- *Corresponding Author:
- P. Zakeri-Milani
Liver and Gastrointestinal Diseases Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
E-mail: pzakeri@tbzmed.ac.ir
| Date of Submission | 18 August 2012 |
| Date of Revision | 10 February 2013 |
| Date of Acceptance | 12 February 2013 |
| Indian J Pharm Sci, 2013;75(2):143-148 |
Abstract
The aim of this work was to develop the best formulations for naproxen suppositories. The effects of different bases and surfactants on the physicochemical characteristics of the suppositories were determined by several tests such as weight variation, melting point, assay, hardness, and release rate. All formulations met the standard criteria for tested physicochemical parameters; weight variation (97-112%), content uniformity (97-105%), melting point (4.66-8.7 min) and hardness tests (>5400 g). Based on release rate studies, hydrophilic, and lipophilic bases without surfactants were not suitable bases for naproxen suppository. Amongst the formulations containing surfactants only Witepsol H15 with 0.5% w/w of Tween 80 and Witepsol W35 with 0.5% of cetylpyridinium chloride were suitable and released nearly complete drug during 30 and 60 min, respectively. This study demonstrates the effects of incorporation of known agents on the in vitro release characteristics of naproxen suppository.
Keywords
Naproxen, polyethylene glycols, suppository, surfactant, Witepsol
Naproxen (NPX), 6-methoxy-α-methyl-2-naphthal ene acetic acid, belongs to an important group of medicines called nonsteroidal antiinflammatory drugs with antiinflammatory, analgesic and antipyretic properties and is widely used in the treatment of rheumatic and other inflammatory diseases and for the relief of mild to moderate pain. Antiinflammatory effects of NPX are generally thought to be related to its inhibition of cyclooxygenase and consequent decrease in prostaglandin concentrations in various fluids and tissues and often preferred to acetylsalicylic acid (aspirin) because of its better absorption following oral administration and fewer adverse effects [1-3]. Oral administration is the route choice for drug administration. However, oral drug delivery becomes impossible in certain cases such as nausea, vomiting or convulsion. In such situations, the rectal route may provide a practical alternative. Melting or liquification of the fatty or hydrophilic base suppository leads to the release of the active drug. The latter process is dependent on rectal environment, drug substance, and suppository base [2,4-10]. Although rectal dosage forms are not common because of cultural and psychological biases, there are several advantages for administration by the rectal route. For example, the absorption site is near the administration site, hence there is a rapid absorption with a rapid increase in plasma drug levels with reduced sideeffects, specifically gastrointestinal irritation and the first pass effect is avoided [11-13]. However, the rectal route also has some potential disadvantages. For instance many drugs are poorly absorbed from rectal mucosa. Moreover a limiting absorbing surface area, dissolution problems due to the small fluid content of the rectum and drug metabolism in microorganisms and rectal mucosa can make it unfavorable route for drug administration. On the other hand, physicochemical and pharmaceutical factors such as solubility, particle size, partition coefficient, pKa, concentration of active substances, composition of the base, melting temperature range, viscosity, spreading in situ of suppository bases affect the rate and extent of drug absorption from suppositories [14-18]. To modulate drug release and absorption from suppositories, several studies have examined the use of surfactants, absorption enhancers, mixed micelles, fatty acid derivatives, and polymers [5,11,19-24]. In general, rectal bioavailability tend to be lower than the corresponding oral values; however, previous studies have demonstrated that the bioavailability of rectal NPX could be similar to its oral form while its side-effects are lower [3]. Therefore to ensure the rapid release of drug from suppositories, in the present study three types of surfactants were incorporated in the formulations and in vitro release characteristics of NPX from hydrophilic and hydrophobic suppository bases were evaluated.
Materials and Methods
NPX was a gift from Pars Daru Pharmaceutical Company, Tehran, Iran. Sodium hydroxide, sodium lauryl sulphate, cetylpyridinium chloride, tween 80, polyethylene glycol 400, 2000, and 6000 were purchased from Merck, Darmstadt, Germany. Witepsol H15 (WH15) and Witepsol W35 (WW35) were procured from Daruphakhsh Pharmaceutical Company, Tehran, Iran. KH2PO4 was obtained from Riedel-de Haen, Hanover, Germany.
Preparation of suppositories
Different types of Witepsols are often used in commercial formula as main fatty bases and various grades of polyethylene glycols (PEG) are used as the main water soluble bases [7,25-27]. Lipophilic and hydrophilic base suppositories were prepared using WH15, WW35, and different combination of PEG 400, 2000 and 6000, respectively. Suppository formulations were produced manually on a small scale by homogenizing NPX (500 mg/suppository) into the melted suppository bases. Accurately weighed quantities of the respective suppository bases, after mixing with PEG 400, 2000 and 6000 (in the case of hydrophilic suppository according to Table 1) were melted on a water bath at an appropriate temperature for each base. Finally NPX powder (500 mg) and/ or additive were incorporated using the displacement values calculations into the melted base along with continuous stirring. The melted mass was poured into the appropriate suppository mould (3 g capacity, Erweka-type 126 ALC, Germany) and allowed to cool at room temperature. Excess base was scraped off after solidification. Before molding of some hydrophilic suppositories (B, C and D combinations), it was necessary to lubricate the moulds with liquid paraffin. Displacement value was calculated based on the following equation: f=(100×(E−G))/(G×x)+1. Where f is the displacement value, E is the weight of the suppository without active substances (the calibration value of the mould for the certain base), G is the weight of the suppository with active substances and x is the active substance content in percentage [28].
| Formulation code | Base composition (%) |
|---|---|
| PEG A | PEG 6000 |
| PEG B | PEG 6000 (75)+PEG 2000 (25) |
| PEG C | PEG 6000 (25)+PEG 2000 (75) |
| PEG D | PEG 6000 (50)+PEG 2000 (50) |
| PEG E | PEG 6000 (75)+PEG 400 (25) |
PEG=Polyethylene glycols
Table 1: Composition of Different Formulations Of Hydrophilic Bases
Physicochemical analysis of suppositories
The weight of five separate suppositories was checked and means weight value was calculated. Melting point was measured by the collodion tube method for three suppositories (Erweka SSP, German). Hardness was measured by the resistance to crushing using hardness tester (Erweka SBT, German). The uniformity of drug content for each base (20 suppositories) was determined by dissolving PEG base suppositories in phosphate buffer at pH 7.2 in 37°, after 30 min stirring, the absorbance was measured by spectrophotometry (Shimadzu 120A, Japan) at a wavelength of 230.2 nm after dilution. The procedure was repeated to determine the uniformity of drug content of lipophilic base suppositories using repeated extraction with phosphate buffer (pH 7.2). To ensure complete extraction of the drug from the bases, blank suppositories without the drug were prepared and subjected to the same analytical procedure to serve as the blank for spectrophotometric determination.
Dissolution rate studies
A number of in vitro dissolution techniques for determination of the dissolution rate of drug substances from suppositories such as dialysis method and through a flow cell method were described in the literature [4,7,8,10,12,15,26,29-33]. In this study, the in vitro dissolution rate of NPX from the suppositories was examined using the basket method (Apparatus I, Erweka-Type DT6R, Germany). The temperature was maintained at 37° and the stirring rate was kept constant at 50 rpm. One thousand millilitre phosphate buffer (pH 7.2), was used as a dissolution medium, and samples of 5 ml test solution were collected manually at specified time intervals for up to 3 h and 4 h for lipophilic and hydrophilic bases, respectively. The withdrawn volume of the sample was replaced with the equal volume of fresh dissolution medium contained at the same temperature. The samples were analyzed for NPX concentration after appropriate dilutions. The US Pharmacopeia describes a UV spectrophotometric method for the assay of NPX tablets and for a dissolution study. In this study, the amount of dissolved NPX was detected at 230.2 nm on a UV spectrophotometer. Calibration curve data were generated using phosphate buffer (pH 7.2), in the concentration range of 0.3125-5 μg/ml (r2=0.999). For investigation of the effect of nonionic, anionic and cationic surfactants on the release rate of NPX, Tween 80, sodium lauryl sulphate, and cetylpyridinium chloride with concentrations of 0.5 and 1% w/w were used in formulations.
Results and Discussion
Variations in weight for the suppositories without surfactant and for the suppositories containing surfactant were in the range of 97-112%, which met the standard criteria (85.0-115.0%). Standard deviations were less than 0.031. The mean content of each suppository preparation was in the range of 97-104% and 97-105% of the labeled value for the suppositories without and containing surfactant, respectively (SD≤14.86), which is in the acceptable range (95-105%) [34]. Mean liquification time for suppositories without surfactant was between 7.7 and 8.7 min (SD≤1.57). The respected range in the presence of surfactants was 4.66-8.66 min (SD≤1.15). Hardness test results showed that hardness of all formulations were more than 5400 g (proper hardness).
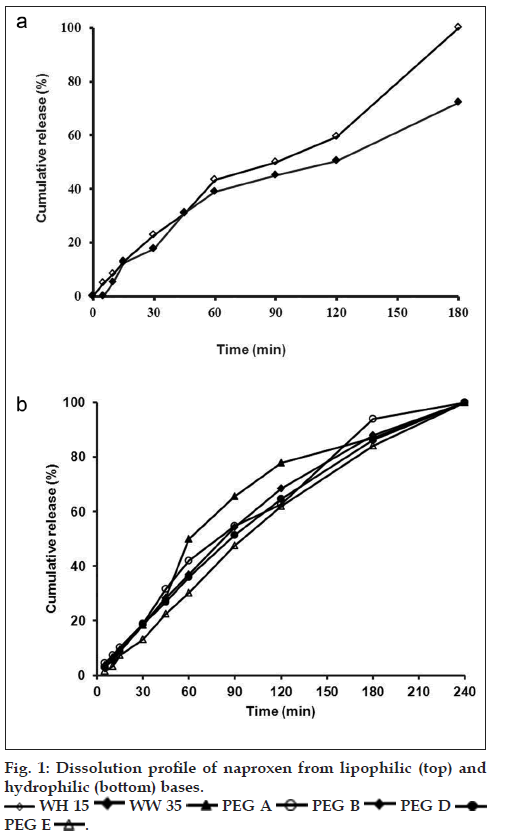
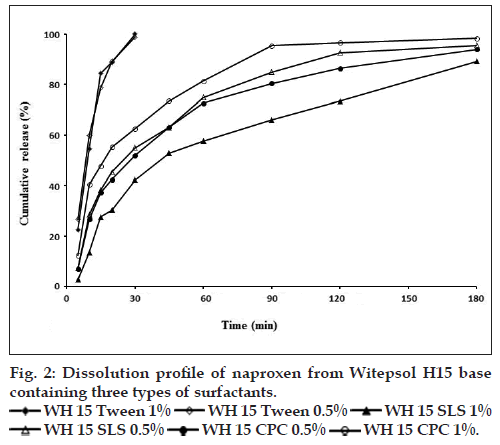
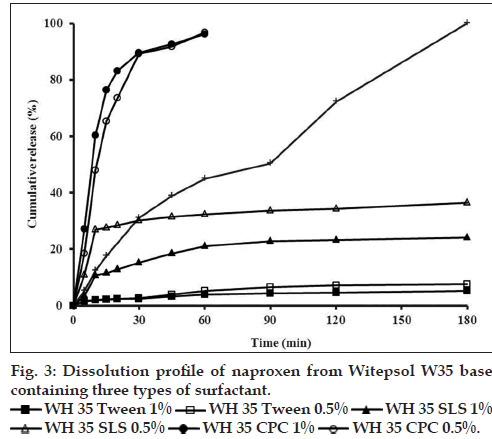
The effect of different suppository bases on the in vitro release of drugs has been described in several investigations [15,35]. In general, drug release from a number of suppository bases depends on the drug solubility in the base, the chemical composition of the base and drug particle size. Numerous studies have shown that drug release from suppository bases is influenced by the presence of other additives in the formulation and may result in an increase or decrease in the rate of release depending on not only the nature of the base but also the additive and its concentration considering the safety, efficacy, and compatibility with other ingredients of the formulation [35,36]. Results of dissolution tests in the present study showed that drug release profiles from both lipophilic bases were approximately the same (fig. 1a) and 100% of drug released after 180 min. Furthermore dissolution rates of five tested hydrophilic bases did not show considerable difference (fig. 1b) and in all formulations the total amount of drug released in 240 min. NPX is a lipophilic drug and it has low solubility in hydrophilic bases like PEGs. Therefore, it is expected to have a tendency to diffuse out the hydrophilic bases into the dissolution medium. However, the findings of this study revealed slow release of NPX from both fatty bases and PEGs. In a study about the crystallization behavior and microstructural evolution of solid dispersions prepared by co-melting method, it was found that for the fast crystallizing drugs like NPX, the interaction between the drug and the PEG matrix slowed down the crystallization rate of NPX and made larger drug domains [37]. It was also found that, drugs with strong interaction in PEG (e.g., NPX/ PEG) favored the inter-lamellar incorporation of NPX in PEG matrix before crystallization of NPX [37]. Moreover, formation of hydrogen bonds between NPX and PEG was also reported [38]. All these interactions change physicochemical behavior of NPX resulting in slower dissolution rate. However, it is obvious that because of long time required for drug release, none of the investigated bases is ideal for NPX suppository formulation per se. Obtained results of release kinetic investigation showed that the release rates of all above formulations were best described with Peppas model (R2>0.96, 0.5<n<1) indicating diffusion and erosion as drug release mechanisms (Table 2). In Peppas model (ln F=ln kp+nlnt), the value of n characterizes the release mechanism of drug. n≤0.50 corresponds to a diffusion mechanism, while 0.50<n<1 communicate to combined mechanism of diffusion and polymer erosion. Fig. 2, illustrates the effect of addition of three types of surfactants into WH15 base. Results showed that tween 80, which has a hydrophilic-lipophilic balance of 14.9, had maximum effect on drug release rate enhancement and 100% of the drug was released in 30 min. The rationale for using tween at concentrations of 0.5 and 1% was to ensure its safety following rectal administration to humans. The mechanism of dissolution enhancement effect of surfactants is complex and not fully understood. The main possible mechanisms could be as a result of their moistening effects which increase the surface area of the suppository mass, and also shortening disintegration times of lipophilic suppositories, which is caused by changing their lipophilic characteristics to a lipohydrophilic nature. However, the enhancement effect was seen to a lesser extent by adding sodium lauryl sulphate. Moreover, addition of cetylpyridinium chloride into suppository base had almost no effect on drug release and couldn’t be considered as suitable additive. Both sodium lauryl sulphate and cetylpyridinium chloride changed drug release kinetic model from Peppas to first order model (R2>0.94) which indicates that the release mechanism alters from diffusion to erosion (Table 2). Fig. 3 depicts the effect of mixing of surfactants into the WW35 base on drug release. It is evident that only cetylpyridinium chloride could enhance dissolution rate of NPX from WW35. In formulations containing sodium lauryl sulphate and tween 80 no significant amount of NPX was released and therefore they are not suitable enhancers for this base. It is known that WW35 is a fatty base with higher hydroxyl value compared to WH15 (40-50 vs. 5-15), having higher monoglyceride contents. The different level of interaction between monoglcerides and polar group of dissolution medium may be added to surfactant addition effect leading to a different drug release rates from the investigated fatty base suppositories [39,40]. On the other hand, it is possible that sodium lauryl sulphate and Tween 80 cause interactions between NPX and WW35 base, which could result in decreased drug release. Fig. 4 compares the release profiles of hydrophilic bases and effect of addition of tween 80 and sodium lauryl sulphate into these bases. Release profiles showed that addition of these surfactants had no significant effect on drug release rate. This result could be a consequence of the presence of surfactants in concentrations around the critical micelle concentration, which prolong drug release because of micellar entrapment of drug [39]. In summary, it can be concluded that hydrophilic bases are not suitable bases for NPX suppository formulation. Results indicated that formulations consisted of WH15 with 0.5% w/w of tween 80 and WW35 with 0.5% of cetylpyridinium chloride were most suitable bases for NPX rectal dosage form. This conclusion is more confirmed by the fact that fatty bases are better from a patient comfort perspective, since, the PEG bases are known to cause rectal mucosa irritation, due to their osmotic effects. However to optimize NPX release from different bases, further investigations using more enhancers and more characterizing techniques like differential scanning calorimeter, infrared spectroscopy are necessary.
| Formulation code | First order | Peppas | |
|---|---|---|---|
| RSQ | n | RSQ | |
| WH15 | 0.946 | 0.808 | 0.992 |
| WW35 | 0.922 | 0.768 | 0.968 |
| PEG A | 0.915 | 0.886 | 0.991 |
| PEG B | 0.942 | 0.882 | 0.993 |
| PEG C | 0.912 | 0.924 | 0.984 |
| PEG D | 0.924 | 0.982 | 0.998 |
| PEG E | 0.936 | 0.964 | 0.993 |
| WH15+SLS 1% | 0.943 | 0.972 | 0.881 |
| WH15+SLS 0.5% | 0.956 | 0.959 | 0.867 |
| WH15+CPC 0.5% | 0.964 | 0.839 | 0.886 |
| PEG A+tween 1% | 0.995 | 0.925 | 0.921 |
| PEG D+tween 1% | 0.992 | 0.815 | 0.954 |
| PEG A+SLS 1% | 0.971 | 0.765 | 0.921 |
WH15=Witepsol H15, WW35=Witepsol W35, PEG=polyethylene glycols, SLS=sodium lauryl sulphate, CPC=cetylpyridinium chloride, RSQ=R squared
Table 2: Kinetic Parameters For Analysis Of Formulations Release Data
Acknowledgements
The authors would like to thank the authorities of Faculty of Pharmacy, Tabriz University of Medical Sciences For their support.
References
- Segura Carretero A, Cruces-Blanco C, RamírezGarcía MI, CañabateDíaz B, Fernández Gutiérrez A. Simple and rapid determination of the drug naproxen in pharmaceutical preparations by heavy atom-induced room temperature phosphorescence. Talanta 1999;50:401-7.
- Healy LO, Murrihy JP, Tan A, Cocker D, McEnery M, Glennon JD. Enantiomeric separation of R, S-naproxen by conventional and nano-liquid chromatography with methyl-beta-cyclodextrin as a mobile phase additive. J Chromatogr A 2001;924:459-64.
- Wilasrusmee S, Chittachareon A, Jirasiritum S, Srisangchai P. Naproxen suppository for perineal pain after vaginal delivery. Int J GynaecolObstet 2008;102:19-22.
- Aiache JM, Islasse M, Beyssac E, Aiache S, Renoux R, Kantelip JP. Kinetics of indomethacin release from suppositories. In vitro-in vivo correlation. Int J Pharm 1987;39:235-42.
- Nakajima T, Takashima Y, Iida K, Mitsuta H, Koishi M. Preparation and in vitro evaluation of sustained-release suppositories containing microencapsulated indomethacin. Chem Pharm Bull 1987;35:1201-6.
- Ibrahim SA, El-Faham TH, ShawkyTous S, Mostafa EM. Formulation release characteristics and evaluation of ibuprofen suppositories. Int J Pharm 1990;61:1-7.
- Nishihata T, Tsutsumi A, Ikawa C, Sakai K. Sustained release suppository of sodium diclofenac: Use of water absorbable polymer. Drug Dev Ind Pharm 1990;16:1675-86.
- Tarimci N, Ermiş D. Sustained release characteristics and pharmacokinetic parameters of ketoprofen suppositories using chitosan. Int J Pharm 1997;147:71-7.
- Azechi Y, Ishikawa K, Mizuno N, Takahashi K. Sustained release of diclofenac from polymer-containing suppository and the mechanism involved. Drug Dev Ind Pharm 2000;26:1177-83.
- Samy EM, Hassan MA, Tous SS, Rhodes CT. Improvement of availability of allopurinol from pharmaceutical dosage forms I-suppositories. Eur J Pharm Biopharm 2000;49:119-27.
- Lootvoet G, Beyssac E, Shiu GK, Aiache JM, Ritschel WA. Study on the release of indomethacin from suppositories: In vitro-in vivo correlation. Int J Pharm 1992;85:113-20.
- Onyeji CO, Adebayo AS, Babalola CP. Effects of absorption enhancers in chloroquine suppository formulations: I. In vitro release characteristics. Eur J Pharm Sci 1999;9:131-6.
- Ryu JM, Chung SJ, Lee MH, Kim CK, Shim CK. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J Control Release 1999;59:163-72.
- Izgu E, Gungor U. The use of natural membranes for in vitro determination of absorption rates of drugs from suppository bases. Int J Pharm 1981;9:107-20.
- Uzunkaya G, Bergişadi N. In vitrodrug liberation and kinetics ofsustained release indomethacin suppository. Farmaco 2003;58:509-12.
- Takatori T, Shimono N, Higaki K, Kimura T. Evaluation of sustained release suppositories prepared with fatty base including solid fats with high melting points. Int J Pharm 2004;278:275-82.
- De Muynck C, Remon JP. Influence of fat composition on the melting behaviour and on the in vitro release of indomethacin suppositories. Int J Pharm 1992;85:103-12.
- Safwat SM, El-Shanawany S. Evaluation of sustained-release suppositories containing microencapsulated theophylline and oxyphenbutazone. J Control Release 1989;9:65-73.
- Fontan JE, Arnaud P, Chaumeil JC. Effects of polysorbate 80 on the pharmacokinetics on a carbamazepine suppository in the rabbit. Int J Pharm 1992;82:67-70.
- Matsuda H, Arima H. Cyclodextrins in transdermal and rectal delivery. Adv Drug Deliv Rev 1999;36:81-99.
- Nishihata T, Sudho M, Kamada A. Investigation of sustained-release suppository of sodium diclofenac in humans. Int J Pharm 1986;33:181-6.
- Taha EI, Zaghloul AA, Samy AM, Al-Saidan S, Kassem AA, Khan MA. Bioavailability assessment of salbutamol sulfate suppositories in human volunteers. Int J Pharm 2004;279:3-7.
- Yoshida T, Itoh Y, Gomita Y, Oishi R. Influence of storage temperature on indomethacin release from fatty-base suppositories in vitro and in vivo. Acta Med Okayama 1991;45:37-42.
- Zuber M, Pellion B, Arnaud P, Chaumeil JC. Kinetics of theophylline release from suppositories in vitro: Influence of physicochemical parameters. Int J Pharm 1988;47:31-6.
- Gattani SG, Amrutkar JR, Kakade KN, Belgamwar VS. Formulation and evaluation of sustained release suppositories of ondansetron in hydrophilic and lipophilic bases. Eur J Parenter Pharm Sci 2010;15:26-32.
- Furuno K, Gomita Y, Yoshida T, Oishi R, Saeki K, Araki Y. In vivo and in vitro release of indomethacin from water-soluble and fatty base suppositories. Acta Med Okayama 1992;46:223-31.
- Thomas NW, Lack LJ, Woodhouse BA, Palin KJ, Gould PL. Formulation of fenbufen suppositories. III. Histology of the rectal mucosa of rats following repeat dosing of fenbufen in witepsol H12 and PEG vehicles. Int J Pharm 1988;44:261-3.
- Luedde M, Luedde KH. The displacement factor and volume of suppositories and suppository masses. Pharm Prax 1961;10:171-7.
- Berkó S, Regdon G Jr, Ducza E, Falkay G, Erós I. In vitro and in vivo study in rats of rectal suppositories containing furosemide. Eur J Pharm Biopharm 2002;53:311-5.
- Yong CS, Oh YK, Kim YI, Kim JO, Yoo BK, Rhee JD, et al. Physicochemical characterization and in vivo evaluation of poloxamer-based solid suppository containing diclofenac sodium in rats. Int J Pharm 2005;301:54-61.
- Yun M, Choi H, Jung J, Kim C. Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. Int J Pharm 1999;189:137-45.
- Victoria MM, David CJ. Thermal and rheological study of lipophilic ethosuximide suppositories. Eur J Pharm Sci 2003;19:123-8.
- Janicki S, Sznitowska M, Zebrowska W, Gabiga H, Kupiec M. Evaluation of paracetamol suppositories by a pharmacopoeial dissolution test – Comments on methodology. Eur J Pharm Biopharm 2001;52:249-54.
- British Pharmacopoeia Commission, British Pharmacopoeia. London: HM The Stationery Office; 2007.
- Miyake M, Minami T, Oka Y, Kamada N, Yamazaki H, Kato Y, et al. Optimization of suppository preparation containing sodium laurate and taurine that can safely improve rectal absorption of rebamipide. Biol Pharm Bull 2006;29:330-5.
- Miyake M, Kamada N, Oka Y, Mukai T, Minami T, Toguchi H, et al. Development of suppository formulation safely improving rectal absorption of rebamipide, a poorly absorbable drug, by utilizing sodium laurate and taurine. J Control Release 2004;99:63-71.
- Qing Z. Crystallization behavior and microstructural characterization of drug/polymer systems. USA: Purdue University; 2011.
- Mura P, Manderioli A, Bramanti G, Ceccarelli L. Properties of solid dispersions of naproxen in various polyethylene glycols. Drug Dev Ind Pharm 1996;22:909-16.
- Oladimeji FA, Omoruyi SI, Onyeji CO. Preparation and in vitro evaluation of suppositories of halofantrine hydrochloride. Afr J Biotechnol 2006;5:1775-80.
- Chicco D, Grabnar I, Skerjanec A, Vojnovic D, Maurich V, Realdon N, et al. Correlation of in vitro and in vivo paracetamol availability fromlayered excipient suppositories. Int J Pharm 1999;189:147-60.
 WH 15
WH 15  WW 35
WW 35 PEG A
PEG A PEG B
PEG B PEG D
PEG D PEG E
PEG E 
 WH 35 Tween 1%
WH 35 Tween 1% WH 35 Tween 0.5%
WH 35 Tween 0.5%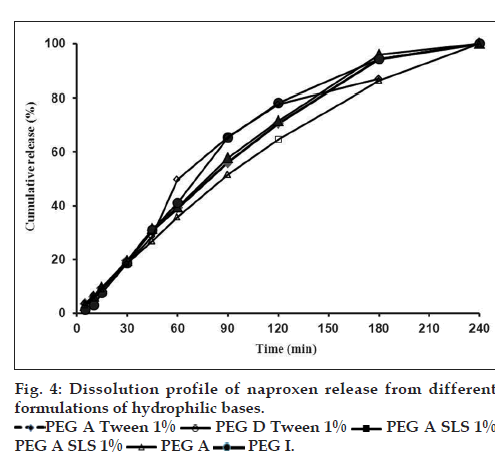
 PEG A Tween 1%
PEG A Tween 1% PEG D Tween 1%
PEG D Tween 1% PEG A SLS 1%
PEG A SLS 1%
 PEG A SLS 1% PEG A
PEG A SLS 1% PEG A  PEG I.
PEG I.



