- *Corresponding Author:
- A. K. Madan
Faculty of Pharmaceutical Sciences, Pt. B. D. Sharma University of Health Sciences, Rohtak-124 001, India
E-mail: madan_ak@yahoo.com
| Date of Submission | 21 June 2016 |
| Date of Revision | 09 November 2016 |
| Date of Acceptance | 17 January 2017 |
| Indian J Pharm Sci 2017; 79(1): 91-104 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Present study pertains to preparation, characterization and evaluation of human guarded chlorpyrifos urea co-inclusion complexes for agricultural use. Chlorpyrifos a widely used organophosphate insecticide was co-included along with long linear chain rapidly complexing agent in urea lattice. A three component powder form comprising of urea as host cum fertilizer, oleic acid as an insect pheromone and an insecticide for killing/ disabling the insects was prepared. Resulting insecticide fertilizer amalgamation offered improvement in safe handling and formulation characteristics. Insecticide was engulfed in tunnels of hexagonal urea and is accordingly not accessible for either contact with skin or for inhalation/odor. Hence, personnel engaged in handling of chlorpyrifos will not be exposed to its toxic effects after co-inclusion in hexagonal urea. Insecticide will be activated/released only after chlorpyrifos urea co-inclusion complex comes in contact with water following switching on of automated water sprinkling system in agricultural fields. Steep reduction in mercaptan like odor of chlorpyrifos was observed following engulfment of chlorpyrifos in hexagonal urea lattice. Minimum proportion of long linear chain rapidly complexing agent required for co-inclusion of insecticide in urea lattice was determined calorimetrically. Chlorpyrifos urea co-inclusion complexes were characterized by Fourier transform infrared spectroscopy, X-ray powder diffraction, differential scanning calorimetry and 1H-nuclear magnetic resonance spectroscopy studies. Regression analysis depicted gradual increase in heat of decomposition with corresponding increase in molar fraction of rapidly complexing agent. Increased heat of decomposition simply indicates better physical stability of complexes. These complexes chlorpyrifos urea co-inclusion complex revealed uniform formulation composition and improved dissolution profile of chlorpyrifos. Studies reveal insecticide fertilizer amalgamation to be a promising approach for formulation of poorly soluble hazardous insecticide into an effective rapidly soluble human guarded insecticide product with improved handling/formulation characteristics at minimal cost.
Keywords
Toxicity, chlorpyrifos, odour reduction, safe handling characteristics, dissolution rate, urea co-inclusion complex
Insecticides are hazardous substances released intentionally into the environment to kill pests. These were mainly responsible for increase in agricultural productivity in 20th century. However, the use of insecticides alters the ecosystems effectively and even ensures its presence/concentration along the food chain. Insecticides are toxic to humans and are responsible for various human health hazards [1]. Insecticides are equally toxic to other species such as fish, birds and mammals [2].
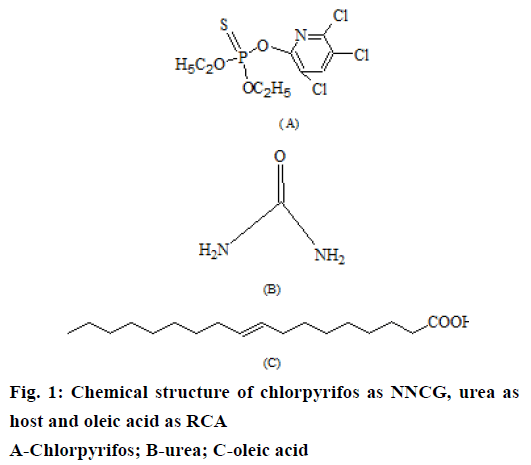
Organophosphates belong to class of contact insecticides. These insecticides target on insect nervous system and kill/disable the insect by disrupting nerve impulses [3]. Chlorpyrifos (CHF), O,O-diethyl 0-3,5,6-trichloro-2-pyridyl phosphorothioate (Figure 1) is an organophosphate insecticide. CHF is effective against a variety of insect pests including, cockroaches, grubs, flea beetles, fire ants, lice, flies and cutworms. It is used as an insecticide for crop protection on cotton, grains, fruit, nut and vegetable crops as well as ornamental/lawn plants. CHF acts on the nervous system of insect pests by interfering with the signals from neurotransmitter acetylcholine [4]. One of the metabolite of CHF, CHF-oxon, binds permanently to acteylcholinesterase preventing this enzyme from deactivating acetylcholine in the synapse. This results in a built up of the concentration of acetylcholine between neurons leading to a longer lasting signal to the next neuron [5,6].
The World Health Organization (WHO) classified CHF as a class II, moderately toxic insecticide for acute effects [7]. Acute toxicity from CHF exposure can result from higher doses [8,9]. The mild poisoning can cause watering of eyes, skin irritation, increased sweating and saliva, nausea, diarrhoea/vomiting, headache and muscle spasms/weakness. Symptoms resulting from severe poisoning include seizures, paralysis, unconsciousness, suffocation and chest discomfort [10,11].
CHF is a white, granular crystalline solid having mercaptan like odour that is practically insoluble in water and is poorly absorbed from soil. Therefore, it is naturally of utmost significance to devise an effective approach for enhancing the solubility profile of CHF so as to improve its bioavailability in soil [12]. Improvement of water solubility of CHF has been achieved through inclusion in cyclodextrins [13-15]. In the current study; an attempt has been made for improving the solubility, odour reduction and safe handling of CHF through co-inclusion of CHF in urea using a modified technique [16-18]. The fertilizer cum insecticide amalgamation in the current study might result in the production of co-inclusion complexes of CHF in urea lattice exhibiting two-in-one product i.e. providing urea as a fertilizer, a convenient source of nitrogen and an insecticide for killing/disabling the insect pests.
The insecticide, CHF with poor solubility in water is complexed with urea lattice in the presence of long chain rapidly complexing agents (RCA). The coinclusion of CHF in hexagonal urea tunnels led to weakening/collapse of urea host lattice, which ensures instantaneous release of the incorporated insecticide on coming in contact with water. Moreover, CHF possessed mercaptan like odour and contains volatile contaminants, which are responsible for the offensive odour of the insecticide. Urea co-inclusion complexes of CHF offered complete incorporation of insecticide in urea lattice leading to a marked reduction in the unacceptable odour of CHF. The mercaptan/offensive odour of the CHF is released only after spraying with water.
CHF is considered as a hazardous substance as per US occupational safety and Health Administration (OSHA) guidelines for chlorpyrifos [19]. As per Environmental Protection Agency (EPA) regulations, safer handling is required by the personnel involved in preparation, processing, transportation, administration and disposal of the insecticide. It is strongly advised that the personnel should wear safety glasses, goggles or face shields, gloves and proper chemical protective clothing during handling operations of an insecticide. Insecticide is engulfed in tunnels of hexagonal urea and is accordingly not accessible for either contact with skin or for inhalation/odour. As a result, personnel engaged in the handling of insecticide will not be exposed to the insecticide after its co-inclusion in urea lattice.
Literature revealed numerous host molecules such as urea, cyclodextrins, thiourea, porphyrins, deoxycholic acid, tri-o-thymotide, hydroquinone, zeolites, calixarenes, cryptophanes and crown ethers for formation of inclusion complexes [20]. Amongst these host molecules, urea has acquired commercial/ industrial prominence. Urea is used as a component of fertilizer providing a cheap source of fixed nitrogen to promote growth of plants [21,22]. Hence, urea being highly soluble, safe, non-toxic, stable, biocompatible, economical and easily available was selected as a host molecule in the current study for performing dual function of acting as an adductor/host as well as a fertilizer for providing cheap source of nitrogen for plants growth.
The guest molecules with long chain length are rapidly incorporated in the host urea channels. Such guests serve as a “pathfinder” and are categorized as RCA. Cyclic compounds or highly substituted compounds do not form complexes with urea and these may be categorized as normally non-complexing guests (NNCG). However, substituted cyclic compounds with sufficient chain length may form complex with urea either alone or in the presence of RCA [23,24].
Dramatic improvement in the dissolution profile of NNCG drugs for oral use as inclusion complexes have been reported. These NNCG drugs included enalapril maleate, glipizide, amiloride hydrochloride, lafutidine, simvastatin and ezetimbe [25-30]. Co-inclusion of NNCG drugs in urea has also been reported for reduction in moisture sensitivity of nicorandil and improvement of safe handling and photostability characteristics of cis-retinoic acid [31,32]. Co-inclusion complex of viscous liquid malathion in urea has been recently reported for improvement of safe handling and formulation characteristics of hazardous insecticide [33].
In the current study, an environment friendly approach has been successfully employed for safe use of CHF through co-inclusion in urea. CHF is a significantly substituted cyclic organic compound, which will not form complex with urea on its own under any known conditions. However, CHF can be rapidly incorporated in urea channels in the presence of a suitable long chain RCA [34]. Hence, oleic acid was adopted as a suitable long chain RCA for co-inclusion of CHF in hexagonal urea lattice. Oleic acid is also reported to function as an insect pheromone, which is emitted by the decaying bodies of number of insects, including bees and ants. Its smell might indicate danger to living insects and triggers the instincts of living workers to remove dead bodies from hive [35].
Materials and Methods
CHF was supplied as a gift sample by Insecticides (India) Ltd, Chopanki, Bhiwadi, Rajasthan. Urea crystals and oleic acid were procured from CDH, while methanol was from Rankem. All other chemicals/ reagents used in the study were of analytical grade.
Preparation of urea co-inclusion complexes of CHF
One gram of CHF was dissolved in 30 ml methanol containing 10 g urea by gentle heating. Subsequently, 1.0 g of oleic acid was incorporated as a long chain RCA to the above solution resulting in the immediate precipitation of the crystals of urea co-inclusion complex upon cooling. The crystals were separated from the mother liquor by filtration, dried and suitably packed [16-18].
Calorimetric method for determination of minimum proportion of RCA
A calorimetric method was employed for determination of minimum proportion of RCA required for coinclusion of CHF in urea host lattice. The said method comprised of two phases. The phase I was utilized for determination of stoichiometric ratio between urea and RCA and phase II for determination of minimum ratio of RCA and CHF for formation of urea co-inclusion complexes [25,30].
Fourier transform infrared spectroscopy (FTIR) analysis
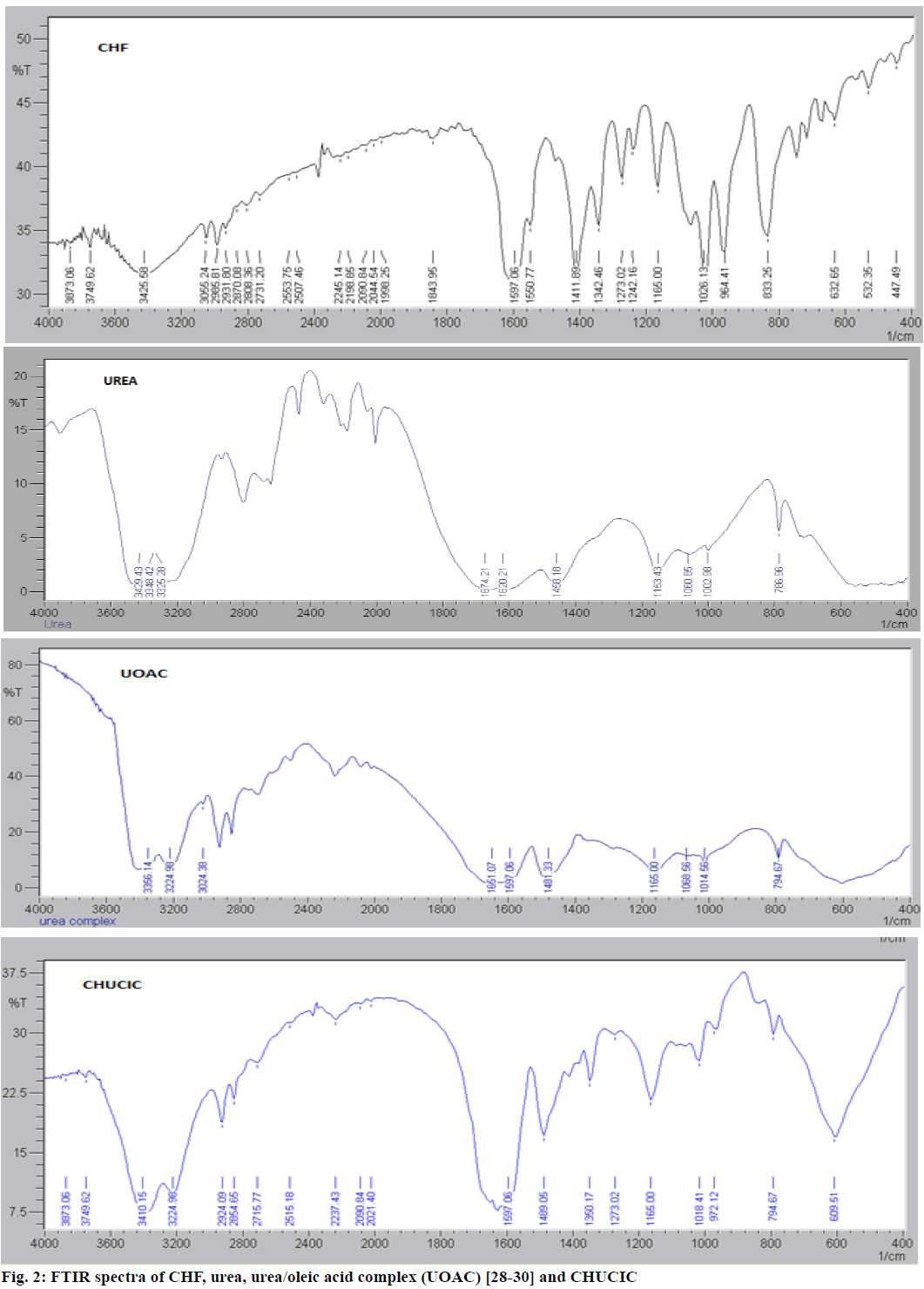
The FTIR spectra of urea, urea/oleic acid complex (UOAC), CHF and CHF urea co-inclusion complex (CHUCIC) were recorded on FTIR (Shimadzu 02205 model) utilizing KBr disc process and all samples were scanned over a range of 400-4000 cm-1.
Differential scanning calorimetry (DSC)
Thermal analysis of urea, UOAC, CHF and CHUCIC was performed using Perkin Elmer DSC 4000 by heating each sample approximately (~5 mg) in an aluminium pan at a scanning rate of 10°/min from ambient temperature to 200° in an atmosphere of nitrogen gas by passing N2 gas at a flow rate of 20 cc/ min.
Powder X-ray diffraction studies (XRD)
X-ray diffraction studies of urea, UOAC, CHF and CHUCIC were conducted using Philips X-ray diffractometer type PW1710 having Cu based tube anode. The operational parameters were Cu-Kα radiations [α1=1.54060Å and α2=1.54443Å], generator tension 45 kV, current 40 mA, divergence slit 1?, intensity ratio 0.500 and scanning rate 2°/min over a 2? range of 0-50°.
Proton nuclear magnetic resonance (1H-NMR) studies
The 1H-NMR spectra of urea and UOAC were performed using Bruker FT-NMR of 400 MHz intensity using deuterated d6-DMSO (d6-dimethyl sulfoxide) (δH=2.5 ppm) as the solvent. The 1H-NMR spectra of CHF, oleic acid and CHUCIC were performed in Bruker Avance II 400 NMR spectrometer (400 MHz) using DMSO as solvent.
Determination of minimum ratio of RCA for coinclusion of CHF in urea
In phase I, a Zimmerschied calorimetric procedure was utilized for calculation of stoichiometric ratio between urea and long chain RCA [36]. Oleic acid was adopted as a suitable linear chain RCA. A Dewar flask possessing a burette and a probe of thermocouple system with capacity for measuring up to 0.0° served the purpose of calorimeter. Hence, 12 g of urea dissolved in 70 ml methanol was gently shaken in the calorimeter until an equilibrium temperature was achieved. The increments of 0.5 ml of oleic acid were successively introduced into the calorimeter using a burette. Temperature was recorded after each addition [28-30]. A graph representing an increase in temperature following incremental addition of oleic acid as a suitable rapidly complexing agent to methanolic solution of urea was plotted.
In phase II, minimum proportion of linear chain RCA required for the formation of co-inclusion complexes of CHF and RCA in urea was determined using modified calorimetric procedure based on the measurement of increase in temperature following addition of oleic acid (RCA) to the methanolic solution of urea and CHF [16-18]. Hence, 12 g urea and 3 g CHF were dissolved in 70 ml methanol and gently shaken in the calorimeter until an equilibrium temperature was recorded. The initial increments of 0.2 ml and subsequently 0.5 ml of oleic acid as a suitable RCA were successively added into the calorimeter. The graph of rise in temperature versus the amount of RCA added to a solution of urea and CHF in methanol was obtained [28-30].
Effect of relative proportion of RCA on heat of decomposition of CHUCIC complexes
Numerous urea CHF-RCA co-inclusion complexes containing different proportions of CHF and RCA (Table 1) were prepared by adding 2 g of guests to 30 ml methanol containing 10 g urea. The resulting crystals upon cooling were separated from the mother liquor by filtration, dried and suitably packed. The urea inclusion complexes were subjected to DSC analysis from ambient temperature to 350° to study the effect of relative proportion of RCA on heat of decomposition of CHUCIC complexes.
| Product | Wt fraction of RCA in guests | Molar fraction of RCA in guests | Onset (°) |
Peak (°) | Final (°) |
Area (mJ) |
Peak height (mW) | Heat of decomposition (J/g) |
|---|---|---|---|---|---|---|---|---|
| CHUCIC-1 | 0.50 | 0.55 | 112.24 | 116.13 | 121.61 | 135.184 | 7.1138 | 26.3674 |
| CHUCIC-2 | 0.60 | 0.65 | 113.05 | 116.08 | 120.93 | 160.372 | 9.7706 | 32.6222 |
| CHUCIC-3 | 0.70 | 0.74 | 107.19 | 116.43 | 122.27 | 171.918 | 8.0388 | 37.8393 |
| CHUCIC-4 | 0.80 | 0.83 | 107.62 | 116.46 | 121.84 | 202.048 | 8.2528 | 39.0459 |
| CHUCIC-5 | 0.90 | 0.92 | 110.74 | 117.04 | 122.27 | 245.051 | 10.2837 | 49.1116 |
| UOAC | 1.00 | 1.00 | 108.17 | 117.84 | 122.15 | 349.059 | 13.8746 | 58.1766 |
Table 1: Thermal Data for Urea Co-Inclusion Complexes Containing Varying Proportions of RCA and CHF
Assay procedure
CHF was determined by spectrophotometrically utilizing LabIndia UV/Vis 3000 spectrophotometer. Both urea and oleic acid did not interfere in the estimation of CHF. The absorbance was recorded of various dilutions of CHF at λmax of 235 nm in methanol for content uniformity analysis and in methanol:distilled water (25:50 v/v) for conducting dissolution rate studies [37].
Aqueous solubility studies
In order to conduct aqueous solubility studies, excess amount of solute i.e. CHF was added to 10 ml of distilled water in 25 ml volumetric flask. This volumetric flask was capped properly and shaken at 25° on a temperature controlled magnetic stirrer (shaking flask method) for 24 h. The saturation was confirmed by the presence of un-dissolved solute. The resulting samples containing un-dissolved solid particles suspended in volumetric flasks were filtered, suitably diluted and analysed spectrophotometrically at 235 nm. A similar procedure was repeated for various co-inclusion complexes (CHUCIC-1 to CHUCIC-5). All the experiments were conducted thrice to precise the measurement.
Content uniformity studies
In order to conduct content uniformity analysis, exactly weighed quantities of randomly drawn ten samples of CHUCIC complexes each containing a quantity equivalent to 10 mg of CHF were dissolved in methanol and suitably diluted. The drug contents were determined at 235 nm in methanol spectrophotometrically [38].
Dissolution rate studies
The dissolution rate studies were attempted using Lab India dissolution apparatus II in 900 ml of distilled water, maintained at 37±0.5° at a speed of 50 rpm. The quantity of CHF equivalent to 10 mg in coinclusion complex (CHUCIC-1 and CHUCIC-2) was added to dissolution medium. Aliquots of 5 ml of the samples were withdrawn at specified time intervals and replenished with fresh distilled water. Subsequently, 2.5 ml of methanol was added to all samples and assayed spectrophotometrically at 235 nm. All the experiments were performed in triplicate [39].
Results and Discussion
In the current study, an attempt has been made successfully for preparation, characterization and evaluation of human guarded CHUCICs. CHF is normally non-complexing substituted cyclic organic compound and is wide enough to be entrapped inside the narrow urea channels. Thus, CHF was incorporated in urea lattice in the presence of a suitable long chain RCA. Hence, addition of oleic acid as a linear chain RCA to a methanolic solution of urea and CHF led to an immediate precipitation of crystals of CHUCIC complex. The present investigation employs insecticide cum fertilizer amalgamation for the preparation of coinclusion complexes of insecticide resulting in twoin- one product for agricultural use i.e. providing urea as a fertilizer and an insecticide for killing/disabling the insects. CHUCIC complexes were characterized by FTIR, DSC, XRD and 1H-NMR. However, further studies are being contemplated for biological evaluation of said products against various pests/ insects commonly encountered in agricultural fields through collaborative work with agricultural scientists.
Figure 2 depicts the FTIR spectra of urea, UOAC, CHF and CHUCIC. FTIR spectrum of urea reveals characteristic peaks of tetragonal urea at 787, 1003, 1150, 1458, 1597, 1659, 3325 and 3429 cm-1. The similar bands depicting important peaks of hexagonal urea appears at 794, 1157, 1015, 1481, 1597, 1658.78, 3225 and 3383 cm- 1 [28-30]. FTIR spectrum of CHF exhibits characteristic bands of CHF at 633 cm-1 (C-Cl stretching); 833 cm-1 (aromatic C-H out of plane bending); 964, 1026 and 1165 cm-1 (P-O-C antisymmetric and C-O stretching); 1273 (C-O stretching); 1412 cm-1(C=C stretching); 1551 cm-1(P=S stretching); 1597 and 1627 cm-1(C=O stretching); 2985 cm-1 (alkane C-H stretching); 3426 cm-1 (N-H stretching) [40]. FTIR spectrum of CHUCIC crystals indicates out-of-phase vibrations at 3410 cm-1and in-phase vibrations at 3224 cm-1, which are prominent peaks of hexagonal urea. Similarly, bands between 1675 and 1590 cm-1 were observed at 1651, 1628 and 1597 cm-1 (due to NH2 bending and C=O stretching vibrations), skeletal out-of-phase bending frequency slightly raised at 794 cm-1 in CHUCIC from 787 cm-1, C-N vibration frequency increased from 1458 to 1489 cm -1, NH2 rocking vibrations raised from 1150 cm-1 in tetragonal urea to 1165 cm-1, symmetric C-N frequency raised at 1018 cm-1 from 1003 cm-1 in urea indicating characteristic peaks of hexagonal urea [41]. Moreover, FTIR spectrum of CHUCIC crystals indicate certain absorption bands of insecticide CHF at 972, 1018 and 1165 cm-1 (P-O-C antisymmetric and C-O stretching); 1273 cm-1 (C-O stretching); 2855 and 2924 cm-1 (alkane C-H stretching) in co-inclusion complex [40]. The presence of characteristic bands of insecticide CHF in co-inclusion complex indicated inclusion of guest moiety (CHF) in CHUCIC complex.
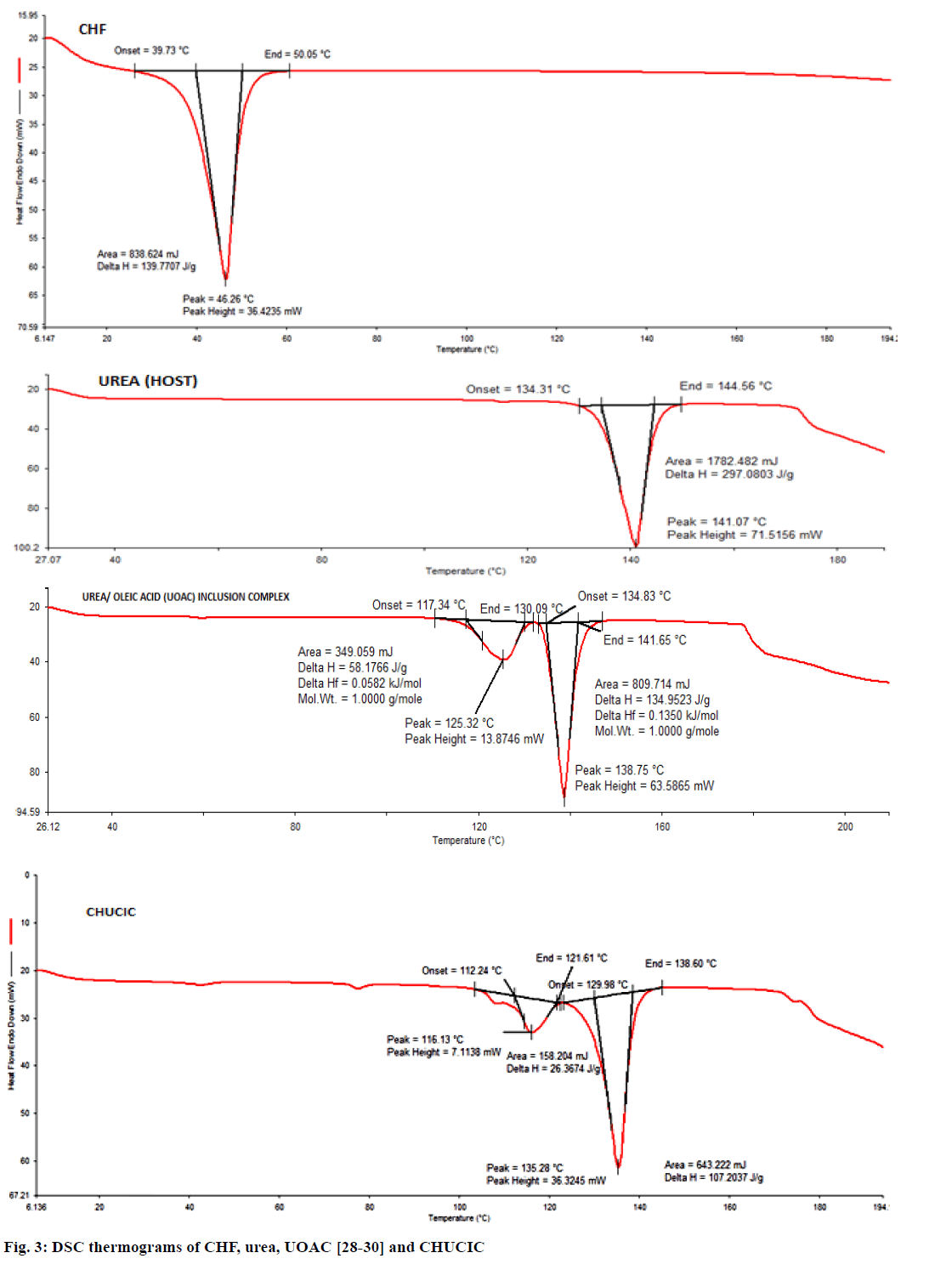
Figure 3 shows the thermograms of urea, UOAC, CHF and CHUCIC. The thermogram of urea displays onset of melting at 134.31° with sharp melting endotherm at a peak temperature of 141.0°. The thermogram of UOAC exhibits melting of urea in two stages. The first stage is attributed to collapse of the hexagonal structure of the UOAC at 117.34° to yield the tetragonal urea while the second step exhibits melting of tetragonal urea at 134.83° [28-30]. Pure CHF displays a melting endotherm in the temperature range of 39.73°-50.05° with a sharp melting endothermic peak at 46.26° [42]. CHUCIC thermogram depicts that urea crystals melt in two stages. The first stage (112.24°) pertains to the decomposition of the hexagonal urea (co-inclusion complex) to yield the guest moiety (CHF) and the tetragonal urea while the second stage (129.98°) is attributed to the melting of tetragonal urea [43]. The disappearance of a sharp melting endotherm of CHF at 46.26° clearly indicates the complete inclusion of the insecticide (CHF) into the urea lattice and presence of insecticide CHF in an amorphous state in CHUCIC complex.
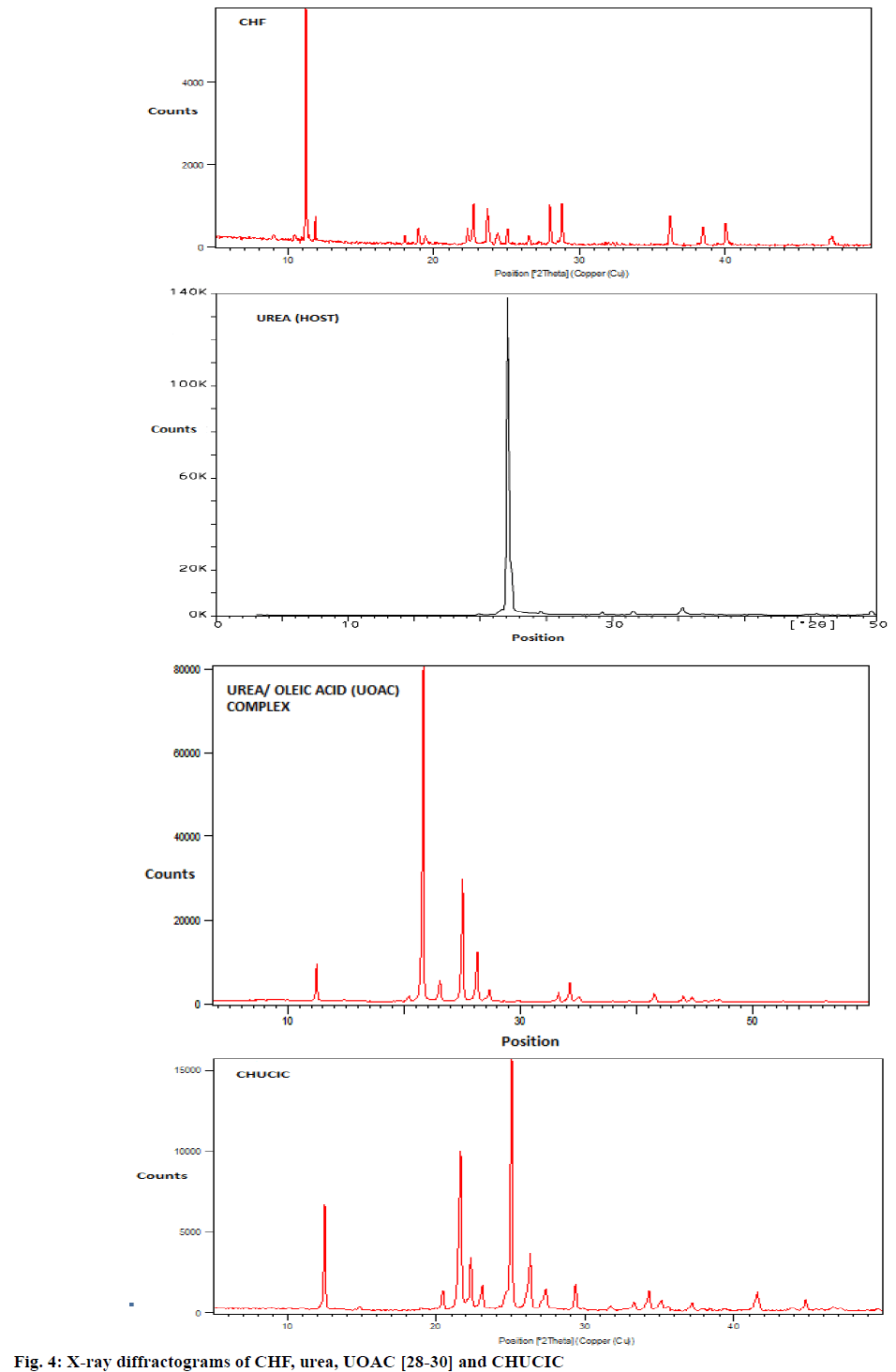
Figure 4 displays powder X-ray diffractograms of urea, UOAC, CHF and CHUCIC. The X-ray powder diffractograms displays interplanar spacings of tetragonal urea at 2.4368, 2.5419, 2.8294, 3.0528, 3.6144 and 4.0198°A. Similarly, interplanar spacings indicating presence of hexagonal urea in UOAC appears at 3.2637, 3.3858, 3.4089, 3.5920, 3.8481, 4.1031 and 7.0769°A [28-30]. Pure CHF exhibits a highly crystalline nature depicted by sharp diffraction peaks at interplanar spacings 7.87743, 7.44938, 3.91461, 3.76072, 3.19315, 3.10483, 2.48390 and 2.34318°A [44]. The diffractograms of CHUCIC co-inclusion complexes depicts important peaks at interplanar spacings 3.2617, 3.29670, 3.3875, 3.55589, 3.8494, 4.1082, 4.1453 and 7.0821°A, which are characteristic peaks of the hexagonal urea [45]. The complete absence of intense peaks of CHF indicates that guest molecules are engulfed inside and isolated from one another in the honey-comb network of urea lattice and do not contribute towards crystallinity of CHF.
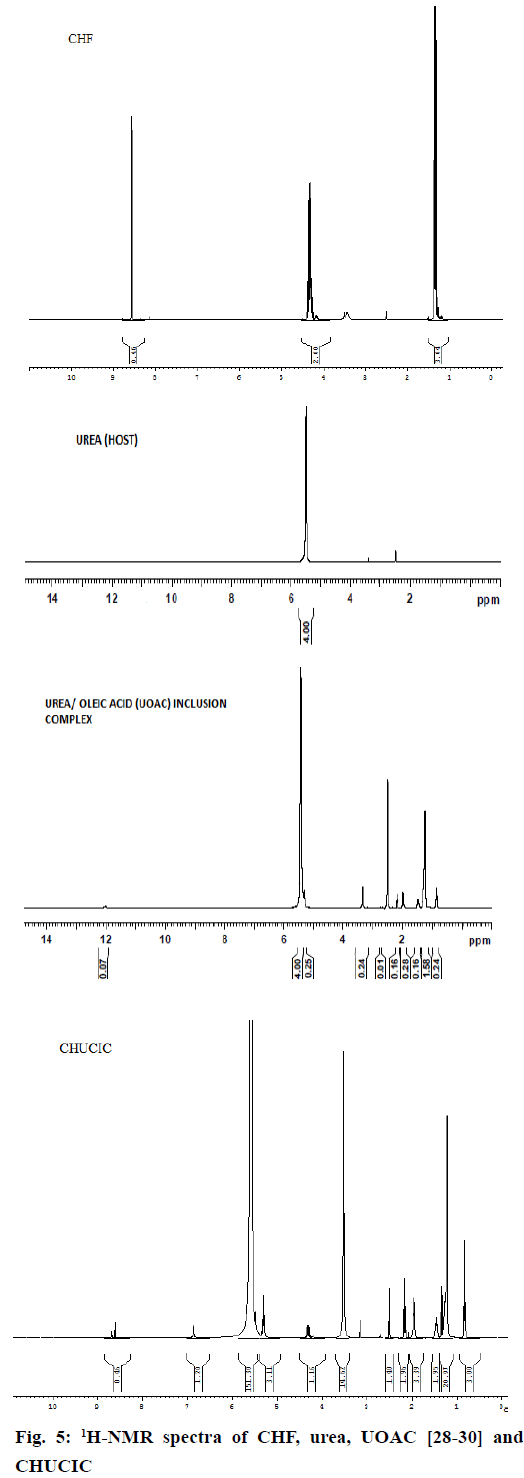
The 1H-NMR spectra of urea, UOAC, CHF, oleic acid and CHUCIC are illustrated in (Figure 5). The transformation of tetragonal urea into hexagonal form of urea in CHUCIC is evident by shift of N–H proton from 5.510 to δH=5.483-5.585 ppm with high prominence due to interaction between (–NH2) group of urea and (–COOH) group of oleic acid. Moreover, O–H proton from carboxylic function of oleic acid at δH=11.8211 ppm deplete in CHUCIC complex indicating that oleic acid is incorporated as a guest in CHUCIC complex. Also, C9H=C10H protons of oleic acid exhibiting peak at δH=5.270-5.347 appeared at 5.284-5.347 ppm in CHUCIC indicating incorporation of oleic acid in CHUCIC [28-30]. Similarly, 20 protons of oleic acid at C3-C7 and C12-C16 at 1.262-1.285 appeared at δH=1.219-1.275 ppm in CHUCIC; C2H2 protons at 2.158-2.196 appeared at 2.152-2.189 ppm; C8H2 and C11H2 protons of oleic acid at 1.948-2.054 appeared at 1.940-2.006 ppm; C17H2 protons at 1.507-1.542 appeared at δH=1.448-1.482 ppm; C18H3 at 0.853- 0.887 appeared at δH=0.818-0.852 ppm in CHUCIC indicating complete inclusion of oleic acid as guest moiety in CHUCIC complex. Most of exposed protons of CHF e.g. 3H-CH3 protons at 1.288-1.371(triplet) appeared at 1.319-1.3547 ppm in CHUCIC; 2H-CH2 protons at 4.275-4.384 (multiplet) appeared at 4.289- 4.349 ppm and 1H-aromatic proton at 8.572-8.574 (singlet) appeared at 8.607-8.694 ppm in CHUCIC [46]. The digitized integral values for integrals printed below the peaks in NMR spectrum of CHUCIC complex were observed to calculate the total area (204.52 mm) and then dividing the total area by total number of hydrogen atoms to determine the actual number of protons in the complex. Thus, 11 protons of CHF, 33 protons of oleic acid and 4 protons of urea appeared in CHUCIC complex indicating the complete incorporation of guest moieties (oleic acid and CHF) in urea to form CHUCIC complex.
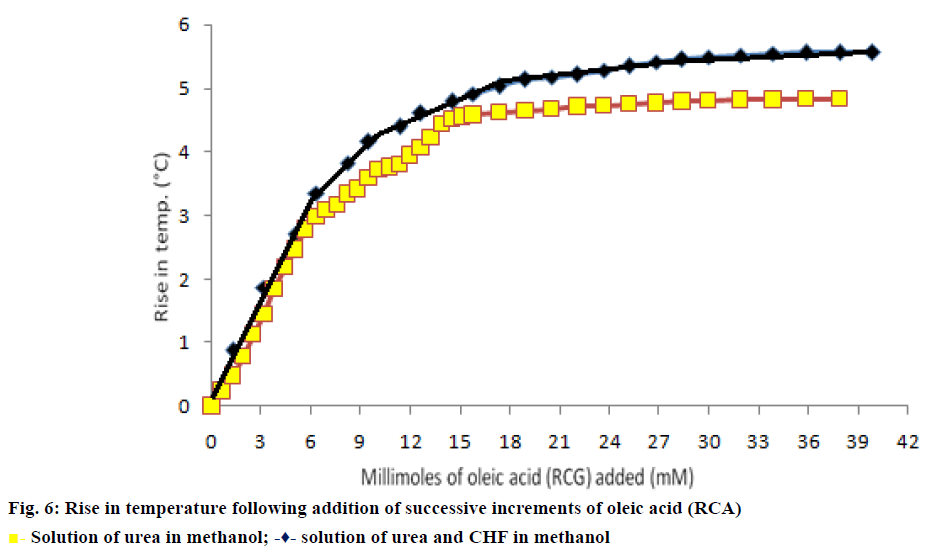
In the calorimetric method, the initial rise curve and final curve were extrapolated in (Figure 6a) to obtain the intersection point to calculate the amount of linear chain RCA required for inclusion with given quantity of urea. The minimum amount of oleic acid required for inclusion in urea was found to be 2.98 g of oleic acid/12 g of urea. This corresponds to 10.55 mmol of oleic acid required for inclusion in 0.20 M of urea [28-30].
In modified calorimetric procedure, the initial rise curve and intermediate transition phase curve of temperature rise were extrapolated (Figure 6b) to obtain an intersection point for obtaining the minimum amount of long chain RCA required for co-inclusion of CHF in urea. The minimum ratio of RCA:CHF required for incorporation of CHF in urea was 0.71:1 g/g. The minimum amount of oleic acid in terms of mmol (mM) required for coinclusion in 8.55 mmol of CHF was found to be 7.57 mmol of oleic acid [28-30].
The curve obtained had a smooth sigmoid shape for addition of oleic acid to methanolic solution of urea (Figure 6a) but the same curve in the presence of the insecticide CHF revealed the following sequence of events, that is, an initial temperature rise, followed by an intermediate transition phase, subsequently second temperature rise curve and then attainment of a final temperature. The second stage of temperature rise is due to the displacement of CHF (NNCG) with RCA as evident by the fact that the overall temperature rise is almost similar to that of RCA alone. The optimum formulation ratio of RCA:CHF calculated in terms of molar ratio required per mole of CHF for co-inclusion in urea was found to be 0.89:1.0 (0.71:1 g/g). After determining minimum ratio of RCA:CHF and urea co-inclusion complexes containing varying molar fractions of RCA in guests (Table 1) were prepared and subjected to further studies.
The overlay of DSC thermograms of co-inclusion complexes containing varying proportions of RCA and CHF is illustrated in (Figure 7). In all these thermograms, a low temperature endotherm was obtained corresponding to the decomposition of the complexed urea and to the release of a guest molecule and the solid tetragonal form of urea. The disappearance of the melting endotherm at 46.26° indicates the inclusion of the insecticide into the host lattice in an amorphous state.
Figure 8 exhibited the plot of heat of decomposition value against the molar fraction of RCA in guests in CHUCIC co-inclusion complexes. The results for heat of decomposition of urea co-inclusion complexes of CHF (CHUCIC) are listed in Table 1. The influence of the molar fraction of RCA on the heat of decomposition was statistically studied and the data exhibited the following relationship, ΔH=66.28R-11.28, (R2=0.947), ΔH is the heat of decomposition (J/g) of CHF urea co-inclusion complex (CHUCIC) and R is the molar fraction of long chain RCA in guests.
The heat decomposition curve shows an increase in the heat of decomposition value (J/G) in various CHUCIC complexes containing increasing molar fractions of long chain RCA and exhibits saturation level up to urea/ oleic acid (UOAC) complex without CHF [28-30]. The plot shows that an increase in molar fraction of linear chain RCA in the complex leads to corresponding rise in the heat of decomposition value with consequent better stability of the urea co-inclusion complex. The models for prediction of value of heat of decomposition of different urea inclusion complexes containing aliphatic guests have already been reported [47]. Since CHF is a bulky substituted aromatic molecule, it is wide enough to be entrapped inside the hexagonal channel formed by urea lattice. However, in the presence of the oleic acid (RCA), CHF is co-included along with linear straight RCA in urea.
Aqueous solubility studies indicated that aqueous solubility of chlorpyrifos increased in various co-inclusion complexes of chlorpyrifos in urea (CHUCIC-1 to CHUCIC-5) in comparison to pure insecticide. This increased solubility may be simply attributed to inclusion of chlorpyrifos in urea (Table 2).
| Product | Solubility (µg/ml) (Mean±SD, n=3) |
|---|---|
| CHF | 18.205±0.654 |
| CHUCIC-1 | 42.718±0.464 |
| CHUCIC-2 | 42.179±0.437 |
| CHUCIC-3 | 40.384±0.629 |
| CHUCIC-4 | 39.641±0.512 |
| CHUCIC-5 | 39.436±0.464 |
Table 2: Aqueous Solubility Data of Chf and Chucic Co-Inclusion Complexes
Content uniformity test listed in United States Pharmacopoeia (USP) was conducted to ensure homogeneity in a batch of low content insecticide. Though USP norms are basically meant for various dosage forms for human use but this test can also indicate extent of uniform distribution of insecticides in formulations for agricultural use. In order to meet the USP criterion of content uniformity, the formulation must possess 85-115% active ingredient of the label claim. The data of content uniformity analysis for different CHUCIC complexes containing varying RCA to insecticide ratios is compiled in Table 3. This table clearly revealed that an insecticide is uniformly distributed in urea co-inclusion complexes of CHF.
| Product | Percent (%) insecticide incorporated* (Mean±SD, n=10) |
|---|---|
| CHUCIC-1 | 92.664±0.320 |
| CHUCIC-2 | 91.344±0.368 |
| CHUCIC-3 | 90.118±0.345 |
| CHUCIC-4 | 89.684±0.361 |
| CHUCIC-5 | 89.172±0.487 |
Table 3: Content Uniformity Data of Chucic Co-Inclusion Complexes
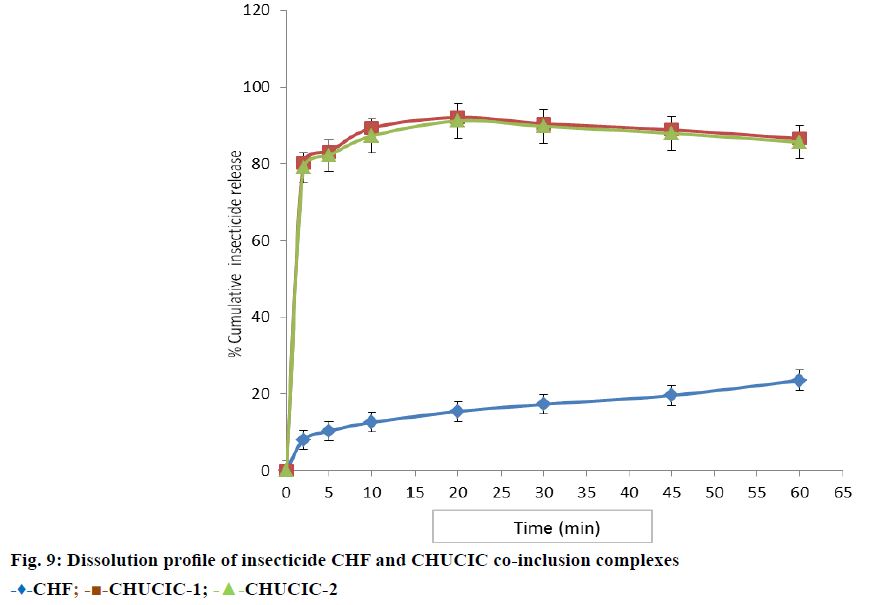
Figure 9 illustrates the dissolution profiles obtained from the experimental values of pure CHF and its urea co-inclusion complexes CHUCIC-1 and CHUCIC-2 in distilled water. Though USP norms are basically meant for dosage forms for human use but this test can also provide vital information with regard to rate at which insecticide will dissolve when exposed to water in agricultural fields. The dissolution profiles were determined by the dissolution percentage (DP) at 2, 5 and 10 min. The amount of the insecticide CHF released in water was found to be quite low. This was evident by determining values of DP exhibiting release of pure insecticide as DP2~8.08%; DP5~10.38% and DP10~12.69% However, co-inclusion of CHF in hexagonal urea resulted in an enhancement in release rate of insecticide as described in Table 4 by release of contents DP2~80.30%; DP5~83.07% and DP10~89.30% (CHUCIC-1); DP2~78.92%; DP5~81.23% and DP10~87.23% (CHUCIC-2). The dissolution profiles of CHUCIC-1 and CHUCIC-2 were found to be almost similar.
| Time (min) | Percent (%) insecticide release (Mean±SD, n=3) | ||
|---|---|---|---|
| CHF | CHUCIC-1 | CHUCIC-2 | |
| 0 | 0 | 0 | 0 |
| 2 | 8.08±2.88 | 80.31±3.85 | 78.92±2.07 |
| 5 | 10.38±2.49 | 83.08±4.32 | 82.15±2.43 |
| 10 | 12.69±1.44 | 89.31±5.67 | 87.23±2.04 |
| 20 | 15.46±2.11 | 92.08±5.22 | 91.15±1.44 |
| 30 | 17.31±1.83 | 90.46±4.91 | 89.77±2.62 |
| 45 | 19.62±2.11 | 88.85±5.28 | 87.92±2.49 |
| 60 | 23.54±2.50 | 86.54±4.84 | 85.61±2.79 |
Table 4: Dissolution Profile of Chf and Chucic Co-Inclusion Complexes
The insecticide release was rapid from co-inclusion complexes of CHF as compared to pure insecticide CHF. The hexagonal urea lattice dissolves rapidly as CHUCIC comes in contact with an aqueous dissolution medium and results in an instantaneous release of an incorporated insecticide. The co-inclusion of a NNCG drug along with linear straight RCA in urea hexagonal tunnels leads to weakening/distortion of urea host lattice and facilitates further dissolution enhancement. However, this immediate release of the insecticide was followed by a subsequent decrease in insecticide contents in solution and DP60 was found to be ~86.53% in CHUCIC-1. Since, as CHF has limited aqueous solubility, the initially released insecticide molecules undergo crystallization in excess of the solubility. However, in normal practice there is plentiful of water available in fields and hence initially released/ activated insecticide molecules on spraying of water in fields/soil will not undergo crystallization because of possible adsorption/absorption in crops/soil. Thus, urea co-inclusion complex formation can be exploited as a valuable technique for the development of rapid release formulation of insecticide CHF.
In current investigation, a successful attempt has been made for preparation, characterization and evaluation of human guarded chlorpyrifos urea co-inclusion complexes. CHF, a moderately toxic class II insecticide was successfully formulated into insecticide- fertilizer co-inclusion complex in order to overcome its toxicity, offensive odour, poor solubility and handling problems. CHF was incorporated in urea host lattice in the presence of a suitable linear chain RCA. CHF, a widely used toxic insecticide is entrapped in tunnels of hexagonal urea lattice and is accordingly not available for inhalation/odour or for contact with skin. Hence, personnel engaged in handling of an insecticide will not be exposed to its toxic effects after its co-inclusion in hexagonal urea. Pests will be exposed to insecticide only after co-inclusion complex of the insecticide comes in contact with water following switching on of an automated water sprinkling system in the agricultural fields. The formation of chlorpyrifos urea co-inclusion complexes was duly confirmed by DSC, FTIR, XRD and 1H-NMR studies. Since, CHF is engulfed in urea channels, therefore, a steep reduction in unacceptable odour of CHF was observed. Thermal/ regression studies reveal that an increase in the molar fraction of long chain RCA in the urea co-inclusion complex leads to rise in heat of decomposition value with consequent improved physical stability. CHUCIC complex revealed uniform formulation composition and improved dissolution profile of CHF. Steep reduction in odour of hazardous insecticide coupled with significant improvement of formulation and safe handling characteristics offer proposed insecticidefertilizer amalgamation technique a vast potential for agricultural use.
Acknowledgements
Authors are thankful to Insecticides (India) Ltd, Chopanki, Bhiwadi (Rajasthan) for providing gift sample of Chlorpyrifos. Authors thank JCDM College of Pharmacy, Sirsa, India for providing facilities for DSC studies. Authors are thankful to Indian Agricultural Research Institute, New Delhi, India for allowing XRD studies. Thanks are also due to SAIF, Panjab University, Chandigarh, India for providing NMR and XRD facilities.
Conflicts of the interest
Authors have not received any funds/financial assistance or grants from any funding agency for conducting the said research. Authors declare that they have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues and risk assessment indicators. Int J Environ Res Public Health 2011;8:1402-9.
- Story P, Cox M. Review of the effects of organophosphorus and carbamate insecticides on vertebrates. Are there implications for locust management in Australia? Wild Res 2001;28:179-93.
- GallowayT, Handy R.Immunotoxicityof organophosphorous pesticides.Ecotoxicol 2003;12:345-63.
- Flaskos J. The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. ToxicolLett 2012;209:81-93.
- Sanchez SF, Canadas F, Flores P, Lopez GM, Cardona D. Long-term functional neurotoxicity of paraoxon and chlorpyrifos: behavioral and pharmacological evidence. NeurotoxicolTeratol 2004;26:305-17.
- Bushnell PJ, Kelly KL, Ward TR. Repeated inhibition of cholinesterase by chlorpyrifos in rats: behavioural, neurochemical and pharmacological indices of tolerance. J Pharmacol ExpTher 1994;270:15-25.
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification (Report) 2009.
- Connors SL, Levitt P, Matthews SG, Slotkin TA, Johnston MV, Kinney HC, et al. Fetal mechanisms in neurodevelopmental disorders. PaediatrNeurol 2008;38:163-76.
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment.Crit Rev Toxicol 2008;38:1-125.
- Jett DA, Navoa RV, Lyons MA. Additive inhibitory action of chlorpyrifos and polycyclic aromatic hydrocarbons on acetylcholinesterase activity in vitro.ToxicolLett 1999;105:223-9.
- Sanchez?Amate MC, Flores P, Sanchez?Santed F. Effects of chlorpyrifos in the plus-maze model of anxiety. Behaviour Pharmacol 2001;2:285-92.
- Wu XM, Yu YL, Li M, Long YH, Fang H, Li SN. Prediction of bioavailability of chlorpyrifos residues in soil to earthworms. J Soil Sci Plant Nutr 2011;11:44-57.
- Zhang B, Qin L, Che G, Li-Meng Z, Fan Z. Molecular recognition of chlorpyrifos by β-cyclodextrin. Chin AgriSci Bull 2010;17:10-17.
- Karim Z, Adnan R. The β-cyclodexrin-chitosan inclusion complex: characterization and application in the removal of pesticides in wastewater. Arch Environ Sci 2012;6:1-12.
- Lucas-Abellan C, Gabaldon-Hernandez JA, Penalva J, Fortea MI, Nunez-Delicado E. Preparation and characterization of inclusion complex of chlorpyrifos in cyclodextrins to improve insecticide formulations. J Agri Food Chem 2008;56:8081-5.
- Madan AK. Microencapsulation of low dose drugs. Ph.D Thesis. IIT, New Delhi: India; 1994.
- Madan AK, Grover PD. A process for preparation of urea based inclusion compounds of vitamin A esters. Indian Patent Number 180627; 1993.
- Madan AK, Bajaj V. A process for preparation of urea based inclusion compounds of vitamin E and its esters. Indian Patent Number 182620; 1994.
- US Department of Labour. Occupational safety and health guidelines for chlorpyrifos 1995;1-8.
- Frank SG. Inclusion compounds. J Pharm Sci 1975;64:1585-1604.
- Marsh KL, Sims GK, Mulvaney RL. Availability of urea to autotrophic ammonia oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil. BiolFert Soil 2005;42:137-45.
- Torello WA, Wehner DJ. Urease activity in a Kentucky bluegrass turf.Agron J 1983;75:654-6.
- Harris KDM. Meldola Lecture: Understanding properties of urea and thiourea inclusion compounds. ChemSoc Rev 1997;26:279-89.
- Smith AE. The crystal structure of urea-hydrocarbon complexes. ActaCrystallogr 1952;5:224-35.
- Thakral S, Madan AK. Urea inclusion compounds of enalapril maleate for the improvement of pharmaceutical characteristics. J Pharm Pharmacol 2007;59:1501-7.
- Thakral S, Madan AK. Urea co-inclusion compounds of glipizide for the improvement of dissolution profile. J InclPhenomMacrocyclChem 2008;60:203-9.
- Thakral S, Madan AK. Adduction of amiloride hydrochloride in urea through a modified technique for the dissolution enhancement. J Pharm Sci 2008;97:1191-1201.
- Dhall M, Madan, AK. Simultaneous improvement in dissolution profile and content uniformity of lafutidine through co-inclusion in urea. J InclPhenomMacrocyclChem2015;82:335-50.
- Dhall M, Madan, AK. Studies on urea co-inclusion complexes of simvastatin for improvement of pharmaceutical characteristics. J InclPhenomMacrocyclChem2015;81:105-20.
- Dhall M, Madan, AK. Steep improvement in dissolution profile of ezetimbe through co-inclusion in urea. J Pharm Invest 2016;46:1-19.
- Thakral S, Madan AK. Urea co-inclusion compounds of 13 cis-retinoic acid for simultaneous improvement of dissolution profile, photostability and safe handling characteristics. J Pharm Pharmacol 2008;60:823-32.
- Thakral S, Madan AK. Reduction in moisture sensitivity/ uptake of moisture sensitive drugs through adduction in urea. J Pharm Innov 2008;3:249-57.
- Dhall M, Madan AK. Conversion of viscous liquid malathion into free flowing solids through co-inclusion in urea for multiple benefits. J InclPhenomMacrocyclChem 2016;86:135-51.
- Dhall M, Madan AK. Urea complexes of chlorpyrifos, malathionbifenthrin and cypermethrin for improving safe handling and other characteristics. Indian Patent No. 201611002986.2016.
- Pumamadjaja AH, Russell RA. Pheromone communication in a robot swarm: Necrophoric bee behavior and its replication. Robotica 2005;23:731-42.
- Zimmerschied WJ, Dinnerstein RA, Weitkamp AW, Marschner, RF. Crystalline adducts of urea with linear aliphatic compounds. IndEngChem 1950;42:1300-6.
- Salem IB, Errami M, Menzi M, Salghi R, Ebenso EE, Hammouti B, et al. Biological, ionizing and ultraviolet radiation and electrochemical degradation of chlorpyrifos pesticide in aqueous solutions. Int J ElectrochemSci 2014;9:342-51.
- Uniformity of dosage units. In: United States Pharmacopeia and National Formulary USP 28–NF 23.Rockville MD: United States Pharmacopeial Convention Inc; 2011. p. 2505-10.
- Brown WL, Policello GA. Antidrift composition. Patent application Number WO2014018070 A1. 2014.
- XiaoG, DongD, LiaoT, LiY, ZhengL, ZhangD, et al. Detection of pesticide (chlorpyrifos) residues on fruit peels through spectra of volatiles by FTIR. Food Anal Meth 2015;8:1341-6.
- Fischer PHH, McDowell CA. The infrared absorption spectra of urea-hydrocarbon adduct. Can J Chem 1960;38:187-93.
- Frederickson HK, Hansen HC, Borggaard O, Pedersen M. Starch encapsulated chlorpyrifos: release rate, insecticidal activity and degradation in soil. J Microencapsul 2002;19:319-31.
- McAdie MG. Thermal decomposition of molecular complexes. Can J Chem 1963;41:2144-53.
- Wu JW, Laird DA. Interactions of chlorpyrifos with colloidal materials in aqueous systems. J Environ Qual 2004;33:1765-70.
- Brodman BW, Radell J. X-ray powder diffraction patterns of some alkanone urea inclusion compounds. SeperationSci 1967;2:139-42.
- Silambarasan S, Abraham J. Ecofriendly method for bioremediation of chlorpyrifos from agricultural soil by novel fungus Aspergillusterreus JAS1. Water Air Soil Pollut 2013;224:1369.
- Thakral S, Madan AK. Topological models for prediction of heat of decomposition of urea inclusion compounds containing aliphatic endocytes. J InclPhenomMacrocyclChem 2012;60:187-92.
 Solution of urea in methanol;
Solution of urea in methanol;  solution of urea and CHF in methanol
solution of urea and CHF in methanol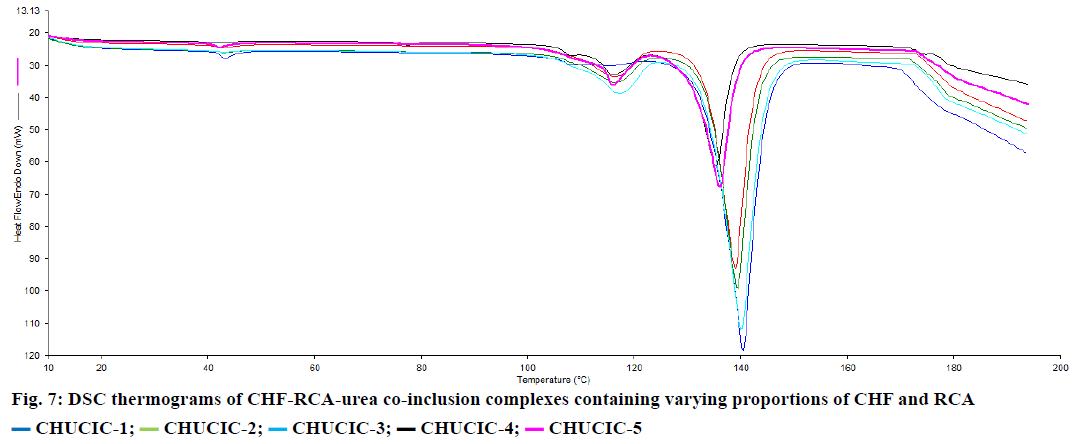
 CHUCIC-1;
CHUCIC-1;  CHUCIC-2;
CHUCIC-2;  CHUCIC-3;
CHUCIC-3;  CHUCIC-4;
CHUCIC-4;  CHUCIC-5
CHUCIC-5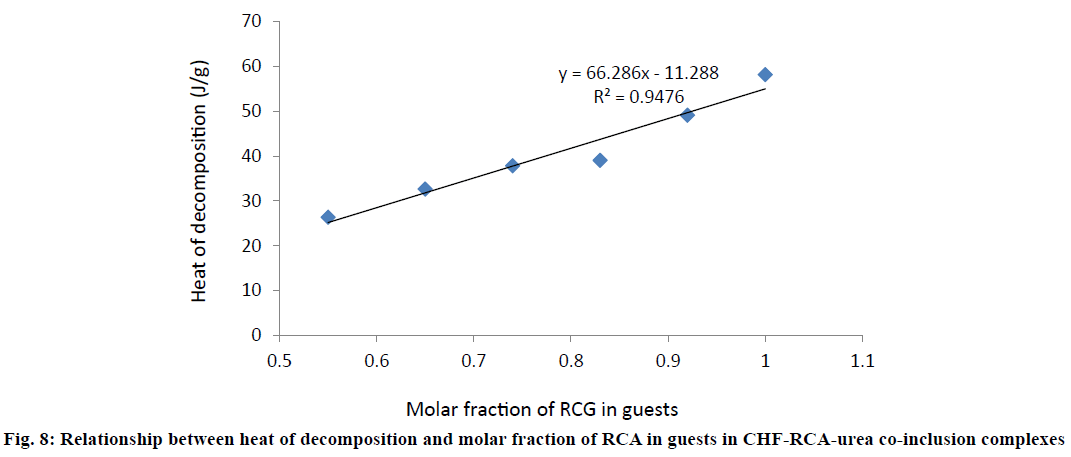
 CHF;
CHF;  CHUCIC-1;
CHUCIC-1;  CHUCIC-2
CHUCIC-2



