppa’s |
Table 3: Model Fitting Of The Release Profile Using Five Different Models (R-Value)
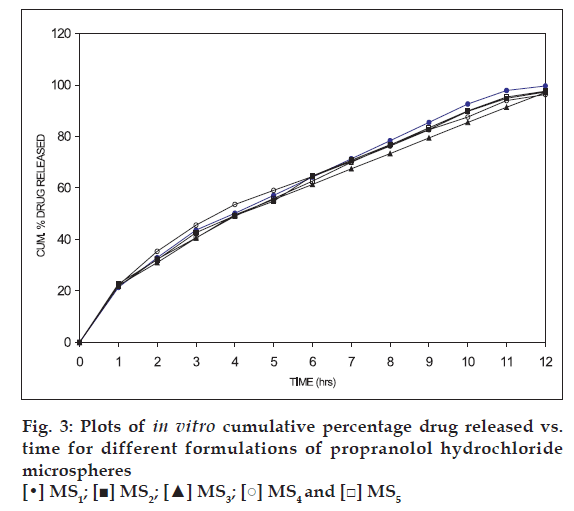
Based on the highest regression values (r) the best-fit model for MS1, MS2 MS3 and MS4was Peppas and MS4 was Higuchi matrix. Further the ‘n’ values for the MS1, MS2, MS3, and MS5 are 0.6237, 0.6011, 0.5990, and 0.6180, respectively. This indicates that the release mechanism followed non-Fickian diffusion. Further the in vitro diffusion study of MS4 using sheep nasal mucosa revealed the controlled release of drug (fig. 4).
The stability studies showed that there was no change in the appearance of the microspheres indicating that the formulations were physically stable at all the conditions to which they were exposed. It was observed that there was slight reduction in the drug content of microspehres which were stored at 400 after storage for 60 d and no change in drug content of the formulations stored at room temperature and at 4°.
In vitro release studies revealed that the formulation stored at 4° showed 96.64% release. The one which was stored at 40° showed 97.13% and room temperature batch showed 97.66% release after 12 h. These results indicate that there was no significant change in drug release from all the formulations.
The gelatin A microspheres exhibited a significant bioadhesive properties and could potentially be used as bioadhesive microspheres for controlled and sustained intranasal systemic delivery of propranolol hydrochloride. Further, there is potential to improve propranolol hydrochloride bioavailability through the nasal route, which could be established by in vivo evaluation of microspheres in animals and/or human volunteers.
Acknowledgements
Authors thank Cipla Ltd., Mumbai and CIFT, Cochi for providing generous quantities of required material and KLES’s College of Pharmacy, Belgaum, for providing necessary facilities to conduct the work.
References
- Rang HP, Dale MM, Ritter JM. In: Pharmacology. 4th ed. London (UK); Churchill Livingstone: 1999.
- Huang CH, Kimura R, Nassar RB. Hussain A. Mechanism of nasal absorption of drugs I: Physicochemical parameters inß uencing the rate of in situ nasal absorption of drugs in rats. J Pharm Sci 1985;74:608- 11.
- Rao PR, Reddy MN, Ramakrishna S, Prakash V. Comparative in vivo evaluation of propranolol hydrochloride after oral and transdermal administration in rabbits. Eur J Pharm Biopharm 2003;56:81-5.
- Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol 2001;53:3-21.
- Pisal S, Shelke V, Mahadik K, Kadam S. Effect of organogel components on in vitro nasal delivery of propranolol hydrochloride. AAPS Pharm Sci Tech 2004;5:63.
- Chein YW, Su KS, Chang SF. Nasal systemic drug delivery. Marcel Dekker Inc: New York; 1989.
- European pharmacopoeia, 4th ed. European directorate for the quality of medicine. London; 2001. p. 1236.
- Morimoto K, Katsumata H, Toshiyuki Y, Iwanaga K, Kakemi M, Tabata Y, et al. Evaluation of gelatin microspheres for nasal and intramuscular administration of solmon calcitonin. Eur J Pharm. Sci 2001;13:179-85.
- Sankar C, Mishra B. Development and in vitro evaluation of gelatin A microspheres of Ketorolac tromethamine for intranasal administration. Acta Pharm 2003;53:101-10.
- Kasper FK, Kushibiki T, Kimura Y, Antonios G, Tabata Y. In vivo release of plasmid DNA from composites of oligo (poly (ethylene glycol) fumarate) and cationized gelatin microspheres. J Control Release 2005;107:547-61.
- Shirui M, Chen J, Wei Z, Liu H, Bi D. Intranasal administration of melatonin starch microspheres. Int J Pharm 2004;272:37-43.
- Martin A, Bustamante P, Chun AH. In: Physical pharmacy: Physical and chemical principles in the pharmaceutical sciences. 4th ed. New Delhi; BI Waverly Pvt Ltd: 1996.
- Huang YC, Yen MK, Chiang CH. Formulation factors in preparing BTM-chitosan microspheres by spray drying method. Int J Pharm 2000;242:239-42.
- Tabassi SA, Razavi N. Preparation and characterization of albumin microspheres encapsulated with propranolol hydrochloride. DARU 2003;11:137-41.
- Patel JK, Patel RP, Amin AF, Patel MM. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS Pharm Sci Tech 2005;6: E49-55.
- Soppimath KS, Aminbhavi TM. Water transport and drug release study from cross linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur J Pharm Biopharm 2002;53:87-9.
- Fandueanu G, Constantin M, Dalpiaz A, Bortolotti F, Cortesi R, Ascenzi P, et al. Preparation and characterization of starch/ cyclodextrin bioadhesive microspheres as platform for nasal administration of Gabexate Mesylate (Foy®) in allergic rhinitis treatment. Biomaterial 2004;25:159-70.
- Rao YM, Devi KM, Rameshachary B. Stability study of Refampicin mucoadhesive nasal drops. Indian J Pharm Sci 1999;61:366-70.
- Kulkarni GT, Gosthamarajan K, Suresh B. Stability testing of pharmaceutical products: An overview. Indian J Pharm Edu 2004;38:194-202-20.
 , MS2
, MS2  , MS3
, MS3 , MS4
, MS4 and MS5
and MS5