- *Corresponding Author:
- B. Guo
Department of Chemical Engineering, Tsinghua University, Beijing-100084, China
E-mail: bh_guo_tsing@126.com
| Date of Submission | 09 June 2016 |
| Date of Revision | 23 March 2017 |
| Date of Acceptance | 21 September 2017 |
| Indian J Pharm Sci 2017;79(6): 930-938 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study was performed to evaluate the feasibility of double emulsion technique combined with membrane emulsification to prepare microspheres for pulmonary delivery. An attempt was made to counter the shortcomings of microspheres; unsuitable size diameters and broad size distribution. Three formulation variables were optimized in order to achieve desired particle size and narrow size distribution. Compared with microspheres prepared by conventional double emulsion technique, the microsphere morphology, aerodynamic particle size, drug encapsulation efficiency and in vitro release were characterized in detail. The particles prepared by double emulsion technique combined with membrane emulsification showed the advantage on controlling the particle size to meet the requirement of respiration, as well as on encapsulation efficiency. Overall, this strategy could be promising for the application of pulmonary delivery.
Keywords
Protein drug, pulmonary delivery, mPEG-PLGA microspheres, membrane emulsification, narrow size distribution
In the past several years, pulmonary drug delivery has attracted much attention as a non-invasive means of drug administration [1]. Due to the characteristic features of the lungs such as sufficient surface area (approximately 100 m2), ample blood supply, avoidance of hepatic firstpass metabolism and low enzymatic metabolism [2], pulmonary drug delivery is used as the route for local as well as systemic diseases [3-5]. However, the vigorously mucociliary clearance in the upper airways and regular respiration could lead to the loss of sustained drug absorption in the lung, which hinders the development of pulmonary drug delivery. It is reported that particles with aerodynamic diameter between 1 and 5 μm could deposit and accumulate in the deep lungs [6]. Therefore, the strategy to control the size of particles could be one of effective ways to circumvent the mentioned problem for pulmonary delivery.
In this paper, membrane emulsification combined with double emulsion technique is developed to manufacture the microspheres for pulmonary delivery. Due to the linear relationship between the diameter of the microsphere and the pore size of the membrane, the size of microspheres could be controlled accurately by using the membrane with desired pore size. Compared with conventional processes to prepare microspheres such as homogenization [7-10], or spray technique [11-13], the size distribution of microspheres produced by membrane emulsification technology is relatively narrow, and the particle size is easy to control. This could increase the particle performance and drug delivery efficiency, and contribute to study the release behaviour of drug [14,15].
Until now, polylactic acid (PLA) and poly lactideco- glycolide (PLGA) microspheres have been widely investigated as the polymer materials to encapsulate the drugs for sustained release. However, the hydrophobic drawback of PLA and PLGA can lead to some common problems, including a high initial burst, protein denaturation caused by the acidic microenvironment followed with degradation of PLA or PLGA [16,17], and adverse reactions, such as inflammation [18]. It is generally known that introduction of hydrophilic blocks could improve the performance of the hydrophobic polymers. Methoxy polyethyleneglycol (mPEG), has been considered as one of the most promising polymers to improve the biocompatibility of the blood contacting materials [19]. Due to the surface modifier of hydrophobic PLA/PLGA, mPEG could improve the stability of the interfacial layer between the oil and water interface [20]. Its amphipathic property could reduce the possibility of aggregation of the encapsulated protein during encapsulation and release [21-23]. Moreover, low-molecular weight mPEG is also a nontoxic, water-soluble polymer, which could be easily excreted from human body. Li et al. [24] exhibited the degradation behaviour and drug release of PLGAmPEG microparticles. Therefore, mPEG-PLGA as the biodegradable material could be used in preparing microspheres.
In this work, mPEG-PLGA microspheres prepared by a double emulsion method followed with a membrane emulsification technique were exploited. In order to obtain microspheres with narrow size distribution and proper aerodynamic diameter, the factors including volume ratio of W1/O/W2, PVA concentration were optimized. For comparison, mPEG-PLGA microspheres fabricated by conventional double emulsion method were also prepared. Then, the formulations were characterized in terms of morphology, encapsulation efficiency (EE), aerodynamic properties and in vitro drug release profiles of the microspheres in order to evaluate the feasibility of this method. Nevertheless, the scope of this study focuses on fabrication, optimization and in vitro characterization of the particles only. The degree to which this microsphere may influence its bioactivity and in vivo performance will be subjects of our future investigations.
Materials and Methods
mPEG-PLGA with a molecular weight of 38 000 Da were purchased from the Dai Gang Company (Shandong, China), in which the mPEG block had a molecular weight of 2000 Da. Polyvinyl alcohol (PVA, degree of polymerization 1700, degree of hydrolysis 87.0-89.0 %) was purchased from Aladdin company. Bovine serum albumin (BSA, Mw 66 kDa) was from Wako (Japan). SPG membrane (with pore size of 5.3 microns) was provided by SPG Technology Co. Ltd. (Japan). Fast membrane emulsification equipment (FMEM-500M) was provided by National Engineering Research Center for Biotechnology (Beijing). Homogenizer was purchased from IKA (Germany). All other reagents were of analytical grade.
Preparation of microspheres
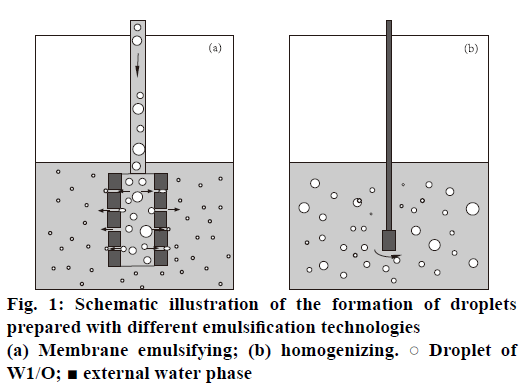
mPEG-PLGA microspheres loading BSA were prepared by water-in-oil-in-water (W1/O/W2) double emulsion-membrane emulsification method. The internal aqueous phase (W1, IAP) containing BSA (20 mg×ml-1) was first emulsified in dichloromethane (organic phase, O) containing 50 mg×ml-1 of mPEGPLGA by homogenizing for 60 s to form a primary emulsion (W1/O). The W1/O was further poured into the external aqueous phase (W2, EAP) containing 1 % w/v PVA and 0.9 % w/v NaCl to prepare coarse double emulsions. These coarse double emulsions were then extruded through the SPG membrane under a high pressure of 150 kPa to achieve the double emulsions with smaller and relatively uniform size (Figure 1a).
mPEG-PLGA microspheres prepared by only double emulsion method was obtained according to the literature [25]. The same procedure was carried out to form the primary emulsion (W1/O) as mentioned above. Then, this emulsion was injected into the external aqueous phase consisting of 1 % w/v PVA and 0.9 % w/v NaCl and homogenized for 5 min to form the secondary emulsion (W1/O/W2, Figure 1b).
These two kinds of resulting double emulsions were stirred for 4 h at room temperature for evaporation of O and hardening of the microspheres. The polymeric microspheres were then washed thrice with water and lyophilized for 48 h to get the powder formulations. Each formulation was prepared in triplicate and stored at 4° for further studies.
EE
The EE of the formulations was determined by micro bicinchoninic acid (micro-BCA) assay (Pierce, USA) [26]. Briefly, the freeze-dried microspheres (10 mg) was added into 1 ml of 1 M NaOH. The BSA concentration in the aqueous phase was measured using a UV/Vis spectrophotometer (SunriseTM, Tescan, Switzerland) at 526 nm. The EE was calculated by the following Eqn. 1: EE (%)=m/m0×100, where m0 was the theoretical amount of BSA added in the microspheres and m was the amount of BSA loaded in the microspheres.
Differential scanning calorimetry (DSC)
DSC experiments were carried out using a DSC 2910 from TA Company (Boston, Massachusetts, USA). The samples were stabilized at 25° in the calorimeter before heating up to 120° with a scanning rate of 5° min-1.
Characterization of microspheres for pulmonary delivery
The microspheres were characterized for their morphology, diameter, the size distribution and the densities. The morphology of the microspheres was examined under Jeol JSM 7401F (Japan) scanning electron microscope (SEM). The volumemean diameter and particle size distribution of the microspheres was measured by laser scattering using a Mastersizer 3000 (Malvern, UK). The span value (SP) was calculated using Eqn. 2 [27]: span=D90 %– D10 %/D50 %, where DN % (N=10, 50 or 90) was the volume percentage of microspheres with diameters. Smaller SP indicated narrower size distribution.
The tapped densities of the particles were determined according to the procedure described previously [28]. An aliquot of particles from each formulation was put to a 10 (±0.05) ml graduated cylinder. The tapped density (ρt) was calculated as the ratio of sample weight (g) and the volume (ml) occupied after tapping until no further change in volume was observed. The theoretical aerodynamic diameter (da) was calculated based on the following Eqn. 3 [29]: da=dp√ρt/ρ0, where dp was the geometric mean diameters of the particle, ρ0 was the standard particle density (1 g×ml-1), ρt was the tapped density.
Flow property test
The Carr's compressibility index and the Hausner’s ratio were calculated to provide a measure of the flow properties. The Hausner’s ratio and Carr's index was determined as follows, Eqn. 4: Hausner’s ratio= ρt/ρb, Eqn. 5: Carr’s index= ρt–ρb/ρt, where ρt and ρb were the tapped density and bulk density of the microspheres, respectively. Angle of repose method [30] was also measured by falling microspheres through a funnel on the horizontal surface. The angle of repose was calculated as follows, Eqn. 6: θ=tan–1(h/r), where θ was angle of repose, h and r were the height and radius of the heap, respectively.
In vitro aerodynamic study
In vitro aerodynamic study was performed using an eight-stage Marc-II Andersen Cascade Impactor (Seak-Mar Environmental Instrument Ltd., Beijing, China). Typically, a 15-20 mg powder formulation was emitted into the impactor using a HandiHaler® (Pfizer) at a flow rate of 28.3 l/min. The aerodynamic process was conducted in triplicate. The amount of powder deposited on each stage was determined by calculating the weight changes of the glass fiber filter paper before and after passing the particle through the impactor. The mass median aerodynamic diameter (MMAD) was determined at the 50th percentile on the percent cumulative amount of particles (probability scale) versus logarithmic effective cut-off diameter (μm) plot. The emitted fraction (EF %) and the fine particle fraction (FPF %) were calculated according to the following relationships [31], Eqn. 7: EF %=mfull–mempty/ mt×100; FPF %=m(da˂4.7μm)/mstages, where mfull and mempty were the weights (mg) of capsule before and after simulating inhalation. m(da <4.7 μm), mt and mstages were the amount of particles with aerodynamic diameters less than 4.7 μm, total amount of particles and the summation of particle amounts collected from stages, respectively.
In vitro protein release studies
To study the in vitro release pattern of encapsulated BSA from the microspheres, 100 mg freeze-dried microspheres was suspended in 20 ml simulated lung fluid (SLF), prepared according to Moss formula plus 0.1 % Tween 80 at pH 7.4 and stirred at 60 rpm at 37±1° [25,32,33]. An aliquot of samples (800 μl) was withdrawn following centrifugation over a period of 28 d at various time intervals. The amount of drug released from the microspheres was measured using micro-BCA assay method as described above.
Calculations and statistics
Results are depicted as mean±SD from at least three different batches of microspheres prepared at the specified conditions. Significance between the mean values was calculated using ANOVA one-way analysis (Origin 7.0 SRO, Northampton, MA, USA). Probability values P<0.05 were considered significant.
Results and Discussion
Since microsphere morphology was important for pulmonary delivery, the formulation variables that could significantly influence the morphology of microspheres were investigated. We first studied the effect of volume ratio of W1/O (1:2.5, 1:5, 1:7.5, 1:10, 1:12.5) while keeping O/W2 ratio (v/v) constant at 1:10 and the concentration of PVA at 1 % (w/v). As shown in Table 1, the volume-based mean size diameters decreased from 2.68±0.04 μm (W1/O, 1:5) to 2.32±0.02 μm (W1/O, 1:7.5) and subsequently increased to 2.51±0.02 μm (W1/O, 1:12.5). Similarly, SP could be observed with a trend that reduced first and then increased. The minimum SP (0.55±0.02) was achieved at W1/O ratio of 1:7.5. Furthermore, SEM photograph in Figure 2 showed that microspheres with the W1/O ratio at 1:7.5 and 1:10 possessed relatively smooth surface.
| W1/O | Volume-based mean diameter (µm±SD) |
Span value (±SD) |
O/W2 | Volume-based mean diameter (µm±SD) |
Span value (±SD) |
Content of PVA (%) | Volume-based mean diameter (µm±SD) |
Span value (±SD) |
|---|---|---|---|---|---|---|---|---|
| 01:02.5 | 2.68±0.04 | 0.65±0.03 | 01:05 | 2.21±0.01 | 0.93±0.02 | 0.25 | 3.10±0.04 | 1.73±0.05 |
| 01:05 | 2.32±0.02 | 0.58±0.02 | 01:07.5 | 2.28±0.03 | 0.69±0.02 | 0.5 | 2.95±0.3 | 1.42±0.03 |
| 01:07.5 | 2.32±0.02 | 0.55±0.02 | 01:10 | 2.35±0.03 | 0.67±0.04 | 1 | 2.35±0.03 | 0.67±0.04 |
| 01:10 | 2.35±0.03 | 0.68±0.04 | 01:12.5 | 2.4±0.04 | 0.68±0.03 | 1.5 | 2.36±0.02 | 0.70±0.04 |
| 01:12.5 | 2.51±0.02 | 1.05±0.04 | 01:15 | 2.51±0.02 | 0.98±0.07 | 2 | 2.36±0.03 | 0.71±0.05 |
BSA-loaded microspheres were prepared by double emulsion method combined with membrane emulsification at different conditions. Volume-based mean size diameters decreased from 2.68±0.04 µm (W1/O, 1:5) to 2.32±0.02 µm (W1/O, 1:7.5) and subsequently increased to 2.51±0.02 µm (W1/O, 1:12.5)
Table 1: Size and size distribution of BSA-loaded microspheres
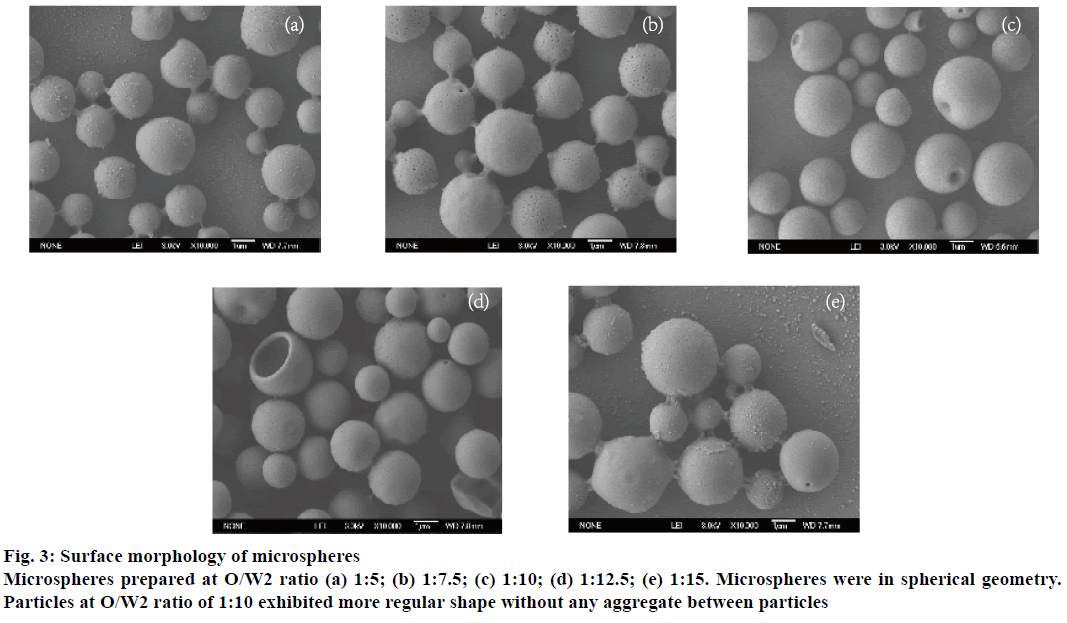
In terms of O/W2 ratio, increasing the ratio from 1:5 to 1:15 at fixed PVA concentration (1 %, w/v) and W1/O ratio (1:7.5) could slightly affect both the particle size diameters and their size distributions. The size diameters were in the range of 2.21±0.01 and 2.51±0.02 μm, and narrowest size distribution (0.67±0.03) was found at O/W2 ratio of 1:10 (Table 1). Besides, the SEM photographs (Figure 3) of these formulations showed that the microspheres were spherical geometry. Particles at O/W2 ratio of 1:10 exhibited more regular shape without any aggregate between particles.
The effect of concentration of PVA was shown in Table 1. Both the size diameters and SP were slightly enhanced when increasing PVA concentration from 1.5 to 2 %. While the diameters of microspheres were significantly decreased with the concentration of PVA increased from 0.25 to 1 %. Interestingly, it was noted that spherical microspheres with smooth surface were observed when PVA content was below 1 %. But the microspheres surface became porous when the PVA concentration was between 1 and 2 % (Figure 4). It was reported that the porous of microspheres was formed during the evaporation of organic solvent [34]. When the PVA concentration in the external water phase was higher, the evaporation rate of solvent in the oil phase was getting lower. Due to the osmotic pressure between the IAP and EAP, the internal water tended to diffuse through the oil layer to the external water phase. Therefore porous spheres were formed. Overall, the results suggested that optimized formulation was 1 % w/v PVA concentration, 1:7.5:75 ratio of W1/O/ W2 for the following experiments.
Figure 4: Morphology of microspheres
Microspheres prepared with different PVA content of (a) 0.25 %; (b) 0.5 %; (c) 1.0 %; (d) 1.5 %; (e) 2.0 %. Spherical microspheres
with smooth surface were observed when PVA content was below 1 %. But the microspheres surface became porous when the PVA
concentration was between 1 and 2 %
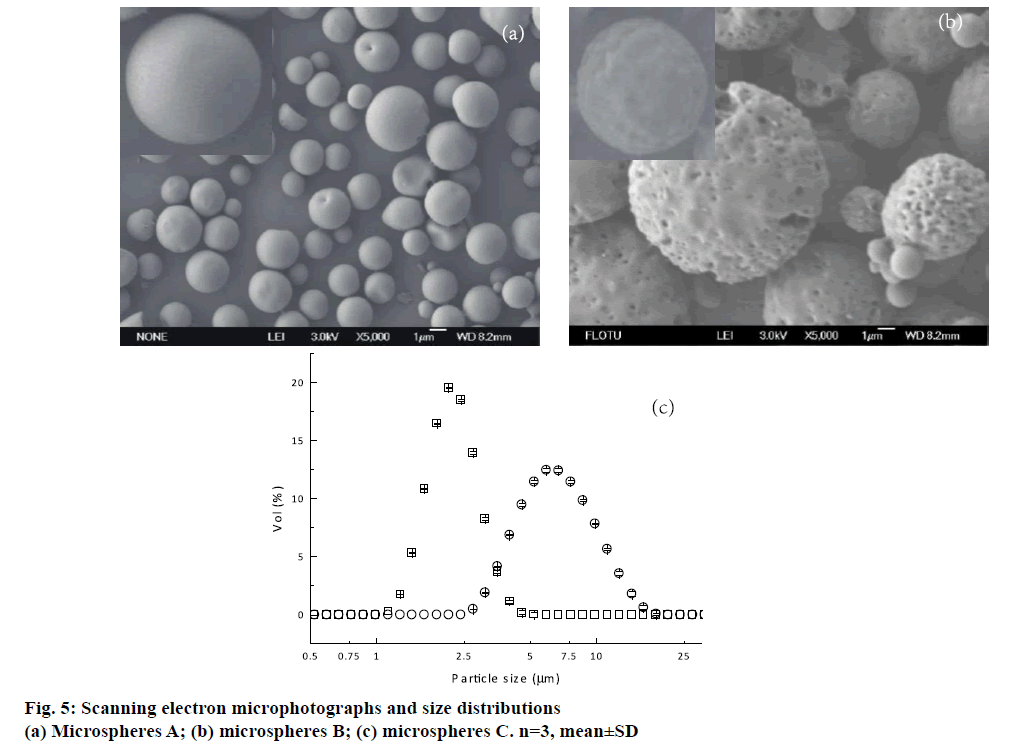
It was well known that EE was an important parameter that should be taken into account. Besides microspheres A prepared by double emulsion technique combined with membrane emulsification technique, microspheres B fabricated by only double emulsion method were also fabricated for comparison. Both of these formulations were prepared with W1/O/ W2 volume ratio of 1:7.5:75 and 1 % PVA. Higher EE was achieved from microspheres A. A possible reason was that the uneven pores existed on the surface of the microspheres B (Figure 5b). As a result, the drug located near the microsphere surface could diffuse towards EAP through the channels and pores during the solidification and washing process.
For pulmonary delivery, the aerodynamic property of microspheres is a key factor, which is influenced significantly by the shape, density, size and size distribution. Therefore, the morphologies of these microspheres prepared by a double emulsion method followed with a membrane emulsification technique A and only double emulsion method B were recorded first. As shown in Figure 5a and b, microspheres A formed regular sphericity with smooth surface. By contrast, the uneven pores and wrinkles on the surface could be observed from microspheres B. A possible reason was that the microspheres A were formed by extrusion through membrane pores. The water in IAP was stably coated by oil phase in the whole emulsification and solidification process. While microspheres B was emulsified by violent homogenization, it could increase the opportunity of collision between the droplets and then coalesced to form the new droplets. Hence, the water in IAP rearranged in the oil layer, and increased the possibility of IAP diffusing towards EAP. It happened during the polymer precipitation and therefore leaded to the particles with non-uniform pores and grooves on the surface. Microspheres B with this surface structure were not desired. It could probably lead to high initial burst release, which brought about surplus drug concentration in vivo at the beginning and decreased drug efficacy in later period.
The size distributions of microspheres A and B were shown in Figure 5c. The volume-based mean diameters (Dv) of microspheres A and B were 2.34±0.02 and 6.95±0.02 μm, respectively. Microspheres A exhibited narrow size distribution, and the SP was 0.58±0.03. However, microspheres B had a broad size distribution with the SP 1.05±0.02. Therefore, a double emulsion method combined with membrane emulsification technique showed the advantage in preparing uniform microspheres for pulmonary delivery. Though the geometric mean diameters of the formulation B were larger than 5 μm, it could not conclude that microsphere B was not suitable for pulmonary delivery. Therefore, flowability and in vitro aerodynamic study were necessary for further discussion. Flow properties of the two formulations were also evaluated in terms of Carr’ index, Hausner’s ratio and angle of repose (Table 2). It was observed that microspheres A had better rheological properties than microspheres B.
| Formulation | Carr's index (±SD) | Hausner's ratio (±SD) | Angle of repose(±SD) |
|---|---|---|---|
| A | 25.00 ± 0.17 | 1.33 ± 0.03 | 41.3° ± 1° |
| B | 33.33 ± 0.28 | 1.50 ± 0.04 | 43.2° ± 1° |
Table 2: Flow properties of the two formulations
The aerodynamic characteristics of the microspheres prepared by double emulsion method combined with membrane emulsification A and only double emulsion method B were assessed in vitro using a dry powder inhaler device and Andersen Cascade Impactor. As shown in Table 3, the MMADs of the formulation A and B were in the range of 1-5 μm, which was desirable for targeting the respiratory region of the human lung. While the MMAD of microspheres A was 1.15 μm, it was preferable aerodynamic diameter (1-3 μm) for lung deposited. Moreover, it should be noted that the theoretical primary aerodynamic diameter was not the same as the MMAD determined by Anderson Cascade Impactor for both kinds of microspheres. Hence, the MMAD as the important index for particle aerodynamic study should be investigated, and could not be absolutely taken place by theoretical calculation method.
| Encapsulation efficiency (%±SD) |
Theoretical aerodynamic diameter (µm±SD) | MMAD (µm±SD) | EF (%±SD) | FPF (%±SD) | |
|---|---|---|---|---|---|
| A | 79.60±5.47 | 1.26±0.04 | 1.15±0.01 | 95.3±0.7 | 83.7±0.7 |
| B | 65.28±4.48 | 4.28±0.07 | 3.94±0.03 | 93.1±0.7 | 33.0±1.1 |
MMAD is the mass median aerodynamic diameter. EF is the emitted fraction and FPF is the fine particle fraction. The MMADs of the formulation A and B were in the range of 1-5 µm, which was desirable for targeting the respiratory region of the human lung. Data are represented as mean±SD
Table 3: In vitro aerodynamic properties of microspheres a and b
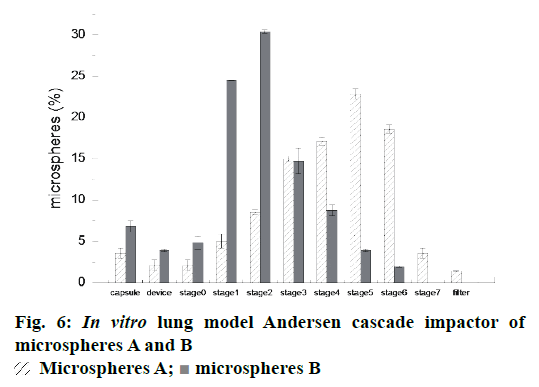
The particle fraction (%) collected on inhaler device, capsule and each stage of Anderson Cascade Impactor was plotted in Figure 6. Between stage 3 and stage 7, a higher percentage of drug deposition was observed from Andersen cascade impactor of microspheres A. This result suggested that more microspheres A were able to effectively deposit in bronchioles and alveoli when a certain amount of powder was inhaled.
Moreover, the microspheres A presented a higher emitted fraction, EF % (95.3 %) than microspheres B (Table 2). Similarly, the fine respirable fraction (FPF %) of microspheres A were found to be higher than that of microspheres B, those were 82.0±2.5 % and 33.0±1.1 %, respectively. It was well known that FRF % was the important parameter, which were promising to evaluate most of the powder deposition in the deep lung. The powders with the higher FPF could achieve better performance in pulmonary delivery. Therefore, the microspheres prepared by double emulsion combined with membrane emulsification technique may have the potential for sustained pulmonary drug delivery in terms of in vitro aerodynamic studies.
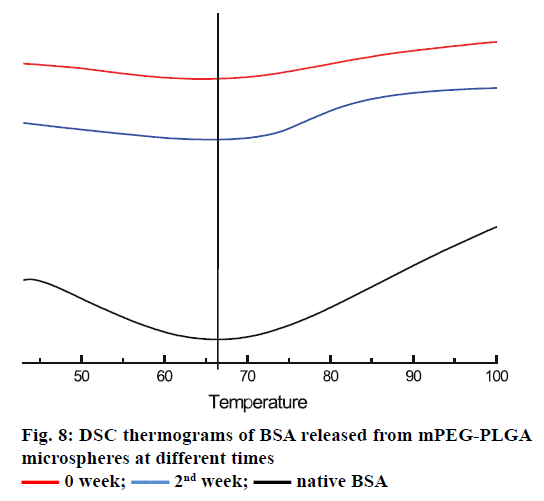
The in vitro release profile of the BSA from microspheres prepared by double emulsion technique combined with membrane emulsification showed a typical trend: initial burst release followed by cumulative drug release, as depicted in Figure 7. In the first 1 h, an initial burst release was 11.43±0.62 %. The burst release was encountered for many drugs encapsulated in PLA and PLA-based microsphere preparations, but there is no evidence that the molecular weight and size of the drugs play a role in causing the burst release [35-38]. It was reported that the burst effect was predominantly attributed to precipitating drugs on the surface of microspheres, during thermodynamically transient phases of nascent microspheres [38]. The cumulative release was performed for 35 d, and about 53.29±0.45 % of BSA was released from microspheres. In order to confirm the structural integrity of the BSA released from the mPEG-PLGA microspheres, DSC test was employed. It can be seen from Figure 8 that the native BSA and BSA released from microspheres showed the same thermal transition temperature. It was indicated that aggregation or hydrolysis did not occur during the preparation and release process.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
The work was supported by the National Natural Science Foundation of China (51073087), Project of Beijing Municipal Education Commission (SQKM201710011004), Beijing Technology and Business University Young Teacher Funding (QNJJ2015-26).
References
- LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol 2003;21:1184-91.
- Tang Cui YC. Development of Pulmonary Delivery Systems. Chin J Pharm 2001;32:560-64.
- Gonda I. The ascent of pulmonary drug delivery. J Pharm Sci 2000;89:940-45.
- Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 2007;6:67-74.
- Gessler T, Seeger W, Schmehl T. Inhaled Prostanoids in the Therapy of Pulmonary Hypertension. J Aerosol Med Pulm Drug Deliv 2008;21:1-12.
- Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, et al. Large porous particles for pulmonary drug delivery. Science 1997;276:1868-71.
- Devrim B, Bozkir A. Preparation and in vitro evaluation of surface-modified poly (lactide-co-glycolide) microparticles as biodegradable drug carriers for pulmonary peptide and protein delivery. J Microencapsul 2014;31:355-62.
- Devrim B, Bozkir A. Preparation and evaluation of double-walled microparticles prepared with a modified water-in-oil-in-oil-in-water (w1/o/o/w3) method. J Microencapsul 2013;30:741-54.
- Li X, Deng X, Huang Z. In Vitro Protein Release and Degradation of Poly-dl-lactide-poly(ethylene glycol) Microspheres with Entrapped Human Serum Albumin: Quantitative Evaluation of the Factors Involved in Protein Release Phases. Pharm Res 2001;18:117-24.
- Ruan G, Feng SS, Li QT. Effects of material hydrophobicity on physical properties of polymeric microspheres formed by double emulsion process. J Control Release 2002;84:151-60.
- Pourshahab PS, Gilani K, Moazeni E, Eslahi H, Fazeli MR, Jamalifar H. Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. J Microencapsul 2011;28:605-13.
- Pilcer G, Vanderbist F, Amighi K. Preparation and characterization of spray-dried tobramycin powders containing nanoparticles for pulmonary delivery. Int J Pharm 2009;365:162-69.
- Alhusban FA, Seville PC. Carbomer-modified spray-dried respirable powders for pulmonary delivery of salbutamol sulphate. J Microencapsul 2009;26:444-55.
- Joscelyne SM, Trägårdh G. Membrane emulsification -a literature review. J Membr Sci 2000;169:107-17.
- Liu R, Huang SS, Wan YH, Ma GH, Su ZG. Preparation of insulin-loaded PLA/PLGA microcapsules by a novel membrane emulsification method and its release in vitro. Colloids Surf B Biointerfaces 2006;51:30-8.
- van de Weert M, Hennink W, Jiskoot W. Protein Instability in Poly(Lactic-co-Glycolic Acid) Microparticles. Pharm Res 2000;17:1159-67.
- Elnaggar YSR, El-Refaie WM, El-Massik MA, Abdallah OY. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J Control Release 2014;180:10-24.
- Wu F, Jin T. Polymer-Based Sustained-Release Dosage Forms for Protein Drugs, Challenges, and Recent Advances. AAPS PharmSciTech 2008;9:1218-29.
- Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Microsphere design for the colonic delivery of 5-fluorouracil. J Control Release 2003;90:313-22.
- Meng FT, Ma GH, Liu YD, Qiu W, Su ZG. Microencapsulation of bovine hemoglobin with high bio-activity and high entrapment efficiency using a W/O/W double emulsion technique. Colloids Surf B Biointerfaces 2004;33:177-83.
- Li X, Zhang Y, Yan R, Jia W, Yuan M, Deng X, et al. Influence of process parameters on the protein stability encapsulated in poly-dl-lactide?poly(ethylene glycol) microspheres. J Control Release 2000;68:41-52.
- Li X, Deng X, Yuan M, Xiong C, Huang Z, Zhang Y, et al. Investigation on process parameters involved in preparation of poly-dl-lactide-poly(ethylene glycol) microspheres containing Leptospira Interrogans antigens. Int J Pharm 1999;178:245-55.
- Li X, Deng X, Yuan M, Xiong C, Huang Z, Zhang Y, et al. In vitro degradation and release profiles of poly-DL-lactide-poly(ethylene glycol) microspheres with entrapped proteins. J Appl Polym Sci 2000;78:140-48.
- Li J, Jiang G, Ding F. Effects of polymer degradation on drug release from PLGA-mPEG microparticles: A dynamic study of microparticle morphological and physicochemical properties. J Appl Polym Sci 2008;108:2458-66.
- Patel B, Gupta V, Ahsan F. PEG?PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release 2012;162:310-20.
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76-85.
- Saikia B, Dutta NN, Dass NN. Extraction of l-phenylalanine in hollow fiber membrane. J Membr Sci 2003;225:1-13.
- Gupta V, Rawat A, Ahsan F. Feasibility study of aerosolized prostaglandin E1 microspheres as a non-invasive therapy for pulmonary arterial hypertension. J Pharm Sci 2010;99:1774-89.
- Learoyd TP, Burrows JL, French E, Seville PC. Chitosan-based spray-dried respirable powders for sustained delivery of terbutaline sulfate. Eur J Pharm Biopharm 2008;68:224-34.
- Shariff A, Pk M, Klk P, MM. Entrapment of andrographolide in cross-linked alginate pellets: I. Formulation and evaluation of associated release kinetics. Pak J Pharm Sci 2007;20:1-9.
- El-Sherbiny IM, Smyth HDC. Controlled Release Pulmonary Administration of Curcumin Using Swellable Biocompatible Microparticles. Mol Pharm 2011;9:269-80.
- Stebounova L, Guio E, Grassian V. Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. J Nanopart Res 2011;13:233-44.
- Gupta V, Davis M, Hope-Weeks L, Ahsan F. PLGA Microparticles Encapsulating Prostaglandin E1-Hydroxypropyl-ß-cyclodextrin (PGE1-HPßCD) Complex for the Treatment of Pulmonary Arterial Hypertension (PAH). Pharm Res 2011;28:1733-49.
- Yang YY, Chia HH, Chung TS. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J Control Release 2000;69:81-96.
- Mohamed F, van der Walle CF. PLGA microcapsules with novel dimpled surfaces for pulmonary delivery of DNA. Int J Pharm 2006;311:97-107.
- Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm 2007;334:137-48.
- Liu DZ, Chen WP, Lee CP, Wu SL, Wang YC, Chung TW. Effects of alginate coated on PLGA microspheres for delivery tetracycline hydrochloride to periodontal pockets. J Microencapsul 2004;21:643-52.
- Xu Q, Czernuszka JT. Controlled release of amoxicillin from hydroxyapatite-coated poly(lactic-co-glycolic acid) microspheres. J Control Release 2008;127:146-53.


 Microspheres A;
Microspheres A;  Microspheres B
Microspheres B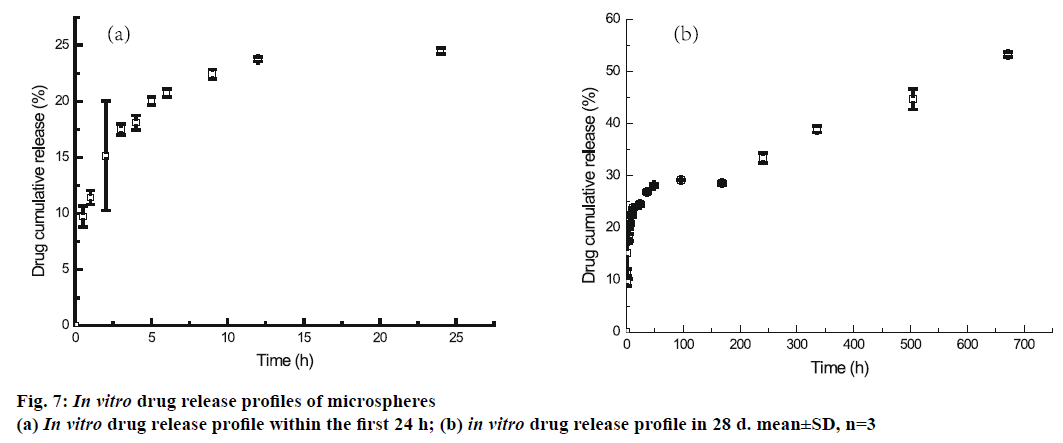
 0 week;
0 week;  2nd week;
2nd week;  native BSA
native BSA



