R. Thadakapally, Arshiya Aafreen, J. Aukunuru*, M. Habibuddin1 and S. Jogala
Department of Pharmaceutics, Mother Teresa College of Pharmacy, Osmania University
1Adept Pharma and Bio science Excellence Pvt. Ltd., Hyderabad, India
- Corresponding Author:
- J. Aukunuru
Department of Pharmaceutics, Mother Teresa College of Pharmacy
E-mail: aukunjv@gmail.com
| Date of Submission | 16 February 2016 |
| Date of Revision | 29 November 2015 |
| Date of Acceptance | 18 December 2014 |
| Indian J Pharm Sci 2016;78(1):65–72 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The aim of present research was to prepare novel serum stable long circulating polymeric nanoparticles for curcumin with a modification to the well known and novel nanoparticle albumin bound technology. polyethylene glycol-albumin-curcumin nanoparticles were prepared using serum albumin and poly ethylene glycol using desolvation technique. Nanoparticles were characterized for encapsulation efficiency, particle size and surface morphology. Drug excipient compatibility was determined using fourier transform infrared spectroscopy. Physical state of the drug in the formulations was known by differential scanning colorimetry. In vitrorelease and solubility of the drug from nanoparticles were determined. In vivo Drug release, tissue uptake and kupffer cell uptake was determined with optimized nanoformulation in rats after intravenous administration. Cell viability assay was determined using breast cancer cell line MD-MB-231. Entrapment efficiency for prepared nanoparticle was above 95%. The polyethylene glycol-albumin-curcumin nanoparticles exhibited an interesting release profile with small initial burst followed by slower and controlled release. Solubility of the drug from the formulation was increased. A sustained release of drug from nanoparticles was observed for 35 days in both in vitro and in vivo studies with the optimized formulation. Polyethylene glycol-albumin-curcumin nanoparticles showed lesser liver and kupffer cell uptake as compared to that of curcumin-albumin nanoparticles suggesting the bestowment of stealthness to nanoparticles with pegylation. Also, the antiproliferative activity of polyethylene glycol-albumin-curcumin nanoparticle formulation was more as compared to native curcumin. Polyethylene glycol-albumin-curcumin nanoparticles thus developed can be conveniently used in breast cancer with improved efficacy compared to conventional therapies and as an alternate to nanoparticle albumin bound technology which is used in producing Abraxane, albumin based breast cancer targeting nanoparticles of paclitaxel.
Keywords
Curcumin, albumin, long circulating polymeric nanoparticles, PEGylation, desolvation, breast cancer
Curcumin, a hydrophobic polyphenol called diferuloyl methane, which is extracted from the rhizome of Curcuma longa (turmeric) belongs to zingiberaceae family[1]. Curcumin has been associated with many pharmacological activities which include antiproliferative, anticancer, antiangiogenic, antidiabetic, antioxidant and antiinflammatory activities. The major limitations of curcumin is rapid systemic elimination, degradation at alkaline pH, limited oral bioavailability[2,3]. To overcome the limitations of curcumin several novel drug delivery carriers has been tagged to curcumin. These delivery systems increased its aqueous solubility, stability, oral bioavailability and achieved controlled and targeted delivery. For instance, Jithan et al., developed curcumin albumin nanoparticles and investigated its application in breast cancer[4]. The results of the study indicated that the solubility of curcumin in aqueous dissolution medium was increased with the nanoformulation compared to its pure solid form and the formulation can be successfully used in breast cancer. Sajomsang et al., developed novel pH responsive micelles for oral curcumin delivery and demonstrated enhanced uptake of the formulation into cervical cells[5]. Madane and Mahajan developed curcumin loaded nanostructured lipid carriers for nasal administration to target brain[6]. The results demonstrated enhanced brain uptake of curcumin after nasal administration of the formulations. In this regard, another technology that can be attempted is stealth nanoparticles. In this study, we particularly focused on pegylated albumin nanoparticles. PEG was introduced in the 90’s to modify the surface of liposomes for improved pharmacokinetics (PK) after intravenous (i.v) administration thereby promoting stealthness[7]. PEG is used to impart the in vivo longevity to drug carriers. PEGylation reduces the protein binding (opsonization) which helps in escape from surveillance of liver, spleen and bone marrow. These are the major reticuloendothelial system (RES). Stealth nanoparticles circulates longer time in the blood; it leads to more accumulation in the tumor, interacts more with target and enhances tumor targeting. Lupi et al., developed pegylated polycaprolactone (PCL) based nanoparticles and demonstrated enhanced biodistribution properties and tumor retention[8]. SenthilKumar et al., developed pegylated polylactidecoglycolide (PLGA) nanoparticles for docotaxel and demonstrated long plasma circulation and enhanced efficacy of the formulation in solid tumors compared to its solution form[9]. Similarly, many such studies can be found in literature with regard to pegylated stealth nanoparticles. This illustrates the success of these types of formulations for clinical use. Thus, similar technology can be attempted for curcumin. This technology particularly focusing at pegylated albumin nanoparticles has not been applied to curcumin as per the best of our knowledge.
Albumin nanoparticle technology is a very familiar technology to research scientists and industrial personnel[4,10]. Nanoparticle albumin-bound (NAB) technology which has been developed by Abraxis BioScience is a novel patented technology. This technology exploits the natural properties of albumin to achieve a safe, solvent-free, efficient and targeted drug delivery. The first formulation that entered the market and became successful is Abraxane. This incorporated paclitaxel in NAB technology. In one of our previous studies, we used similar technology to develop curcumin-albumin (CA) nanoparticles[4]. Solubility of the drug was enhanced with this formulation and the formulation was more effective in breast cancer compared to solution form of the drug. In this study, we further aimed at improvement of the formulation by conjugating pegylation to NAB technology. Therefore, the main objective of this study was to prepare and evaluate serum stable long circulating PEG-albumin-curcumin (PAC) nanoparticles intended to be administered in breast cancer, capable of improving the therapeutic index of the drug by increasing its permeability, solubility and tumour accumulation.
Materials and Methods
Curcumin was purchased from Yucca enterprises, Mumbai. Curcumin contained 94% curcuminoid content. Solubility in ethanol was 1 mg/ml and in DMSO was >11 mg/ml. Bovine serum albumin (BSA) and PEG (6000, 4000, and 1500) were purchased from S. D. Fine Chem., Mumbai and all other chemicals and reagents used were of highest purity grade and commercially available. Magnetic stirrer, cyclomixer, ultracentrifuge, and centrifuge from (Remi equipments Pvt. Ltd) were used. A bath sonicator from (Merck) was used. A Shimadzu UV/Vis spectrophotometer, A Waters HPLC was used for in vitro drug release and drug content assay studies.
Preparation of PAC nanoparticles:
PAC nanoparticles were prepared by desolvation technique. Polymeric solution was prepared by taking bovine serum albumin 200 mg and different polyethylene glycols (1500, 4000, 6000) were dissolved in 2.0 ml of purified distilled water (Table 1). Drug solution was prepared by weighing required amount of curcumin 100 mg and dissolved in 8 ml of ethanol. Prepared drug solution was added drop wise to the polymeric solution under magnetic stirring (500 rpm). To this, 0.11 ml of 8% glutraldehyde in water (v/v) was added to cross-link the desolvated BSA nanoparticles and cross linking process was performed for over 24 h. The obtained suspension was subjected to differential centrifugation for 5 cycles and redispersion of the pellet to the original volume in distilled water. Each redispersion step was carried out using bath sonicator. The obtained suspension was lyophilized using freeze dryer (Mini lyodel, Delvac, Chennai, India). The dried particles were collected as PAC nanoparticles. Curcumin-albumin (CA) nanoparticles were also prepared as a control for this study. In this case, PEGs were not added to the aqueous phase. The procedure of preparation of nanoparticles is as described by Jithan et al., 2011[4].
Characterization of PAC nanoparticles:
| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| Curcumin (mg) | 100 | 100 | 100 |
| BSA (mg) | 200 | 200 | 200 |
| PEG (6000) (mg) | 20 | - | - |
| PEG (4000) (mg) | - | 20 | - |
| PEG (1500) mg | - | - | 20 |
| Ethanol (ml) | 8 | 8 | 8 |
| Distilled water (ml) | 2 | 2 | 2 |
| Glutaraldehyde (8% v/v) (ml) | 0.11 | 0.11 | 0.11 |
Table 1: Composition and amount of materials used in the preparation of curcumin nanoparticles.
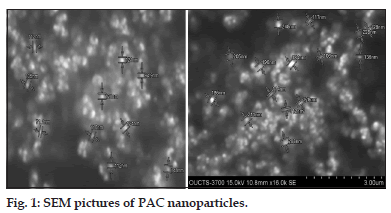
Surface morphology and particle size were characterized by scanning electron microscopy (SEM) (S-3700). The nanoparticle formulations F1, F2, and F3 were taken and spread over and after complete drying images of the samples were captured and analyzed.
Fourier transform infrared spectroscopy:
FTIR spectra of various samples which include curcumin, bovine serum albumin, PEG 6000, PEG 4000, PEG 1500, nanoparticulate formulations F1, F2, and F3 were obtained using FTIR spectrophotometer. About 5 mg of sample is mixed with dry KBr IR powder and grounded to obtain fine powder. This was compacted under pressure for approximately 3 min and a spectrum was obtained over a wave number region of 400-4000 cm-1 to record the characteristic peaks of different samples.
Differential scanning colorimetry:
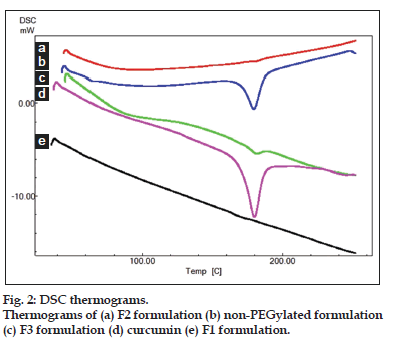
Differential scanning colorimetry is a thermal analytical method used to study thermal transition and to study the physical change of drug from one state to other state. About, 10 mg of samples such as curcumin, F1, F2, and F3 were analyzed in an open aluminium pan and heated at scanning rate of 10o min between 0o and 400o. Magnesia was used as the standard reference material. The thermal transitions were then recorded.
Zeta potential:
The Zeta potential is used to measure the electric charge at the surface of the particles, indicating the stability of nanoparticles. The Zeta potential was measured by using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Zeta potential was characterized for all the three formulations F1, F2, and F3.
Drug entrapment efficiency:
Drug entrapment efficiency for samples F1, F2, and F3. were determined. At the end of the the centrifugation step, the supernatants was obtained and analyzed for the drug using UV/Vis spectrophotometer. The amount of the drug encapsulated is the drug that is subtracted from the initial amount taken with that of the amount obtained in the supernatants. Encapsulation Efficiency (EE) is calculated using the formula, Encapsulation Efficiency=(Amount of the initial drug–Amount of the drug in the supernatants)/(Amount of the initial drug)×100.
In vitro drug release:
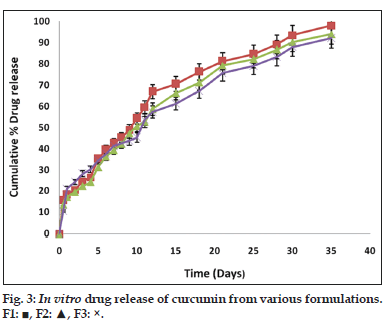
An aliquot of 10 mg of PAC nanoparticles suspended in 5 ml of phosphate buffer were taken in two side open test tube. To one side of the test tube a dialysis membrane (12,000 to 14,000 Mwt cut off) was attached. This was placed in a beaker containing 100 ml of phosphate buffer pH 7.4 (PBS). The PBS was made 1% ascorbic acid and 0.1% butylated hydroxyl toluene to prevent degradation. Entire set up was kept for magnetic stirring at room temperature at 100 rpm. The samples were withdrawn at time intervals such as 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 24 h, 2 days till 35 days. Five milliliter of the medium was withdrawn and replaced with 5 ml of fresh medium of phosphate buffer pH 7.4. The samples were filtered by using 0.2 μ sterile filter and its absorbance was measured by using UV/Vis spectrophotometer at λmax 425 nm.
Solubility studies:
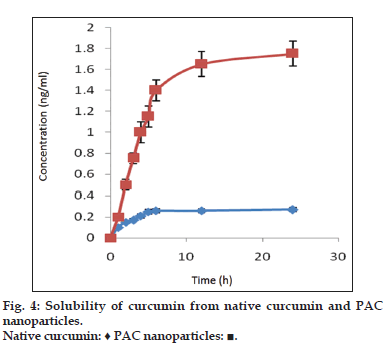
The solubility of native curcumin and curcumin from PAC nanoparticles was investigated using the USP rotating paddle dissolution apparatus at 100 rpm and 37±0.5°. A 100 mg of the formulation was weighed accurately and added to 500 ml of release medium. An aliquot of 5 ml of samples were withdrawn using a syringe filter (0.2 μ sterile filter) at various time intervals such as 30 min, 1, 2, 4, 6, 8, 12, 24 h replaced with fresh dissolution medium. The samples were analyzed using UV/Vis spectrophotometer at 425 nm.
Pharmacokinetics:
Animal experiments were conducted after taking approval from institutional animal ethics committee, Geetanjali College of Pharmacy, Hyderabad (IAEC No.1684/PO/a/12/CPCSEA). The protocols used were as per CPCSEA guidelines. The study was conducted using 12 Wister rats divided into 3 groups (n=4), each rat was injected via lateral vein. Native curcumin dissolved in ethanol and PEG was injected for group 1, CA nanoparticles were injected into Group 2 and optimized PAC nanoparticles were injected for group 3.
The method of preparation of the formulations that were administered is as described as, curcumin solution: solutions of curcumin (10 mg/ml) for intravenous administration to the rats was prepared by dissolving the drug in 0.5 ml of a 50:50 mixture of polyethylene glycol (PEG) 400 and 95% ethanol. CA and PAC nanoparticular suspension: CA and PAC nanoparticles (containing 10 mg of the drug) were suspended in 0.5 ml normal saline (0.9% w/v) for intravenous injection to rats.
Ten mg of the drug was selected in pharmacokinetic studies as the same amount of the drug yielded good results in our earlier studies[4]. Curcumin levels were determined at 1, 3, 6, 12, 24 h and 2, 3, 4 days up to 35 days in group 1 and group 3. The assay was performed using a HPLC method previously validated for curcumin[11]. Drug levels from group 1, group 2 and group 3 in various tissues like liver, kidney, brain were determined by isolating tissues from the rats at the end of the kinetic study. The tissues were chopped into small pieces and minced with dichloromethane. The resulted solution was evaporated to dryness and reconstituted with mobile phase and concentrations of the drug in the tissues were analyzed by performing HPLC with mobile phase methanol:water (20:80). Kupffer cells were obtained from liver samples by the previousl published method and curcumin levels in these cells were also obtained[12].
Cell viability assay:
The cell viability assay with pure curcumin and optimized PAC nanoparticles was carried out in MDA-MB-231 cell lines following the protocol previously described[4]. The method is described in detail as below.
MDAMB231 and BT 549 cell lines were grown as adherent in DMEM medium supplemented with 10% fetal bovine serum, 100 μg / ml penicillin, 200 μg/ml streptomycin, 2 mM L-glutamine, and culture was maintained in a humidified atmosphere with 5% CO2.
Preparation of samples for cytotoxicity:
Formulations and blank nanoparticles were dispersed/ dissolved in sterile PBS, PEG and ethanol to desired concentrations for the treatment as follows.
Cytotoxicity evaluation:
Cytotoxicity of formulations was determined by MTT assay based on mitochondrial reduction of yellow MTT tetrazolium dye to a highly colored blue formazan product. 1x104 Cells (counted by Trypan blue exclusion dye method)) in 96- well plates were incubated with formulations with series of concentrations for 48 h at 37° in DMEM with 10% FBS medium. Then the above media was replaced with 90μl of fresh serum free media and 10 μl of MTT reagent (5 mg/ml) and plates were incubated at 37° for 4 h, there after the above media was replaced with 200 μl of DMSO and incubated. The absorbance was measured at 570 nm on a spectrophotometer (spectra max, Molecular devices).
Statistical analysis:
All experiments were done more than four times and the data were expressed as mean ± standard deviation and Tukey’s post-hoc test was done to analyze significance of difference between different groups using the statistical analysis software package SPSS (version 16.0, IBM, USA).
RESULTS
Fourier transform infrared spectroscopy studies were conducted to know the drug excipient interactions and the presence of drug and polymer such as curcumin, bovine serum albumin and PEG in the formulations. The spectra of curcuminoid and polymer are not characteristically different from the spectra of prepared PAC nanoparticles. The peaks appearing at 1508.38 cm-1 for curcumin, 1558 cm-1 for bovine serum albumin, 1111.03 cm-1 for PEG 6000, 11.09 cm-1 for PEG 4000 and 1500, which also appeared in the PAC based curcumin nanoparticles which represents the chemical stability of curcumin in the formulation (fig not shown). Particle size and surface morphology of the PAC nanoparticles was conducted by SEM. The average size of the particles F1, F2 and F3 were found to be 198, 179 and 112 nm, respectively. The surface morphology of the particles was found to be smooth and spherical (fig. 1). The results obtained from the DSC were shown in the fig. 2. This study was conducted to know the physical state of curcumin in the PEGylated nanoparticle formulations. The DSC results confirm that the drug is in amorphous state in the formulations. Zeta potential was conducted for all the formulations and the results obtained were found to be -33±5, -31±7, -32±3 mV for the four formulationsF1, F2, and F3, respectively. The results obtained by the drug entrapment efficiency were shown in the Table 2. The drug was entrapped or encapsulated into the PAC nanoparticles were 96.6, 96 and 95.8% for formulationsF1, F2, and F3, respectively. In vitro drug release studies were conducted for 45-days to obtain the complete release of the drug from the PEGylated nanoparticles. The results obtained from the release of nanoparticles for 35 days were shown in the (fig. 3) and was found to be 99.2, 93.56 and 90.2% forF1, F2, and F3 formulations. It is observed that formulations F1, F2, and F3 which were PEGylated by using different molecular weights of PEG shows slow and sustain release. Solubility of the pure curcumin has enhanced from 0.2 ng/ml to 1.8 ng/ml (fig. 4) upon formulating into PAC nanoparticles. The release of the drug after 2 h from the PEGylated nanoparticles were found to be 2.6, 3.4 and 4%, respectively for the three formulations. All these PEGylated nanoparticles show fast release initially due to the drug present on the surface of the nanoparticles was released and later the drug present from the matrix is released to obtain a sustain release.
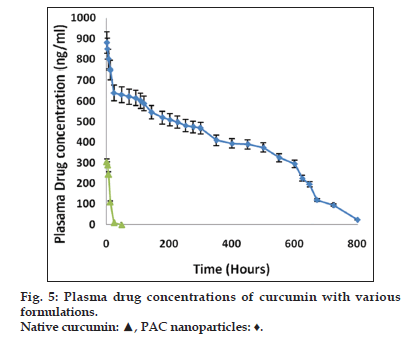
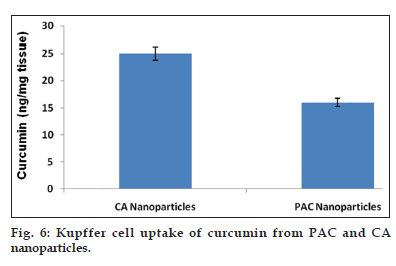
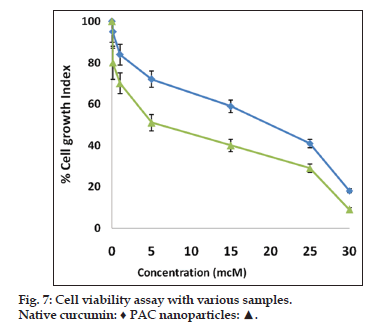
Nanoparticulate curcumin was designed to achieve prolonged systemic drug levels with optimized formulation. In this study native curcumin and PAC nanoparticles of 10 mg dose were injected and plasma levels of curcumin were determined for 45 days. A HPLC method previously developed and validated was used. There was no internal standard in the method. The minimum detection and minimum quantification of curcumin with the HPLC method used was 0.25 and 0.4 μg/ml, respectively. The results showed maximum serum availability of 880 ng/ml of curcumin from PEGylated nano particles was observed after 1 h. whereas 300 ng/ml of native curcumin was observed with curcumin dissolved in ethanol (fig. 5). Our results suggested the slow release of curcumin from PAC nanoparticles and sustained plasma levels were achieved for 35 days with higher plasma levels noticed for PAC nanoparticles. Upon intravenous administration of pure curcumin, the drug disappeared rapidly in plasma and within 1 h the drug totally disappeared in plasma. AUC of the curcumin with PAC nanoparticles was several fold higher compared to that of the naked curcumin. Tissue uptake studies indicated that with CA nanoparticles, liver and KC uptake was increased compared to PAC nanoparticles (fig. 6 and Table 3). This suggest bestowment of stealthness to PAC nanoparticles compared to that of CA nanoparticles. The antiproliferation efficacy of free curcumin and PAC nanoparticles on breast cancer cell line (MDA-MB-231) were determined at different concentrations 0, 20, 40, 60, 80, 100, 120 μM for about 72 h. The results showed that PAC nanoparticles was more antiproliferative than that of native curcumin (fig. 7). Blank nanoparticles did not demonstrate any cytotoxic effects. This suggests that improved formulation against breast cancer has been developed in the form of PAC nanoparticles.
| Formulation | Percent drug encapsulated |
|---|---|
| F1 | 96.6±2.5 |
| F2 | 96±2.3 |
| F3 | 95.8±3.5 |
Table 2: Drug entrapment efficiency of curcumin nanoparticles.
Discussion
The aim of this study was to develop a better nanoparticulate formulation for curcumin that can take the molecule from bench to bedside. Curcumin is a molecule of potential use in various diseases. However, its use is restricted due to poor membrane absorption and high systemic metabolism. Its origin lies in turmeric, an ancient herbal product used for traditional and religious purposes in India. Toxicological studies clearly indicated that it is a very safe molecule and as a reason it has been investigated by various scientists for its potential to treat various diseases. In all these cases, poor membrane absorption was a major limitation. Its oral bioavailability is very low as well as its diffusion into tissues of therapeutic interest is very low. This has been attributed to its very low solubility in biological media. Its membrane permeation has been addressed in few studies. It has poor membrane permeability and this factor also has been attributed to its poor oral bioavailability[13]. In other words, its poor bioavailability is due to its poor solubility as well as its poor membrane permeation. However, in in vitro pharmacological assays it demonstrated very potent activity and thus this molecule of religious sanctity was not restricted to the shelves of chemistry scientists. Several animal studies and clinical trials conducted demonstrated potent promise with this molecule[3,4,11]. However, transfer of this molecule to the bedside is still a challenge. In this regard, our group also addressed this challenge previously by developing curcumin-albumin (CA) nanoparticles using another version of NAB technology and investigated this formulation to eradicate the problems with curcumin delivery and bring this molecule to the forefront in the arsenal to treat various diseases especially cancers[4]. The results of the earlier study indicated that a successful CA formulation has been developed and this can be used to treat breast cancer. In this study, we improvised our earlier study using pegylated formulation in the form of PAC nanoparticles.
PAC nanoparticles were successfully prepared using desolvation technique. The particles formed were of nanosize as illustrated with the results of zeta sizer and SEM pictures. SEM pictures also indicated that the particles are spherical in shape. Thus scanning electron microscopy results conforms the formation of PAC nanoparticles and also shows reduction in the size of the particles which enhances the solubility and permeability of the drug. From FTIR studies, it is clear that PEG has been incorporated into nanoparticles. Thus, the formulation can be considered as pegylated curcumin-albumin nanoparticles. The results of FTIR conforms that there are no interactions between drug and excipients used. Zetapotential was determined after fabrication of nanoparticles and the values suggest sufficient physical stability of nanoparticles[14]. The ratio of drug and excipients have no significant effect on the charge of the particles. Significant entrapment of curcumin in nanoparticles was achieved and as the molecular weight of PEG decreased there was a decrease in entrapment efficiency of the particles. In in vitro release studies, it has been demonstrated that the nanoparticles released the drug for over a period of 35-days. This suggests the possibility of sustained release of the drug in vivo as well. The solubility of the drug from the formulation was also enhanced when compared to the pure drug. This suggests that the formulation leads to enhancement in Cmax thereby enhanced the therapeutic activity of the formulation. This was further confirmed by in vivo pharmacokinetic studies. In rats, upon intravenous administration, the drug was released for over a period of 35 days with enhanced Cmax and AUC. Both the values of Cmax and AUC for PAC nanoparticles of this study were more when compared to that of CA nanoparticles of our earlier study. Thus, a better formulation has been prepared in this study when compared to our earlier study. Also, the drug was released for 35 days which indicate the extended release than that was obtained with CA nanoparticles of earlier study. The tissue uptake studies indicated that liver uptake of curcumin was more when compared with PAC nanoparticles than that of CA nanoparticles. Similarly, Kupffer cell uptake was less with PAC nanoparticles when compared to that of CA nanoparticles. This suggests bestowment of stealthness to the PAC nanoparticles compared to that of CA nanoparticles. In biological assay, PAC nanoparticles demonstrated significant anticancer activity when compared to the native drug. This is a breast cancer cell line and as a reason it can be concluded that nanoparticles intended to treat breast cancer have been successfully developed in this study.
| Tissue | Native curcumin | PAC nanoparticles | CA nanoparticles |
|---|---|---|---|
| (nmol/g) | (nmol/g) | (nmol/g) | |
| Liver | 7.5±2 | 24±6 | 31±4 |
| Lung | 1.8±0.6 | 4.5±2 | 5.6±0.9 |
| Brain | 0.5±0.1 | 1.1±0.8 | 1.6±0.4 |
Table 3: Curcumin levels in various tissues at the end of pharmacokinetic study.
Acknowledgements
The authors of the manuscript would like to acknowledge the Management of Mother Teresa College of Pharmacy for providing necessary facilities for conduction of work. The authors would also acknowledge Rapaka Sai Kishore, Geetanjali College of Pharmacy, Hyderabad, for assisting during the conduction of animal experiments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett 2008;267:133-64.
- Gong C, Deng S, Wu Q, Xiang M, Wei X, Li L, et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013;34:1413-32.
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: From kitchen to clinic. BiochemPharmacol 2008;75:787-809.
- Jithan A, Madhavi K, Madhavi M, Prabhakar K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Investig 2011;1:119-25.
- Sajomsang W, Gonil P, Saesoo S, Ruktanonchai UR, Srinuanchai W, Puttipipatkhachorn S. Synthesis and anticervical cancer activity of novel pH responsive micelles for oral curcumin delivery. Int J Pharm 2014;477:261-72.
- Madane RG, Mahajan HS. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv 2015 Jul 14:1-9. (Epub Ahead of Print)
- Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res 2003;42:463-78.
- Lupi M, Colombo C, Frapolli R, Ferrari R, Sitia L, Dragoni L, et al. A biodistribution study of PEGylated PCL-based nanoparticlesin C57BL/6 mice bearing B16/F10 melanoma. Nanotechnology 2014;25:335706.
- Senthilkumar M, Mishra P, Jain NK. Long circulating PEGylatedpoly(D,L-lactide-co-glycolide) nanoparticulate delivery of Docetaxel to solid tumors. J Drug Target 2008;16:424-35.
- Lluch A, Alvarez I, Muñoz M, Seguí MÁ, Tusquets I, García-Estévez L. Treatment innovations for metastatic breast cancer: Nanoparticle albumin-bound (NAB) technology targeted to tumors. Crit Rev OncolHematol 2014;89:62-72.
- Viswanath G, Jithan A, Reddy VM. Development of new delivery strategies to increase bioavailability of curcumin. Int J Pharm SciNanotechnol 2009;1:335-40.
- Elthuri I, Bonepally C, Kokkula S, Thadkapally R, Aukunuru J. Preparation and optimization of kupffer cell targeted catechin loaded spherical particles. Turk J Pharm Sci 2013;10:35-47.
- Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm 2011;77:275-82.
- Thadkala K, Nanam PK, Rambabu B, Sailu C, Aukunuru J. Preparation and characterization of amorphous ezetimibenanosuspensions intended for enhancement of oral bioavailability. Int J Pharm Investig 2014;4:131-7.