- *Corresponding Author:
- A. Vinayagam
Department of Chemistry, Sathyabama University, Chennai-600 119, 1Department of Chemistry, DKM College for Women, Thiruvallur University, Vellore-632 001, India
E-mail: vinayaga_star@yahoo.co.in
| Date of Submission | 05 February 2014 |
| Date of Revision | 24 October 2014 |
| Date of Acceptance | 22 January 2015 |
| Indian J Pharm Sci,2015;77(1):91-95 |
Abstract
Four major compounds were separated and identified from the methanol extracts of Nerium indicum flowers (Arali) using HPLC and mass spectral data. Through mass data, the chemical structures were elucidated as: trans5-O-caffeoylquinic acid (1), quercetin-3-O- rutinoside (2), luteolin-5-O-rutinoside (3) and luteolin-7-O-rutinoside (4). In addition, the cis isomers of 5-O-caffeoylquinic acid in Nerium indicum flowers were confirmed by Mass, HPLC and UV. The structures of these compounds confirmed with the help of liquid chromatography mass spectrometry.
Introduction
any plants produce chlorogenic acids in which esterification occurs at positions 3, 4 and 5 of the quinic acid moiety. Esterification at position 1 is less frequent, but 1-acyl chlorogenic acids are found in some Asteraceae [1-3].Flavonoids and phenolic acids have protective role in carcinogenesis, inflammation, atherosclerosis, thrombosis and have high antioxidant capacity. Furthermore, flavonoids have been reported as aldose reductase inhibitors blocking the sorbitol pathway that is linked to many problems associated with diabetes [4-8]. Flavonoids interact with various enzymatic systems. Their inhibition of the enzymes cyclooxygenase and lipooxygenase results in a decrease of platelet activation and aggregation,protection against cardiovascular diseases, cancer chemoprevention and their antiinflammatory activity [9-13]. Many other biological activities are attributed to flavonoids and phenolic acids: antiviral, antimicrobial, antihepatotoxic, antiosteoporotic, antiulcer, immunomodulatory, antiproliferative and apoptotic activity [14-22].
The purpose of this research was to separate and identify phenolic compound and flavonoids from Nerium indicum flowers. A sensitive, accurate and specific method coupling high performance liquid chromatography (HPLC) with diode array detector (DAD) and electrospray ionization mass spectrometry (MS) was developed for the separation and identification of phenolic acid and flavonoids, in the methanolic extract of Nerium indicum flowers. The molecular masses of phenolic acid and flavonoids were assigned by electrospray ionization using ion trap mass spectrometry in negative mode.
Materials and Methods
Trans 5-O-caffeoylquinic acid (1), quercetin-3- O-rutinoside (2), luteolin-5-O-rutinoside (3) and luteolin-7-O-rutinoside (4) were separated from the plant material, its purity was checked by HPLC and structure elucidated by MS spectral data. Acetonitrile and methanol were HPLC grade from Merck (Darmstadt, Germany). Water was purified by a Milli-Q system from Millipore (Milford, USA).
Analyses were performed on Agilent 1200 chromatograph equipped with a diode array detector and mass detector in series (Agilent Technologies, Waldbronn, Germany). A Phenomenex LUNA -C18, 4.6×150 mm, particle size 5 μm with suitable guard column was employed for the separation. The binary mobile phase consisted of solvents A as 0.1% formic acid in water and solvents B as acetonitrile. The gradient elution started with 10% B and changed to 75% B in 20 min, then reached 95% B in 27 min. After each run the chromatographic system was set to 10% B in 4 min and equilibrated for 4 min. The flow rate was 1.0 ml/min and Injection volume was 5 μl.
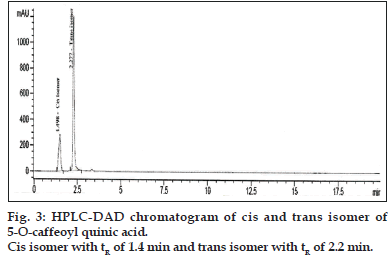
Separation of trans and cis isomer of 5-O-caffeoylquinic acid was attempted by using a Zorbax SB phenyl, 4.6×100 mm, particle size 1.8 μm with suitable guard column. The binary mobile phase consisted of solvents A as 0.1% formic acid in water and solvents B as acetonitrile. The isocratic elution started with 20% B and 80% A. The flow rate was 0.8 ml/min and Injection volume was 5 μl.
Spectral data for all peaks were recorded in the range of 200-400 nm. The mass detector was an ion trap spectrometer (Agilent LC/MSD Trap XCT) equipped with an electrospray ionization interface and controlled by LCMSD software. The ionization conditions were adjusted at 300° and 3.5 kV for capillary temperature and voltage, respectively. The nebulizer pressure was 40 psi and the nitrogen flow rate was 8 l/min. Collision-induced fragmentation experiments were performed in the ion trap using helium as a collision gas, with voltage cycles from 0.3 up to 2 V. All mass spectrometry data were recorded in negative ion mode.
The screening was performed in full scan covering the range from m/z 100 up to 1000; multiple reaction monitoring (MRM) mode. UV spectra were recorded on Agilent UV/Vis 8453 diode array spectrophotometer.
Plant material
The flowers of Nerium indicum were collected from the surroundings of Vellore, in November 2013, and identified at the Department of chemistry, Thiruvallur University. The plant material was air dried, smashed into powder and stored in a desiccator.
Extraction and isolation
The flowers of Nerium indicum (50 g) were extracted 3 times with MeOH under reflux for 3 h. Evaporation of the solvent under reduced pressure provided the MeOH extract (5.1 g). To separate the compound, preparative liquid chromatography was done using acetonitrile-formic acid-water as mobile phase and the octadecylsilane as a stationary phase.
Results and Discussion
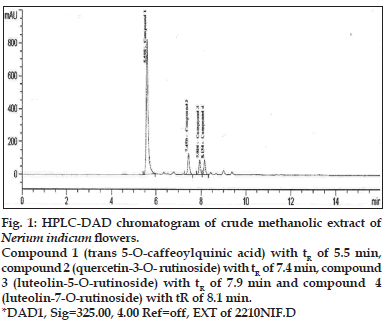
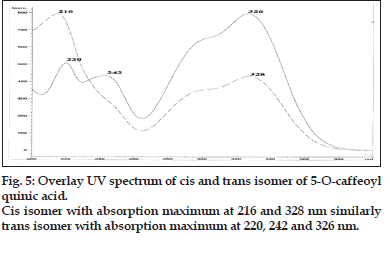
A method coupling HPLC with diode-array detector (DAD) and electrospray ionization mass spectrometry with an ion trap analyzer was optimized for the separation and identification of phenolic acid in the extract of Nerium indicum flowers. Different mobile phase compositions were screened to obtain chromatograms with good resolution within an acceptable time of analysis. Formic acid, as solvent A, and acetonitrile, as solvent B, were chosen for the gradient elution. Especially the formic acid and acetonitrile are volatile and thus compatible with LC/ MS system. Formic acid (lower pH values) ensures better sample separation but shortens the HPLC column lifetime and affects ESI ionization. 325 nm were chosen as monitoring wavelengths according to absorption maxima of analytes. A typical chromatogram showing the separation of 4 components is presented in fig. 1.
Fig. 1: HPLC-DAD chromatogram of crude methanolic extract of Nerium indicum flowers. Compound 1 (trans 5-O-caffeoylquinic acid) with tR of 5.5 min, compound 2 (quercetin-3-O- rutinoside) with tR of 7.4 min, compound 3 (luteolin-5-O-rutinoside) with tR of 7.9 min and compound 4 (luteolin-7-O-rutinoside) with tR of 8.1 min. *DAD1, Sig=325.00, 4.00 Ref=off, EXT of 2210NIF.D
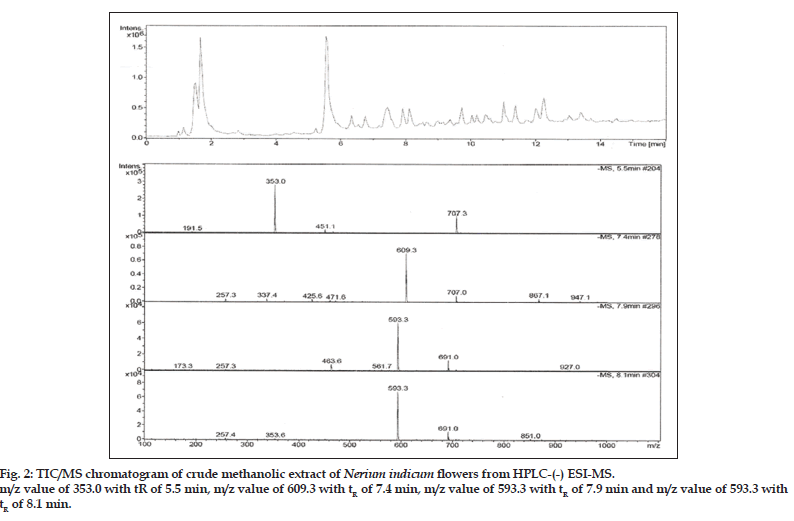
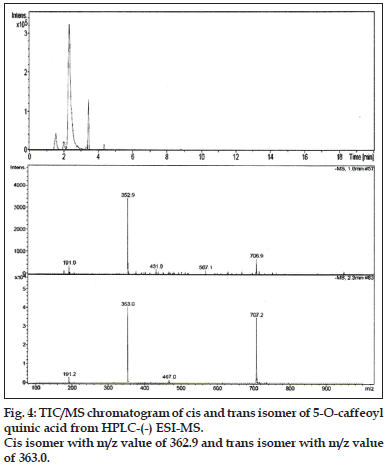
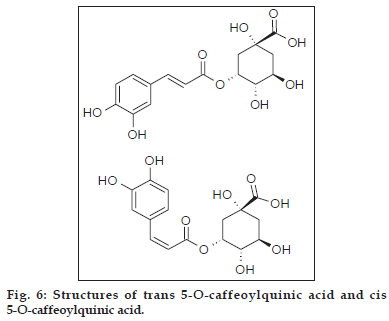
Compound 1 observed as a [M-H] - ion peak at m/z 353 in the ESI mass spectrum (fig. 2) and based on literature data [23] compound 1 was confirmed as a 5-O-caffeoyl quinic acid. By using Zorbax SB Phenyl column able to separate cis and trans isomer of 5-O-caffeoyl quinic acid (fig. 3) gave an [M-H]-ion peak at m/z 353 in the ESI mass spectrum (fig. 4). UV spectra also suggest that trans isomer is more absorption value compared to cis isomer (fig. 5). Mass spectral value of compound 2 showed a quasi-molecular ion [M-H] - at m/z 609, compared with literature data [24] compound 2 was identified as a quercetin-3-O-rutinoside (fig. 2).
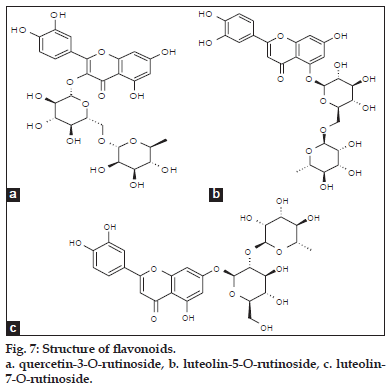
ESI-MS of compound 3, 4 showed a quasimolecular ion [M-H] - at m/z 593, both are same mass value only position is different and based on literature data [25] compounds 3 and 4 identified as luteolin-5-O-rutinoside and luteolin-7-O-rutinoside (fig. 2).
Based on the above data two phenolic acids, trans 5-O-caffeoylquinic acid and cis 5-O-caffeoylquinic acid (fig. 6). Three flavonoid glycosides, quercetin- 3-O-rutinoside, luteolin-5-O-rutinoside and luteolin- 7-O-rutinoside (fig. 7) were identified from crude methanolic extract of Nerium indicum flowers.
References
- Papetti A, Daglia M, Aceti C, Sordelli B, Spini V, Carrazzone C, et al.Hydroxycinnamic acid derivatives occuring in Cichoriumendiviavegetables. J Pharm Biomed Anal 2008;48:472-6.
- Clifford MN, Wu W, Kuhnert N. The chlorogenic acids of Hemerocallis. Food Chem 2006;95:574-8.
- Perrone D, Farah A, Donangelo CM, De Paulis T, Martin PR. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem 2008;106:859-86.
- Tapiero H, Tew KD, Ba GN, Mathe G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed Pharmacother 2009;56:200-7.
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481-504.
- Hung TM, Na MK, Thoung PT, Su ND, Sok DE, Song KS, et al. Antioxidant activity of caffeoylquinic acid derivatives from the roots of Dipsacusasper Wall. J Ethnopharmacol 2006;108:188-92.
- Yonathan M, Asres K, Assefa A, Bucar F. In vivoantiinflammatory andantinociceptive activities of Chelianthes farinose. J Ethnopharmacol 2006;108:462-70.
- Bonita JS, Mandarano M, Shuta D, Vinson J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol Res 2007;55:187-98.
- Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr 2004;59:113-22.
- Sadhu SK, Okuyama E, Fujimoto H, Ishibashi M, Yesilada E. Prostaglandin inhibitory and antioxidant components of Cistuslaurifolius, a Turkish medicinal plant. J Ethnopharmacol 2006;108:371-8.
- Kong LD, Abliz Z, Zhou CX, Li LJ, Cheng CH, Tan Rx. Glycosides and xanthine oxidase inhibitors from Conyzabonariensis. Phytochemistry 2001;58:645-51.
- Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenase by flavonoids: Structure-activity relations and mode of action. Biochem Pharmacol 2003;65:773-81.
- Al-Fayez M, Cai H, Tunstall R, Steward WP, Gescher AJ. Differential modulation of cyclooxygenase-mediated prostaglandin production by the putative cancer chemopreventive flavonoids tricin, apigenin and quercetin. Cancer ChemotherPharmacol 2006;58:816-25.
- Li Y, But PP, Ooi VE. Antiviral activity and mode of action of caffeoylquinic acids from Scheffleraheptaphylla (L.) Frodin. Antiviral Res 2005;68:1-9.
- Sousa A, Ferreira IC, Calhelha R, Andrade PB, Valentão P, Saebra R, et al. Phenolics and antimicrobial activity of traditional stoned tableolives ‘alcaparra’. Bioorg Med Chem 2006;14:8533-8.
- Kim KH, Kim YH, Lee KR. Isolation of quinic acid derivatives and flavonoids from the aerial parts of Lactucaindica L. and their hepatoprotective activity in vitro. Bioorg Med ChemLett 2007;17:6739-43.
- Oh H, Kim DH, Cho JH, Kim YC. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J Ethnopharmacol 2004;95:421-4.
- Innocenti G, Vegeto E, Dall’Acqua S, Ciana P, Giorgetti M, Agardi E, et al. In vitro estrogenic activity of Achilleamillefolium L. Phytomedicine 2007;14:147-52.
- Hamauzu Y, Irie M, Kondo M, Fujita T. Antiulcerative properties of crude poloyphenols and juice of apple, and Chinese quince extracts. Food Chem 2008;108:488-95.
- Ha CL, Weng CY, Wang L, Lian TW, Wu MJ. Immunomodulatory effect of Glossogynetenuifolia in murine peritoneal macrophages and splenocytes. J Ethnopharmacol 2006;107:116-25.
- Pieroni A1, Pachaly P, Huang Y, Van Poel B, Vlietinck AJ. Studies on anti-complementary activity of extracts and isolated flavones from Ligustrumvulgare and Phyllyrealatifolia leaves (Oleaceae). J Ethnopharmacol 2000;70:213-7.
- Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin induced-apoptosis is mediated by the activation of PKCd and caspases in leukaemia cells. BiochemPharmacol 2006;72:681-92.
- Bashir A. Antioxidant activity and phenolic compounds from ColchicumluteumBaker (Liliaceae). Afr J Biotechnol 2010;9:5762-6.
- N’Dri D, Calani L, Mazzeo T, Scazzina F, Rinaldi M, Rio DD, et al. Effects of Different Maturity Stages on Antioxidant Content of Ivorian Gnagnan (Solanumindicum L.) Berries. Molecules 2010;15:7125-38.
- Plazonic A, Bucar F, Maleš Ž, Mornar A, Nigovic B, Kujundžic N. Identification and Quantification of Flavonoids and Phenolic Acids in Burr Parsley (Caucalisplatycarpos L.), Using High- Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Mass Spectrometry. Molecules 2009;14:2466-90.