- *Corresponding Author:
- S. A. Khan
Department of Chemistry, School of Science, University of Management & Technology, Lahore-54770, Pakistan
E-mail: shakilahmad56@gmail.com
| Date of Submission | 02 January 2017 |
| Date of Revision | 30 April 2017 |
| Date of Acceptance | 19 December 2017 |
| Indian J Pharm Sci 2018;80(1):173-180 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Silver is commonly used antibacterial material, and showed improved results when doped to less expensive ZnO nanoparticles. Chemical reduction method with zinc acetate as host and silver nitrate as dopant precursor was used. The surface area of particles was enhanced by calcination in different atmospheric conditions. Antibacterial activity of synthesized nanoparticles was evaluated against different Gram-negative and Gram-positive bacterial strains. Minimum inhibitory (6 to 21 mM) and minimum bactericidal concentrations (23 to 47 mM) indicated that antibacterial activity of nanoparticles was increased by silver doping and calcination in oxygen atmosphere. The X-ray diffraction, scanning electron microscopy and energy dispersive X-ray spectroscopic data further confirmed the hypothesis. Present study confirmed that oxygen treated silver doped zinc oxide nanoparticles could have pharmacological applications as alternative for antibiotics and disinfectants.
Keywords
Calcination, minimum inhibitory concentration, minimum bactericidal concentration, oxygen atmosphere, silver doping
Antibiotic-resistant pathogens attracted the attention of numerous researchers as these pathogens are responsible for several untreatable infections. Lot of articles reported improved therapies of antibacterials. It is reported that a single effective antibiotic can resist over 70 % of infections caused by bacteria [1]. Nanotechnology is a rapidly progressing field with several applications especially in biosciences. Nanoparticles have additional properties as compared to the parent material. These additional properties are due to surface/volume ratio of nanoparticles, which is inversely proportional to particle size [2,3].
Zinc oxide (ZnO) is an n type (II to IV) semiconductor. It has a large band gap i.e. 3.37 eV, for optical pumping and has low threshold power at 25°, abundant in nature and environment friendly. Its high exciton binding energy even at high temperature provides excitonic emission as good as at low temperature [4]. This semiconductor has several applications such as transparent conductive coatings [5], electrodes for dyesensitized solar cells [6], gas sensors [7], field emission materials [8], antimicrobial agents, electrical devices and in gas sensors [9]. In addition, ZnO nanoparticles also possess huge technological importance. It has quasi-one-dimensional structure and a small diameter (10-100 nm). Especially such a small diameter gives more dominating position in the field of research. At nano size, these particles probably have attractive physical properties, which are quite different from their bulk material [10].
Doping of ZnO assists in getting good quality crystals with enhanced optical, electrical as well as ferromagnetic properties. In practical devices, Mn, Co, Ag, V, Ni introduced as dopants into doped ZnO and as a result diluted magnetic semiconductors (DMS) formed. This DMS characterized on the basis of strong coupling of d electrons of transition metal ions with sp electron of ZnO. Impurities affect the conductivity and optical properties of doped ZnO. Now-a-day’s semiconductors of Ag-doped ZnO have potential applications such as novel memory and optical devices. Many new pharmaceutical products are based on nano-sized drug particles [11]. Recently, our group reported that ZnO nanoparticles could be used as antibiotics and preservative for food provided their biosafety and toxicity is evaluated [12]. Nano ZnO with a lot of transition metal ions is a non-toxic and a favourable semiconductor material with the property of ferromagnetism at ambient temperature [13].
Ag nanoparticles are mostly used as inorganic antibacterial agents. Ag nanoparticles release Ag+ ions in aqueous solution and these Ag ions showed a broad spectrum of antibacterial activities [14-16]. It has wide application in the field of medical devices, in topical preparation, in textile and in dental materials. These nanoparticles are also used to saturate bandages, which resist the growth of bacteria on injury [17]. Thus, Ag nanoparticles with ZnO may have antibacterial properties according to their different particle size. ZnO nanoparticles can be prepared with low cost and can be prepared by sol gel method, chemical preparation and by hydrothermal reaction. First step in nanoparticle synthesis is the formation of nuclei. To obtained uniform sized nanoparticles all the nuclei should be formed at the same time. In this research work all the nuclei have same sized as well as subsequent growth because of same conditions were applied. As a result, nanoparticles of mono size could be obtained. It’s evident that if the nucleation occurs in short time, it has advantages. Further, change in size can be monitored and altered by using different growth process. Kinetics of growth process may increase or decrease nucleation. Uniform sized nanoparticles can be obtained by controlling kinetics of growth process [18]. Calcinations atmosphere process plays a vital role for the synthesis of uniform sized nanoparticles. This article reports a simple route for the synthesis of Ag/ZnO nanoparticles under different atmospheric conditions and their structural, morphological, textural properties were evaluated using X-ray diffraction (XRD), scanning electron micrograph (SEM) and antibacterial activity against various bacterial strains.
Materials and Methods
The current research work was carried out at the Biochemistry Laboratory, Department of Biochemistry, University of Agriculture, Faisalabad and Chemistry Laboratory, Department of Chemistry, University of Management and Technology Lahore, Pakistan. Analytical grade zinc nitrate hexahydrate (Zn(NO3)2.6H2O), sodium hydroxide (NaOH), silver nitrate (AgNO3), nutrient agar, nutrient broth, absolute ethanol, were procured from Sigma-Aldrich.
Synthesis of ZnO nanoparticles
Metal-doped and un-doped ZnO nanoparticles can be synthesized by sol-gel method, vapour deposition, solution route method and hydrothermal method. Among these methods, solution routes method is commonly used because it is simple and low cost technique. Here simple solution route method was used for the synthesis of ZnO nanoparticles [19]. For the synthesis of un-doped ZnO, 0.1 M Zn(NO3)2.6H2O and 0.1 M NaOH solutions were prepared in distilled water. NaOH solution was then added drop wise in Zn(NO3)2.6H2O solution with continuous stirring for 2 h. The solution was kept overnight for settlement and filtered. The residue was washed several times with distilled water and the final product was in the form of a white precipitate. The white precipitate was dried in an oven at 110° for 2 h and then ground. Precipitate was divided in two portions. One portion was calcined at 300 to 800° for about 35 to 80 min in a muffle furnace in oxygen atmosphere while the second portion was calcined at 300 to 800° for about 35 to 80 min in a muffle furnace in air atmosphere. During the drying process, Zn(OH)2 converted into ZnO completely. The above process was repeated once again to prepare Agdoped ZnO powder and only 0.3 M AgNO3 solution was added in zinc nitrate solution.
Characterization of ZnO nanoparticles
Both nanoparticles, un-doped and Ag-doped ZnO were characterized by phases and compositional analysis. Measurement of the particle size of ZnO nanoparticles was done by XRD (Panalytical X’Pert Pro) while the morphology of the synthesized powder was determined by SEM (Jeol, 5910LV).
Preparation of un-doped and Ag-doped ZnO nanoparticles for antibacterial activity
First of all ZnO and Ag-doped ZnO nanoparticles sterilized at 160° for 3 h separately [20]. Half a gram of each nanoparticle preparation was taken, 0.1 g of acacia gum, 2 to 3 ml ultrapure water (Milli-Q®, Millipore Corporation, Bedford, MA) were added and mixed well in a mortar and pestle. Obtained suspension was sonicated for 40 min and considered as the stock solution and was further diluted. Diluted suspension was used for bacterial susceptibility evaluation.
Bacterial strains
Four bacterial strains were used including Gramnegative bacteria, Escherichia coli (PCSIR-B-67) and Gram-positive bacteria, Staphylococcus aureus (ATCC-6538), Bacillus subtilis (PCSIR-B-248) and Streptococcus pyogenes (ATCC-19615). These bacterial strains were obtained from PCSIR laboratories complex, Lahore, Pakistan. The cell suspensions were used for antibacterial activity contained 105 colony forming units (CFU) ml-1.
Agar well diffusion assay
Activity of nanoparticles was checked against different bacterial strains by agar well diffusion method [21,22]. Bacteria was prepared freshly for each experiment by inoculating the nutrient broth at 37° for 24 h. Sterile pipettes were used for the transfer of 50 ml molten agar to broth culture (0.5 ml) of test organisms and these were mixed properly and transfer into sterile petri dish. Wells were then bored with sterile cork borer (6 mm in diameter) into the plates with seeded organisms. ZnO solution of various concentrations was poured with 50 μl of test solution separately to the wells for study. After 24 h of incubation, the inhibition zones were measured. Each experiment was repeated thrice and the inhibition zones were measured and result shown as the mean±standard deviation.
Determination of minimum inhibitory concentration (MIC)
The MIC and minimum bactericidal concentration (MBC) were verified by NCCLS; 2000, recommended method with few modifications. MIC and MBC were determined aerobically and incubated at 37° for 24 h. The sample contained in 5 ml Muller-Hinton (MH) broth (Difco, USA) with approximate 5×109 CFU bacterial cells and control group has zero to increased concentration of ZnO nanoparticles. MIC is where no visible growth of bacteria in concentration of tube could be seen.
Determination of MBC
For MBC determination, 100 μl samples were transferred from every tube to MH agar plate and incubated for 24 h under aerobic conditions [23]. MBC is the lowest concentration where no growth was observed. In this test, it was considered that if agar plate population is less than 10 then it should be considered as the growth is zero. All measurements were repeated thrice.
Statistical analysis
Analysis of data was carried out using Microsoft Excel-2016 software. All computations were executed in triplicate and the results were expressed as mean±SEM (n=3). Antimicrobial assay was computed with suitable dilutions for each sample and different statistical technique such as Duncan multiple range method. One-way analysis of variance was used for analysis of data obtained from different samples. P values <0.05 were taken as indicative of statistical significance.
Results and Discussion
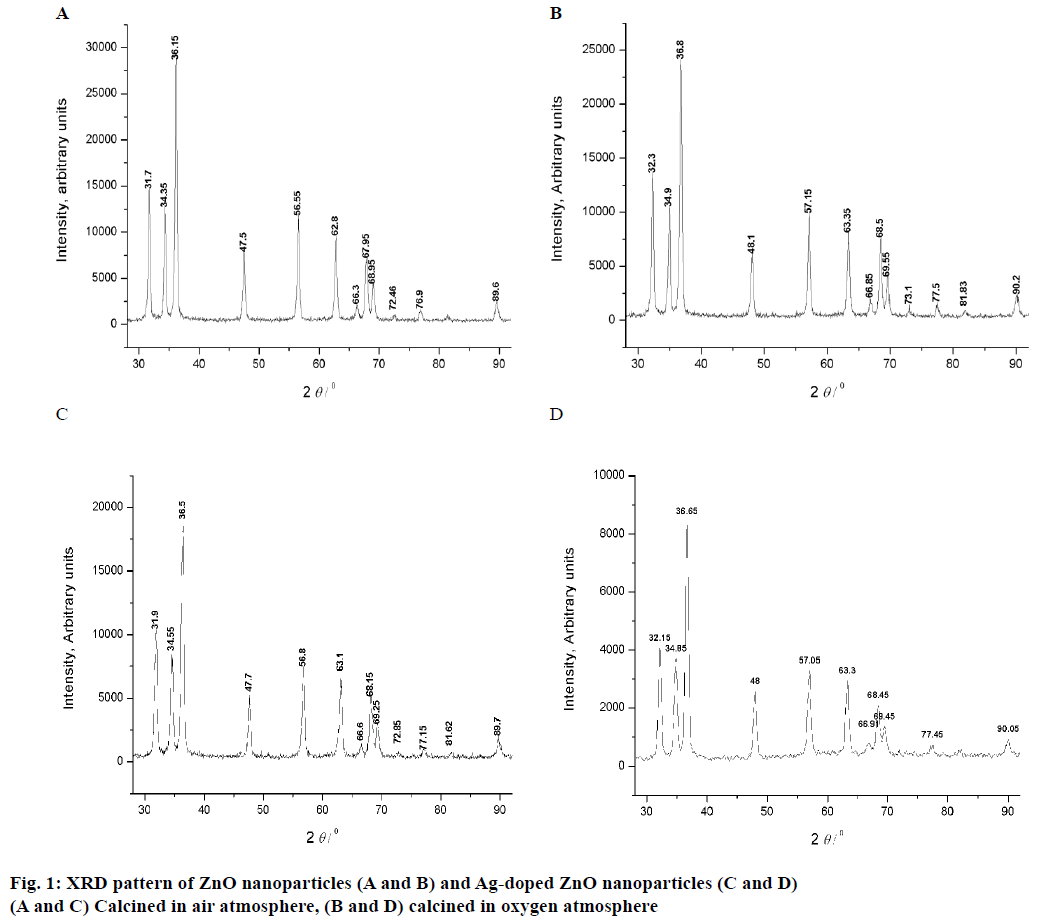
The XRD pattrens of un-dopped ZnO (air and O2 anealed at 300 to 800°) are shown in Figure 1A and B, and 3 mol % Ag-dopped ZnO (air and O2 anealed at 300 to 800°) powders are shown in Figure 1C and D. XRD peaks of all samples were same as the hexagonal wurtzite ZnO data (JCPDS. No: 36-1451). The diffraction peaks showed that ZnO powder is crystalline in nature. The results confirmed the high purity of synthesized powders as there were no peaks of impurity. Thus, addition of Ag into ZnO host material was not affected the wurtzite structure [24]. The crystallite sizes of all the samples were estimated by using Scherrer formula and the values are listed in Tables 1 and 2: D = 0.89 λ/βcosθ.
| Sample A* | |||||
| 2 θ (deg) of the intense peak | FWMH intense peak (θ2-θ1) | FWMH intense peak (β) radians | Grain size (D), nm | Average grain size (nm) | specific surface area (m2/g) |
| 36.1 | 0.42 | 0.0075 | 19.4 | 19.6 | 54.7 |
| 34.3 | 0.42 | 0.0075 | 19.3 | ||
| 31.7 | 0.43 | 0.0075 | 19.2 | ||
| 56.5 | 0.44 | 0.0077 | 20.4 | ||
| Sample B* | |||||
| 2 θ (deg) of the intense peak | FWMH intense peak (θ2-θ1) | FWMH intense peak (β) radians | Grain size (D), nm | Average grain size (nm) | specific surface area (m2/g) |
| 36.8 | 0.44 | 0.0077 | 19.0 | 20.2 | 53.0 |
| 32.3 | 0.39 | 0.0070 | 20.7 | ||
| 34.9 | 0.43 | 0.0075 | 19.4 | ||
| 57.1 | 0.42 | 0.0073 | 21.6 | ||
Table 1: The Grain Size and Specific Surface Area of ZnO Nanoparticles.
| Sample C* | Sample D* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2θ (deg) of the intense peak | FWMH intense peak (θ2-θ1) |
FWMH intense peak (β) radians |
Grain size (D), nm |
Average grain size, nm |
2θ (deg) of the intense peak | FWMH intense peak (θ 2-θ1) |
FWMH intense peak (β) radians |
Grain size (D) nm |
Average grain size, nm |
| 31.9 | 0.5 | 0.0094 | 15.3 | 15.4 | 32.1 | 0.6 | 0.011 | 13.1 | 13.3 |
| 34.5 | 0.5 | 0.0092 | 15.8 | 34.8 | 0.7 | 0.012 | 12.1 | ||
| 36.5 | 0.5 | 0.0096 | 15.2 | 36.6 | 0.6 | 0.0098 | 14.9 | ||
| 57.1 | 0.7 | 0.012 | 13.1 | ||||||
Table 2: The Grain size of Ag Doped ZnO Nanoparticles.
Specific surface area is a scientific value that is used for the determination of type and properties of materials [25]. It has especial importance in the determination of adsorption, reactions on surface of material and heterogeneous catalysis. Specific surface area can be calculated by following Eqn., S = 6×103/Dpρ, where, S= specific surface area, Dp= size of the particles, ρ= density of ZnO (5.6 g/cm3). By using this Eqn. particle size and specific surface areas of ZnO powder were analysed and reported (Table 1).
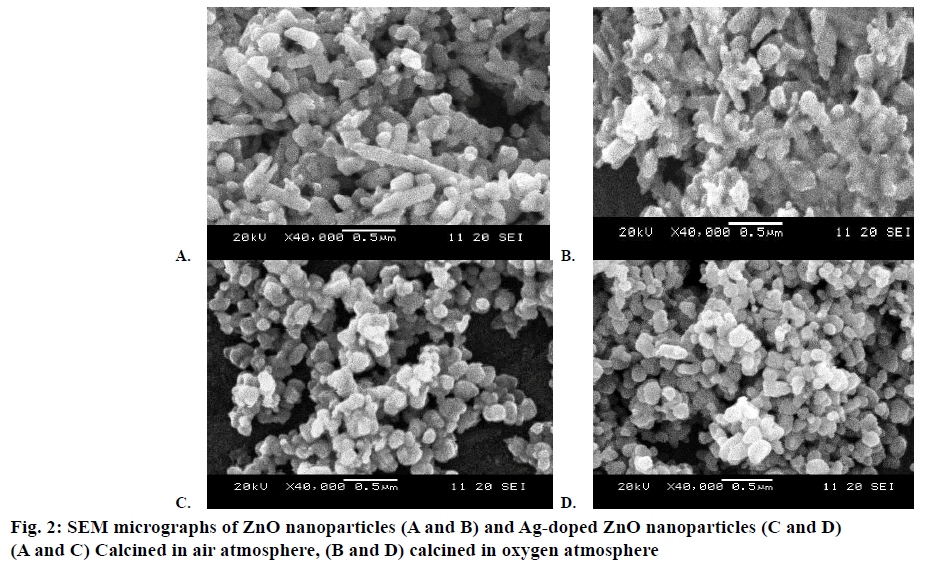
The morphological information of all the samples was obtained using SEM. Figure 2A and B showed the SEM images of the pure (air and O2 annealed). Figure 2C and D showed the SEM images of Ag-doped ZnO (air and O2 annealed) nanoparticles, respectively. Some bigger particles in both samples of pure ZnO nanoparticles might be attributed because of aggregating of smaller particles. Results indicated that both samples of Agdoped ZnO nanoparticles were spherical in shape and smaller in size than the pure ZnO particles. The particle size of oxygen annealed Ag-doped ZnO nanoparticles is smaller than all the other samples. XRD analysis confirmed the doping of Ag on ZnO. There were found a close agreement with the results of SEM as well as XRD analysis to each other that confirmed the successful synthesis of un-doped ZnO and Ag-doped ZnO nanoparticles.
The antibacterial activity of ZnO nanoparticles and Agdoped ZnO nanoparticles was compared as function of increasing concentrations, Ag doping and atmospheric conditions by evaluating the zone of inhibition. Inhibition zone sizes (mm) were noted against all bacteria tested with un-doped ZnO nanoparticles and Ag-doped ZnO nanoparticles and shown in Table 3.
| Bioactive agent | Zone of inhibition* (diameter, mm) | ||||
|---|---|---|---|---|---|
| S. pyogenes | B. subtilis | S. aureus | E. Coli | ||
| ZnO A* | 100 µl | 10±0.6 | 12±1.3 | 12±0.9 | 9±1.3 |
| 200 µl | 11±1.2 | 16±0.7 | 15±0.5 | 10±0.7 | |
| 300 µl | 13±0.9 | 18±0.9 | 17±0.4 | 14±0.4 | |
| 400 µl | 14±0.1 | 20±0.2 | 19±0.7 | 15±0.6 | |
| 500 µl | 16±0.8 | 23±0.4 | 22±1.6 | 17±1.4 | |
| ZnO B* | 100 µl | 12±0.3 | 15±0.9 | 13±1.2 | 11±1.1 |
| 200 µl | 13±0.5 | 17±0.6 | 16±0.7 | 12±0.4 | |
| 300 µl | 15±0.7 | 19±0.8 | 19±0.3 | 16±0.8 | |
| 400 µl | 17±0.3 | 21±0.5 | 23±0.9 | 18±0.1 | |
| 500 µl | 20±0.2 | 25±1.2 | 25±0.9 | 20±0.7 | |
| Ag/ZnO C* | 100 µl | 14±0.3 | 18±0.9 | 16±1.2 | 15±1.1 |
| 200 µl | 17±0.5 | 20±0.6 | 19±0.7 | 17±0.4 | |
| 300 µl | 18±0.7 | 22±0.8 | 22±0.3 | 20±0.8 | |
| 400 µl | 21±0.3 | 25±0.5 | 27±0.9 | 21±0.1 | |
| 500 µl | 23±0.2 | 26±1.2 | 30±0.9 | 24±0.7 | |
| Ag/ZnO D* | 100 µl | 16±0.7 | 20±0.4 | 18±0.8 | 17±1.3 |
| 200 µl | 19±0.9 | 21±0.5 | 21±0.5 | 20±0.2 | |
| 300 µl | 18±0.2 | 25±1.2 | 25±0.8 | 23±0.6 | |
| 400 µl | 23±0.3 | 28±0.5 | 28±0.1 | 25±0.5 | |
| 500 µl | 26±0.2 | 32±1.4 | 33±0.4 | 28±0.1 | |
| Gentamycin | 100 µl | 15±0.2 | 18±0.3 | 17±0.6 | 14±0.8 |
| Methicillin | 100 µl | 16±0.5 | 19±0.7 | 22±0.3 | 15±0.1 |
Table 3: Zones of Inhibitions of ZnO, Ag/ZnO Nanoparticles Against Different Bacterial Strains
Results showed that among concentrations (100- 500 μl) tested at all level, best growth inhibition was observed with 500 μl concentration of Ag-doped ZnO nanoparticles against S. aureus (33±0.4) and the least with 100 μl concentration of ZnO nanoparticles against E. coli (9±1.3). This is due to three factors that involved to showing increased antibacterial activity i.e. increasing in concentration, Ag doping and nanoparticles calcination in oxygen that lead to the nanoparticles to show best bactericidal activity. The suspended ZnO nanoparticles were used to study relative antibacterial activity towards four bacterial strains. Quantitative analysis was performed in terms of the MIC and MBC. A standard procedure is applied, which is appropriate for inorganic metal oxide composite like Ag-doped ZnO nanoparticles. MBC is the lowest concentration of a compound (μg/ml) that could kill more than 99 % of bacteria present, while MIC is the concentration where solution showed turbidity. MIC is reciprocal of antibacterial activity as low MIC represented higher antibacterial activity. It was observed that both MIC and MBC have shown inverse relationship between the particle size and activity. These findings were summarized (Table 4). The MIC was commonly observed in the range of 6 to 21 mM while MBC is from 23 to 47 mM (depending on the particular bacterial strain). Gram-negative bacteria E. coli (MIC: 21 mM and MBC: 47 mM) was more resistant than Gram-positive bacterial strains especially, B. subtilis (MIC: 6 mM and MBC: 25 mM).
| Bacterial strain | MIC (mM) | MBC (mM) | ||||||
|---|---|---|---|---|---|---|---|---|
| ZnO nanoparticles | Ag/ZnO nanoparticles | ZnO nanoparticles | Ag/ZnO nanoparticles | |||||
| A* | B* | C* | D* | A* | B* | C* | D* | |
| S. pyogenes | 15±0.5 | 14±0.2 | 13±0.1 | 11±0.5 | 40±0.9 | 38±0.3 | 35±0.5 | 32±0.7 |
| B. subtilis | 11±0.3 | 10±0.7 | 8±0.2 | 6±0.3 | 32±0.7 | 31±0.9 | 29±0.6 | 25±0.6 |
| S. aureus | 9±0.4 | 10±0.3 | 9±0.6 | 8±0.7 | 27±0.5 | 27±0.8 | 25±0.4 | 23±0.5 |
| E. Coli | 21±0.6 | 19±0.2 | 18±0.4 | 16±0.8 | 47±0.9 | 45±0.4 | 41±0.7 | 36±0.2 |
Table 4: Minimum Inhibitory and Minimum Bacterial Concentrations of ZnO, Ag/ZnO Nanoparticles Against Different Bacterial Strain
Gram-positive and Gram-negative bacteria are mostly used in literature for the determination of antibacterial activities [26,27]. These bacterial strains have different structures and chemical composition of their cell wall. The outer membrane of Gram-positive bacteria is different from Gram-negative bacteria. Gramnegative bacteria have peptidoglycan in its outer layer so they show staining and also helpful for protection from outer substances while this layer was absent in Gram-positive bacteria. Different mechanism of ZnO powders as antibacterial agent has been proposed several times [28]. This action of ZnO powder is might be due to release of Zn+2 ions from zinc oxide or by penetration of nanoparticles by destruction of cell membrane. This action might be present due to the formation of oxidative species from the surface of ZnO but actual mode of action is still in progress and not clears. Literature reported that these reactive species (•O2–, •OH and H2O2) released by ZnO can damage the peptidoglycan outer layer of bacteria [27,29].
Literature reported that Ag nanoparticles act as antibacterial agent by damaging the outer layer of bacteria [30]. The metal surfaces (with positive charged) help to bind with the negative surface part of bacteria and enhanced bactericidal effect [31] or might be it is due to the ability of Ag nanoparticles to fragment the cell membrane of bacteria by stimulate pits and gaps [32,33]. Proper function of outer cell well is helped by bacterial proteins and cytoplasm. Permeability and respiratory functions might be disturbed due to the presences of Ag nanoparticles and leads to disturb the cell well and at the end cell lysis. It has been also reported that enzyme metabolic process disturbed when Ag+ interact with disulphide or sulfhydryl groups present in enzyme and can cause cell death [34]. The calcinations in oxygen atmosphere decreases the particles size, which further increases surface area of nanoparticles and enhance antibacterial properties. The reason of decreased size in oxygen atmosphere than in air may be due to nucleation of particles at same time, which results in homogeneous small size nuclei than their subsequent growth in homogeneous oxygen rich environment.
Infectious diseases remain a challenging problem for human from a long time. Microbes developed resistance to some common disinfectant and antibiotics. Metal and metal oxide nanoparticles considered more suitable alternates to resist bacteria. Present work concluded that Ag-doped ZnO nanoparticles have ability to destroy bacteria and serve as better antimicrobial agent than ZnO against all the microorganisms (B. subtilis, S. pyogenes, E. coli and S. aureus) tested. It is concluded that metal oxide with minimum toxic effects could have wide utility in future for treating the different infectious conditions. Further the doping of a metal on the metal oxide increases antibacterial efficacy. It is reported that Ag nanoparticles are nontoxic and widely used as antimicrobial agent, but it is also suggested that it is hazardous to the environment due to their small size and unpredictable properties [35]. From this investigation it can be anticipated that costeffective antimicrobial agents like synthesized Agdoped ZnO nanoparticles might serve as alternatives to traditional antibiotics and could have great potential future use in pharmaceutics and medicine and possess lot of scope for advanced research in the areas of sterile coatings and wound dressings.
Acknowledgements
This work was supported by the Department of Chemistry, School of Science, University of Management and Technology, Lahore, Pakistan.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M.Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol2011;6:933-40.
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2007;2:MR17-71.
- Requejo IJ, Coso RD, Solis J, Gonzalo J, Afonso CN. Role of surface-to-volume ratio of metal nanoparticles in optical properties of Cu: Al2O3nanocomposite films. ApplPhysLett 2005;86:193104-3p.
- Yadav BC, Srivastava R, Dwivedi CD, Pramanik P. Synthesis of nano-sized ZnO using drop wise method and its performance as moisture sensor. Sens Actuators A-Phys 2009;153:137-41.
- Minami TJ. Transparent and conductive multicomponent oxide films; prepared by magnetron sputtering. J Vac SciTechnol A1999;17:1765-72.
- Ahsanulhaq Q, Hossain MF, Faiz M, Tabet N, Alam MW, Reddy NK. Fabrication of well-aligned and dumbbell-shaped hexagonal ZnOnanorod arrays and their dye sensitized solar cell applications. J Alloys Compd 2010;503:L40-3.
- Ahsanulhaq Q, Kim JH, Lee JS, Hahn YB. Electrical and gas sensing properties of ZnOnanorod arrays directly grown on a four-probe electrode system. ElectrochemCommun 2010;12:475-8.
- Farid JS, Ramin Y, Mahendra AM, Dilip SJ. Sn-ZnOnanoneedles grown on Zn wire as a pointed field emitter and switching device. Materials Lett 2013;111:181-4.
- Pivin JC, Socol G, Mihailescu I, Berthet P, Singh F, Patel MK, et al. Structure and magnetic properties of ZnO films doped with Co, Ni or Mn synthesized by pulsed laser deposition under low and high oxygen partial pressures. Thin Solid Films 2008;517:916-22.
- Sondi I, Salopek-Sondi B.Silver nanoparticles as antimicrobial agent: a case study onE. colias a model for Gram-negative bacteria. J Colloid Interface Sci 2004;275:177-82.
- Jo YK, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Disease 2009;93:1037-43.
- Siddique S, Shah ZH, Shahid S, Yasmin F. Preparation, characterization and antibacterial activity of ZnO nanoparticles on broad spectrum of microorganisms. ActaChimSlov 2013;60:660-5.
- Herng TS, Lau SP, Yu SF. Magnetic anisotropy of ferromagnetic copper doped ZnOnano needles.ApplPhysLett 2007;90:032509-032509-3.
- Kawashita M, Tsuneyama S, Miyaji F, Kokubo T, Kozuka H, Yamamotto K. Antibacterial silver containing silica gel prepared by sol-gel method. Biomaterials 2000;21:393-8.
- Lin WC, Chen CN, Tseng TT, Wei MH, Hsieh JH, Tseng WJ. Micellar layer-by-layer synthesis of TiO2/Ag hybrid particles for bactericidal and photocatalytic activities. J Eur Ceram Soc 2010;30:2849-57.
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli.Appl Environ Microbiol 2007;73:1712-20.
- Kassaee MZ, Akhavan A, Sheikh N, Sodagar A. Antibacterial effects of a new dental acrylic resin containing silver nanoparticles. J ApplPolymSci2008;110:1699-703.
- Ozin GA, Arsenault AC, Cademartiri L.Nanochemistry: A chemical approach to nanomaterials, 2nd ed. London: Imperial College Press; 2009. p. 58.
- Chauhan R, Kumar A, Chaudhary RP.Photocatalytic studies of silver doped ZnO nanoparticles synthesized by chemical precipitation method. J Sol-Gel SciTechnol 2012;63:546-53.
- Raju BN, Kumar SS, Prasad VSRK, Ramji K, Synthesis and characterization of high pure ZnOnano particles by conventional methods. Int J NanotechnolAppl 2010; 4:199-05.
- Nagarajan P, Rajagopalan V. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. SciTechnolAdv Mater 2008;9:035004-(7pp).
- Khan SA, Shahid S, Jameel M, Ahmad A. In vitroantibacterial, antifungal and GC-MS analysis of seeds of mustard brown. Int J Pharm Chem 2016;6:107-15.
- https://clsi.org/media/1632/m07a10_sample.pdf.
- Reddy BS, Reddy SV, Reddy NK. Physical and magnetic properties of (Co, Ag) doped ZnO nanoparticles. J Mater Sci: Mater Electron 2013;24:5204-10.
- Alagar M, Theivasanthi T, Kubera RA. Chemical synthesis of nano-sized particles of lead oxide and their characterization studies. J ApplSci 2012;12:398-01.
- Yang M, Mao J, Zhang X, Xue T, Hou T, Wang L, et al. Preparation and characteristics of Ag/nano-ZnO composite antimicrobial agent. Nanoscience 2006;11:44-8.
- Fang M, Chen JH, Xu XL, Yang PH, Hildebrand HF. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int J Antimicrob Agents 2006;27:513-17.
- Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, et al. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res 2008;42:4591-602.
- Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles-an antibacterial study. SciTechnolAdv Mater 2008;9:035004(7pp).
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, et al. Silver nanoparticles: partial oxidation and antibacterial activities. J Proteome Res 2006;5:916-24.
- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methodsand literature.Int J Nanomedicine2012; 7: 2767-81.
- Yun H, Kim JD, Choi, HC, Lee CW. Antibacterial activity of CNT-Ag and GO-Ag nanocomposites against Gram-negative and Gram-positive bacteria. BullKorean ChemSoc 2013;34:3261-4.
- Iavicoli L, Fontana V, Leso V, Bergamaschi A. The effects of nanomaterials as endocrine disruptors.Int J MolSci 2013;14:16732-801.
- Egger S, Lehmann RP, Height MJ, Loessner MJ, Schuppler M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl Environ Microbiol 2009;75:2973-76.
- Braydich SL, Hussain S, Schlager J, Hofmann MC. In vitrocytotoxicity of nanoparticles in mammalian germ line stem cells. ToxicolSci 2005;88:412-19.