- *Corresponding Author:
- R. K. Maheshwari
Department of Pharmacy, S. G. S. I. T. S., 23 Park Road, Indore-452 003, India
E-mail: rkrkmaheshwari@yahoo.co.in
| Date of Submission | 12 May 2007 |
| Date of Revision | 31 May 2010 |
| Date of Acceptance | 25 September 2010 |
| Indian J Pharm Sci, 2010, 72 (5): 649-651 |
Abstract
Highly concentrated aqueous solutions of various hydrotropic agents like sodium benzoate, sodium salicylate, sodium acetate, sodium citrate, nicotinamide and sodium ascorbate have been observed to enhance aqueous solubilities of a large number of poorly water-soluble drugs. In the present investigation hydrotropic solubilization technique has been employed to solubilize poorly water-soluble aspirin (analgesic and antipyretic drug) by 0.5 M ibuprofen sodium solution to carry out titrimetric analysis of aspirin in tablet dosage form. Results of analysis by proposed method and Phamacopoeial method are very comparable. Proposed method is new, rapid, simple, accurate, and reproducible. Statistical data proved the accuracy, reproducibility and the precision of proposed method.
Keywords
Aspirin, hydrotropy, ibuprofen sodium, titrimetry
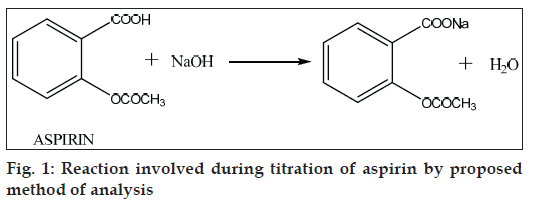
Hydrotropic solubilization involves addition of large amount of a second solute to increase the aqueous solubility of the first solute. Concentrated aqueous solutions of a large number of hydrotropic agents were successfully used to enhance the aqueous solubility of many poorly water-soluble drugs [1-15]. Sodium benzoate, sodium salicylate, sodium acetate, sodium citrate, nicotinamide and sodium ascorbate are most popular examples of hydrotropic additives. A dramatic increase in the solubility of aspirin (acetyl salicylic acid) in hydrotropic solution of 0.5 M ibuprofen sodium solution was observed. Enhancement in solubility of aspirin in 0.5 M ibuprofen sodium solution was more than 5 fold as compared to solubility in distilled water. Therefore, it was thought worthwhile to solubilize aspirin in hydrotropic solution to carryout titrations with sodium hydroxide solution. Back titration method of Pharmacopoeia [16] is time consuming. Proposed method is rapid and involves direct titration according to the eqn depicted in fig. 1.
All chemicals and solvents used were of analytical grade. Aspirin was obtained as gift sample from Shree Pharmaceuticals, Indore. Tablet formulations were purchased from the local market.
For the preparation of 0.5 M ibuprofen sodium solution, 10 g sodium hydroxide was dissolved in 200 ml distilled water. Ibuprofen (51.6 g) was added little at a time and stirred continuously to dissolve. After complete addition of ibuprofen, the pH was adjusted between 7.5-8.0 with additional sodium hydroxide solution to assure complete neutralization of ibuprofen, then volume was made up to 250 ml with distilled water.
Solubility of aspirin was determined at 27±1º. An excess amount of drug was added to screw capped 30 ml glass vials containing distilled water and 0.5 M ibuprofen sodium solution, separately. These vials were shaken mechanically for 12 h at 27±1º in an orbital flask shaker (Khera Instrument Pvt. Ltd., India). These solutions were allowed to equilibrate for 24 h and then centrifuged for 5 min at 2000 rpm (Remi, India). The supernatant of each vial was filtered through a Whatman filter paper No. 41. Filtrates were analyzed for drug contents to determine the solubilities.
For the quantitative estimation of aspirin tablet formulations using the proposed method of analysis, twenty tablets were weighed and finely powdered. An accurately weighed quantity of tablet powder equivalent to about 500 mg of aspirin was taken in a conical fl ask. Seventy-five milliliters of 0.5 M ibuprofen sodium solution was added and the fl ask was shaken for about 5 min to solubilize aspirin from tablet powder. Then, titration was performed with 0.5 M sodium hydroxide using phenolphthalein solution as indicator. Blank determination was performed for necessary correction and amount of aspirin was calculated (Table 1) Aspirin content was thus determined by using the factor; each ml of 0.5 M sodium hydroxide is equivalent to 90.08 mg of aspirin.
| Tablet | Label claim per | Method of | Percent label claim estimated* | Percent coefficient | Standard error |
|---|---|---|---|---|---|
| formulation | tablet (mg) | analysis | (mean±S.D.) | of variation | |
| I | 150 | PM | 97.73 0.559 | 0.572 | 0.322 |
| BPM | 98.51 0.899 | 0.913 | 0.519 | ||
| II | 75 | PM | 100.66 1.443 | 1.434 | 0.833 |
| BPM | 99.87 2.007 | 2.010 | 1.159 |
*Average of three determinations, BPM - British Phamacopoeial method, PM – ibuprofen sodium method.
Table 1: Results Of Analysis Of Commercial Aspirin Tablets With Statistical Evaluation
Using British Pharmacopoeial method [16] of analysis, tablet powder equivalent to 0.5 g of aspirin was transferred to a conical flask containing 30 ml of 0.5 M sodium hydroxide and the mixture was boiled gently for 10 min. Excess of alkali was titrated with 0.5 M hydrochloric acid using phenophthalein solution as indicator. Operation was repeated without substance being examined. The difference between the titrations represented the amount of alkali required. Aspirin content was thus determined (Table 1) using the factor; each ml of 0.5 M sodium hydroxide is equivalent to 45.04 mg of aspirin.
For validation and reproducibility of the proposed method, recovery studies were performed by adding definite known quantities of aspirin pure drug (spiked drug) in preanalyzed tablet powder (as shown in Table 2) and determining the aspirin content by the proposed method (mentioned earlier). The results of analysis are presented in Table 2. Each type of analysis was done in triplicate.
| Tablet formulation | Amount of aspirin in preanalzed | Pure aspirin added | Percent recovery estimated Percent coefficient | Standard | |
|---|---|---|---|---|---|
| tablet powder taken (mg) | (spiked) mg | (mean±S.D.) | of variation | error | |
| I | 250 | 25 | 101.38 2.208 | 2.178 | 1.275 |
| 250 | 50 | 98.65 0.820 | 0.831 | 0.473 | |
| II | 250 | 25 | 99.33 1.762 | 1.774 | 1.017 |
| 250 | 50 | 99.68 0.811 | 0.814 | 0.468 | |
*Average of three determinations, BPM - British phamacopoeial method, PM – Ibuprofen sodium method.
Table 2: Recovery Studies For Spiked Concentration Of Aspirin Added To The Preanalysed Tablet Powder
Results of solubility analysis indicated that enhancement in solubility of aspirin in 0.5 M ibuprofen sodium solution was found to be more than 5 fold as compared to the solubility in distilled water. This hydrotropic solubilization phenomenon was utilized in titrimetric analysis to solubilize poorly water-soluble aspirin to carryout titration with sodium hydroxide solution by use of direct titration method.
It is evident from Table 1 that the mean percent label claims of aspirin estimated in tablet dosage form by British Phamacopoeial method is 98.51 (formulation-I) and 99.87 (formulation-II). In proposed method of analysis, the mean percent label claims of aspirin estimated by use of 0.5 M sodium ibuprofen were 97.73 (formulation-I) and 100.66 (formulation-II) which are close to 100. Also the results of analysis by proposed method are very comparable to the results of analysis by standard British Phamacopoeial method. This indicates the accuracy of the proposed method. Validation of proposed method is further confirmed statistically by low values of standard deviation, % coefficient of variation and standard error (Table 1).
Accuracy, reproducibility and precision of the proposed method are further confirmed by recovery studies. The values of the mean percent recovery estimated were 101.38 and 98.65 in case of formulation-I and 99.33 and 99.68 in case of formulation-II (Table 2). These values are close to 100 indicating the accuracy of the proposed method. Accuracy, reproducibility and precision of the proposed method is further confirmed by low values of standard deviation, % coefficient of variation and standard error (Table 2).
British Phamacopoeial method to analyze aspirin tablets is time consuming. Proposed method to analyze aspirin tablet is rapid method. British Phamacopoeial method to analyze aspirin tablets requires boiling step while proposed method does not require boiling step. Thus, it may be concluded that proposed method to analyze aspirin in tablet dosage form using solution of hydrotropic additive (0.5 M ibuprofen sodium solution) is new, rapid, simple, accurate, reproducible and precise and can be successfully employed in routine analysis of aspirin in tablets. In future, attempts can be made to solubilize other water insoluble drugs in concentrated solutions of hydrotropic additives to carryout titrations precluding the use of costlier, unsafe, pollution producing organic solvents.
Acknowledgements
The authors are thankful to M/s. Shree Pharmaceuticals, Indore for supplying gift sample of aspirin bulk sample.
References
- Maheshwari RK. A Novel Application of Hydrotropic Solubilization in the Analysis of Bulk Samples of Ketoprofen and Salicylic acid. Asian J Chem 2006;18:393-6.
- Maheshwari RK. New Application of Hydrotropic Solubilization in the Spectrophotometric Estimation of Ketoprofen in Tablet Dosage Form. Pharma Review 2005;3:123-5.
- Maheshwari RK. Application of Hydrotropic Solubilization in the Analysis of Aceclofenac. Asian J Chem 2006;18:1572-4.
- Maheshwari RK. Analysis of Frusemide by Application of Hydrotropic Solubilization Phenomenon. Indian Pharmacist 2005;4:55-8.
- Maheshwari RK. Spectrophotometric Determination of Cefixime in Tablets by Hydrotropic Solubilization Phenomenon. Indian Pharmacist 2005;4:63-8.
- Maheshwari RK. Novel Application of Hydrotropic Solubilization in the Spectrophotometric Analysis of Tinidazole in Dosage Form. Asian J Chem 2006;18:640-4.
- Maheshwari RK. Spectrophotometric Analysis of Amoxycillin in Tablets using Hydrotropic Solubilization Technique. Asian J Chem 2006;18:3194-6.
- Maheshwari RK, Chaturvedi SC, Jain NK. Application of Hydrotropic Solubilization Phenomenon in Spectrophotometric Analysis of Hydrochlorothiazide Tablets. Indian Drugs 2005;42:541-4.
- Maheshwari RK, Chaturvedi SC, Jain NK. Titrimetric Analysis of Aceclofenac in Tablets Using Hydrotropic Solubilization Technique. Indian Drugs 2006;43:516-8.
- Maheshwari RK, Chaturvedi SC, Jain NK. Application of Hydrotropic Solubilization Phenomenon in Spectrophotometric Analysis. Indian Drugs 2005;42:760-3.
- Jain NK, Agrawal RK, Singhai AK. Formulation of aqueous injection of carbamazepine. Pharmzie 1990;45:221-4.
- Jain NK, Patel VV. Hydrotropic Solubilization. Eastern Pharmacist 1986;29:51-4.
- Etman MA, Hada AH. Hydrotropic and Cosolvent Solubilization of Indomethacin. Acta Pharm 1999;49:291-8.
- Poochikian GD, Cradock JC. Enhanced Chartreusin Solubility by Hydroxyl Benzoate Hydrotropy J Pharm Sci 1979;68:728-32.
- Darwish A, Florence AT, Saleh AM. Novel Application of Hydrotropic Solubilization in the Spectrophotometric Analysis. J Pharm Sci 1989;78:577-81.
- British Pharmacopoeia, London: Her Majesty’s Stationary Office; 2002. p.1943.