- *Corresponding Author:
- V. R. Patel
Baroda College of Pharmacy, Parul Group of Institutes, Limda, Waghodia, Vadodara‑391 760
E‑mail: vish_rx@yahoo.com
| Date of Submission | 14 May 2012 |
| Date of Revision | 19 January 2013 |
| Date of Acceptance | 22 January 2013 |
| Indian J Pharm Sci 2013;75(1):106-109 |
Abstract
Manjisthadi churna has been traditionally used in the Ayurvedic system of medicine and by traditional medical practices of India to treat hyperlipidemia. A rapid, simple and accurate method with high performance thin layer chromatography has been developed to standardised Manjisthadi churna using rubiadin, sennoside and ellagic acid as markers. Methanol extract of Manjisthadi churna were used for high performance thin layer chromatography on silica gel plates. The Rf of rubiadin, sennoside-A and ellagic acid were found to 0.48, 0.23 and 0.72, respectively with densitometric scanning at 280 nm and the calibration plot were linear in the range of 100-600 ng of markers. The correlation coefficients were higher than 0.99 were indicative of good linear dependence of peaks area on concentration. The rubiadin, sennoside-A and ellagic acid contents in Manjisthadi churna were found to be 0.014, 0.038 and 0.534% w/w, respectively. This method permits reliable quantification of rubiadin, sennoside-A and ellagic acid with good resolution and separation of the same from other constitutes of the extract of Manjisthadi churna. Recovery value from 95.66-102.33% showed the reliability and reproducibility of the method. The proposed high performance thin layer chromatography method for simultaneous quantification of markers in Manjisthadi churna can be used for routine quality testing.
Keywords
Ellagic acid, high performance thin layer chromatography, Manjisthadi churna, Rubiadin, Sennoside
Standardisation and analysis of the chemical marker of the Ayurvedic and other polyherbal formulation is always very big problem. Standardisation of herbal formulations is essential in order to assess the quality of drugs, based on the concentration of their active principles [1]. Ayurveda, the ancient system of Indian medicine has gained worldwide attention due to its safety and efficacy. However, one of the impediments in the acceptance of the ayurvedic medicines is the lack of standard quality control profiles. Standardisation and development of reliable quality protocols for Ayurvedic formulations using modern techniques of analysis is extremely important [2,3]. In majority of the cases, the traditional extraction, separation and quantification technique remain failure in term of accuracy, precision and reproducibility of the method [4]. The advances in chromatographic techniques made it possible to quantify the chemical constituents in a mixture with comparatively little clean‑up using high performance thin layer chromatography (HPTLC) [5].
Manjisthadi churna is an Ayurvedic preparation mentioned in the chapter Trutiya Sanskar of Ayurvedic literature Bruhad Ausadhiya Shuchi Patra: Therapeutic Index [6]. Manjisthadi churna has been also used by traditional medical practices of India to treat hyperlipidemia. It consists of the mixture of the fine powder of the dried stem of Manjistha (Rubia cordifolia Linn., F.‑Rubiaceae) [7], dried leaf of Svarnapatri (Cassia angustifolia Vahl., F.‑Leguminosae) [8], dried pericarp of fruits of Haritaki (Terminalia chebula Retz. F.‑Combretaceae) [9] and dried root of Trivrit (Operculina turpethum Linn., Syn. Ipomoea turpethum, F.‑Convolvulaceae) [10]. Traditionally it is widely used for the treatment of skin disease, hyperlipidemia and in constipation. Rubiadin [11], sennoside‑A [12] and ellagic acid [13] are the main active markers present in the Manjistha, Svarnapatri and Haritaki, respectively and are mainly responsible for their aid pharmacological action.
In the present investigation, we have developed simple, optimised and validated HPTLC method for the standardization of Manjisthadi churna. Three chemical markers were selected, one from each medicinal herbs used as raw materials. The method was validated on the basis of its selectivity, linearity, precision, accuracy, limit of detection (LOD) and limit of quantification (LOQ) according to International Conference on Harmonization (ICH) requirements.
Analytical grade toluene, ethyl acetate, methanol and formic acid were obtained from Merck, Darmstadt, Germany. Rubiadin, sennoside‑A and ellagic acid were obtained from Natural remedies, Banglaru, India. Individual components of Manjisthadi churna were procured from M/s Yucca Enterprise, Mumbai, India and authenticated by botanist and comparison with herbarium specimens. The drugs were cleaned, dried and powdered separately and passed through 80 # sieve. These all powders were mixed well in equal proportion uniformly to prepared Manjisthadi churna.
Four grams of powder from Manjisthadi churna and 1 g of its three ingredients, Manjistha, Svarnapatri and Haritaki were extracted three times with 100 ml methanol. The extract were combined and concentrated at reduce temperature (50o) on rotary evaporator (Equitron rotevar, Medica Instrument Mfg. Co., Mumbai, India) upto 100 ml. Prior to use, all sample were filtered through a 0.45 µm nylon membrane filter. Ten milligrams of the rubiadin, sennoside and ellagic acid were dissolved separately in 10 ml of methanol. One milliliter of this was further diluted to 10 ml in volumetric flask with methanol. This was serve as a standard stock solution (100 ng/µl).
Optimum chromatographic conditions were obtained after running different mobile phase. Many different mobile phase and scanning wavelength were tried for the best separation of peaks. Chromatography was performed at 25±2°, relative humidity 40%, on 10×10 cm aluminum backed HPTLC plates coated with 0.2‑mm layers of silica gel 60F254 (E. Merck). Solutions (5 μl) were applied to the plates as bands 6 mm wide by use of a Camag (Muttenz, Switzerland) Linomat V sample applicator equipped with a 100 μl syringe (Hamilton, Bonaduz, Switzerland). Linear ascending development to approximately 80 mm from the point of application was performed with toluene:ethyl acetate:methanol:formic acid (10:9:6:5 v/v) as mobile phase in a Camag 20×10×4 cm glass twintrough chamber previously saturated with mobile phase vapor for 25 min. After development, the plate was dried in hot air oven at 105° for 5 min. Densitometric scanning in absorbance mode at 280 nm was then performed by use of a Camag thin layer chromatography (TLC) scanner‑III linked to Wincats software (V 1.4.3.6336, Camag, Muttenz, Switzerland). The slit dimensions were 5.00×0.45 mm and the scanning speed 20 mm/s. For calibration, stock solutions of rubiadin, sennoside‑A and ellagic acid of different concentration (100‑600 ng/band) were applied to the HPTLC plate to prepare a calibration plot and to check for reproducibility. Peak areas were recorded and calibration plots were prepared by plotting average peak area against concentration. Peak area and amount of standards data were treated by linear least‑square regression analysis.
The method was validated according to ICH [14] guideline for linearity, precision, accuracy, selectivity, LOD and LOQ. Selectivity was checked using an extract of Manjisthadi churna and a mixture of standards in order to optimise separation and detection. Linearity of the method was performed by analysing a standard solution of markers by the proposed method in the concentration range 100‑600 ng/spot. The accuracy of the proposed method was determined by a recovery study, carried out by adding standard markers in the Manjisthadi churna extract. The samples were spiked with three different amounts of standard compounds prior to extraction. The spiked samples were extracted in triplicate and analysed under the previously established optimal conditions. The obtained average contents of the target compounds were used as the actual values in order to calculate the spiked recoveries. Precision was determined by repeatability and interday and intraday reproducibility experiments of the proposed method. A standard solution containing three markers was injected six times; Manjisthadi churna was also extracted six times to evaluate the repeatability of the extraction process. The mean amounts and standard deviation (SD) value of each constitute were calculated. The LOD and LOQ of markers compounds were calculated at signal‑to‑noise ratio of approximate 3:1 and 10:1 respectively.
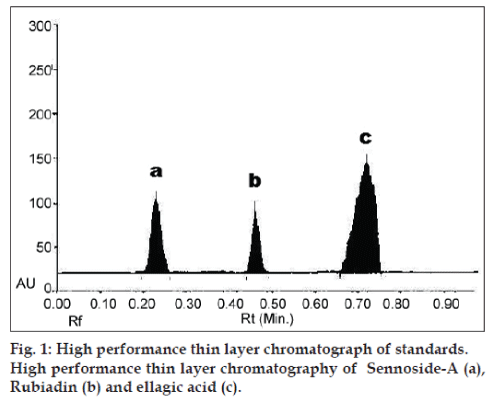
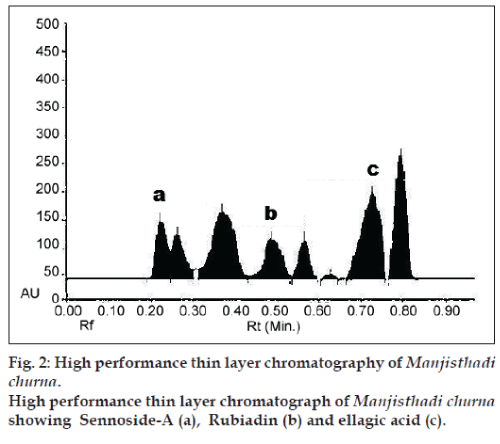
A densitometric methods were developed using HPTLC for the quantification of three marker compounds from Manjisthadi churna. Solvent systems were optimised to achieve best resolution of the marker compounds from the other components of the sample extracts. Several methods tried to seperat out markers from extract such as 2‑propanol:ethyl acetate methanol:formic acid (17:19:12:2), methanol:acetic acid:formic acid (20:1:80). Of the various solvent system tried, the one containing toluene:ethyl acetate:methanol:formic acid (10:9:6:5) gave the best resolution of rubiadin (Rf=0.48), sennoside-A (Rf=0.23) and ellagic acid (Rf=0.72) in the presence of other compounds in the sample extract and enabled the quantification of marker compounds. The amount of markers found in Manjisthadi churna were 0.014±0.03% w/w (rubiadin), 0.038±0.06% w/w (sennoside‑A) and 0.534±0.05% w/w (ellagic acid) respectively. The HPTLC chromatogram obtained for Markers and Manjisthadi churna are shown in figs. 1 and 2, respectively.
For qualitative purposes, the method was evaluated by taking into account the precision in the Rf and selectivity of marker compounds. A high repeatability in the Rf time was obtained for both, standards and extracts even at high concentration. LOD and LOQ values for rubiadin 45 and 114 ng/spot, sennoside-A 30 and 99 ng/spot and for ellagic acid 22 and 66 ng/ spot, respectively. Linear correlation was obtained between peak area and concentration of two markers in the range of 100‑600 ng/spot. Values of the regression coefficients (r2) of the markers were higher than 0.99, thus confirming the linearity of the methods. The high recovery values (95.66‑102.33%) indicated a satisfactory accuracy. Relative standard deviation (RSD) of all the parameters was less than 2.24% for the degree of repeatability, indicating the high repeatability of the proposed method. The low coefficient of variation values of intraday and interday precision reveals that the proposed method is precise. The result of validation parameters were shown in Tables 1‑3. Therefore, this HPTLC method can be regarded as selective, accurate and precise.
| Markers | Conc. range (ng/spot) | Rfa | Regression equation | R2 | LOD (ng/spot) | LOQ (ng/spot) |
|---|---|---|---|---|---|---|
| Rubiadin | 100‑600 | 0.48±0.01 | y=13610x+10658 | 0.999 | 55 | 184 |
| Sennoside | 100‑600 | 0.26±0.08 | y=361.36x+104.71 | 0.995 | 30 | 99 |
| Ellagic acid | 100‑600 | 0.72±0.04 | y=321.62x+136.53 | 0.997 | 20 | 66 |
aMean±SD (n=6), LOD=Limit of detection, LOQ=Limit of quantification
Table 1: Summary of developed high performance thin layer chromatography method.
| Markers | Contentsa (mg/g) | Added amount (mg) |
Recorded amounta (mg) |
Recovery ratea (%) |
RSD (%) |
|---|---|---|---|---|---|
| Rubiadin | 0.14±0.03 | 0.1 | 0.235±0.11 | 95.66±1.08 | 1.12 |
| 0.2 | 0.337±0.18 | 98.33±2.04 | 2.07 | ||
| 0.3 | 0.437±0.07 | 98.89±0.77 | 0.77 | ||
| Sennoside | 0.38±0.06 | 0.3 | 0.681±1.07 | 100.33±0.73 | 0.73 |
| 0.4 | 0.774±2.04 | 98.55±1.55 | 1.57 | ||
| 0.5 | 0.876±1.08 | 99.10±1.83 | 1.84 | ||
| Ellagic acid |
5.34±0.05 | 3 | 8.409±0.17 | 102.33±2.30 | 2.24 |
| 5 | 10.337±0.02 | 100.75±0.71 | 0.7 | ||
| 7 | 12.488±1.10 | 101.98±1.08 | 1.06 |
aMean±SD (n=3)
Table 2: Repeatability and recovery tests for the markers in manjisthadi churna.
| Compound | Intradayb | Interdayc | ||
|---|---|---|---|---|
| Contentsa (% w/w) |
RSD (%) |
Contentsa (% w/w) |
RSD (%) |
|
| Rubiadin | 0.014±0.03 | 1.54 | 0.014±0.02 | 1.12 |
| Sennoside | 0.038±0.06 | 1.81 | 0.037±0.09 | 0.22 |
| Ellagic acid |
0.534±0.05 | 2.17 | 0.531±0.04 | 2.21 |
aMean±SD (n=6), bSample were analyzed six times a day, cSample were analyzed once a day over six consecutive days
Table 3: Precision of developed method for markers in manjisthadi churna.
The results indicate that Manjisthadi churna contains a number of markers that may be responsible for its therapeutic activity. The developed HPTLC method will assist in the standardisation of Manjisthadi churna using biologically active chemical markers. The proposed HPTLC methods for simultaneous estimation of rubiadin, sennoside‑A and ellagic acid from Manjisthadi churna seems to be accurate, precise, reproducible and repeatable. Manjisthadi churna also contained a number of other constitute, which are currently the subject of further investigation, apart from those standards studied. With the growing demand for herbal drugs and with increased belief in the usage of herbal medicine, this standardisation tool will help in maintaining the quality and batch to batch consistency of this important Ayurvedic preparation.
Acknowledgements
The authors thank to S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Mehsana and Parul Institute of Pharmacy and Research (formally known as Baroda College of Pharmacy) Vadodara for providing facility to carry out this research.
References
- Ong ES. Extraction methods and chemical standardization of botanicalsand herbal preparations. J Chromatogr B AnalytTechnol Biomed LifeSci 2004;812:23‑33.
- Asokar LV, Kakkar KK, Chakra OJ. Glossary of Indian MedicinalPlants with Active Principles. New Delhi: Publication and InformationDirectorate; 1992. p. 122.
- Ravishankara MN, Shrivastava N, Padh H, Rajani M. HPTLC methodfor the estimation of alkaloids of Cinchona officinalisstem bark andits marketed formulations. Planta Med 2001;67:294‑6.
- Dhalwal K, Biradar YS, Rajani M. High‑performance thin‑layerchromatography densitometric method for simultaneous quantitation ofphyllanthin, hypophyllanthin, gallic acid, and ellagic acid in Phyllanthusamarus. J AOAC Int 2006;89:619‑23.
- Gupta AK, Tandon N, Sharma M. Quality Standards of IndianMedicinal Plants. Vol. 1. New Delhi: Indian Council of Medical Research; 2003. p. 10‑50.
- Acharya T. ChurnaPrakran, Rastantrasar and SiddhrayogSangrah.Vol. 1, 1974. p. 672.
- Anonymous. The Ayurvedic Pharmacopoeia of India. 1st ed., vol. 3,part 1. New Delhi: Govt. of India, Ministry of Health and FamilyWelfare; 1986. p. 112‑4.
- Anonymous. The Ayurvedic Pharmacopoeia of India. 1st ed., vol. 1.part 1. New Delhi: Govt. of India, Ministry of Health and FamilyWelfare; 1986. p. 140‑1.
- Anonymous. The Ayurvedic Pharmacopoeia of India. 1st ed., vol. 1.part 1. New Delhi: Govt. of India, Ministry of Health and FamilyWelfare; 1986. p. 62‑3.
- Anonymous. The Ayurvedic Pharmacopoeia of India. 1st ed., vol. 3.part 1. New Delhi: Govt. of India, Ministry of Health and FamilyWelfare; 1986. p. 214‑5.
- Wu LJ, Wang SX. 6‑Methoxygeniposidic acid: An iridoid glycosidefrom Rubiacordifolia. Phytochemistry 1991;30:1710‑1.
- Müller BM, Kraus J, Franz G. Chemical structure and biologicalactivity of water‑soluble polysaccharides from Cassia angustifolialeaves. Planta Med 1989;55:536‑9.
- Anonymous. The Indian Pharmaceutical Codex: Indigenous Drugs.Vol. 1. New Delhi: Council of Scientific and Industrial Research; 1953.p. 154‑6.
- Anonymous. International Conference on Harmonization: Guideline onValidation of Analytical Procedure‑Methodology. Geneva: InternationalConference on Harmonization; 1996.