- *Corresponding Author:
- H. A. Raj
Pharmaceutical Quality Assurance Division, Pharmacy Department, Faculty of Technology and Engineering, Kalabhavan, The M. S. University of Baroda, Vadodara - 390 001, India
E-mail: sjrajput@rediffmail.com
| Date of Submission | 25 November 2005 |
| Date of Revision | 29 August 2006 |
| Date of Acceptance | 17 April 2007 |
| Indian J Pharm Sci,2007, 69 (2): 314-316 |
Abstract
An extractive spectrophotometric method was developed for the estimation of tegaserod maleate. This method is based on the formation of a yellow colored ion-pair complex with bromo cresol green in phthalate buffer (pH 2.5). The complex was extracted into chloroform and absorbance measured at 415 nm. The calibration curve was found to be linear in the range of 1 to 30 μg/ml. The molar absorptivity and Sandell sensitivity values were found to be 2.9775 × 104l mol-1cm-1 and 0.2264 ×10-2 μg/cm2/0.001, respectively. The limit of detection and limit of quantification were found to be 0.0524 μg/ml and 0.1749 μg/ml, respectively. The method was successfully applied for assay in tablet formulation and the recovery was found to be in good agreement with label claim. The method was found to be precise, accurate and sensitive.
Introduction
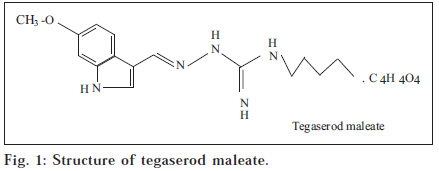
Tegaserod maleate (TM), is 3-(5-methoxy-1H-indole-3-ylmethylene)-N-pentyl carbazimidamidehydrogen maleate [1] (fig. 1). It is a selective 5-HT4 receptor partial agonist with (fig. 1). It is a selective 5-HT4 receptor partial agonist with premotile activity in the gastrointestinal tract. TM is used for the treatment of irritable bowel syndrome [2]. Literature survey revealed the availability of only two method for the analysis of TM which used an HPLC-MS techniques [3,4]. The main objective of the work was to develop simple, fast, inexpensive, sensitive and accurate method, which could be applied to analyze TM in pure form and in pharmaceutical dosage form.
All the reagents used were of analytical grade. Bromocresolgreen (0.01% w/v) was prepared by dissolving 25 mg of dye in water, and diluted to 500 ml with the same solvent. Acid phthalate buffer of pH 2.5 was prepared as per IP procedure [5,6]. All spectral measurements were made on an Hitachi UV-1601 UV/Vis Spectrophotometer with matched 1-cm quartz cells.
A stock standard solution of tegaserod maleate (1 mg/ml) was prepared in methanol and further dilutions were made with the same solvent to get the working standard solution of 100 μg/ml. Suitable aliquots of standard solution (0.1 to 3.0 ml) were transferred into a series of 100 ml separating funnels and to each were added 5.0 ml pf acid phthalate buffer (pH 2.5) and 1 ml of bromo cresol green (0.01% w/v) and mixed. The yellow colored complex was extracted with two portions (5,3 ml) of chloroform. The extract was dried over anhydrous sodium sulphate and collected in 10 ml volumetric flasks; volume was made up to mark with chloroform and the absorbance was measured at 415 nm against a reagent blank. The calibration curve was prepared by plotting absorbance v/s concentration of tegaserod maleate in μg/ ml. The concentration of the unknown was read from the calibration graph or decided from the regression equation derived using the Beer’s law data. The robustness of the method was studied by varying different experimental condition and the results were found to be depended upon change in pH. The stability of the colored solution was assessed by measuring the absorbance of same solution after every 10 min and the solution found to be stable for 15 min
Twenty tablets (Tegibs-6 of Torrent Pharmaceutical Ltd.) were weighed accurately and triturated to a fine powder.The powder equivalent to 10 mg of tegaserod maletate was weighed and transferred to a beaker. To this 15 ml of methanol was added and stirred using a magnetic stirrer for 15 min; the solution was then filtered through a Whatman No 42 filter paper into a 25 ml volumetric flask. The filter paper was washed twice with 3 ml portion of methanol and the volume was made upto the mark with methanol. The stock solution was diluted and analyzed as described above.
The protonated drug will react with the acidic anionic dye to form the colored ion-pair which is easily miscible in organic layer.TM+ +BCG→TM+ BCG–.As the pH change form acidic to alkali, color change form yellow to blue, Maximum absorbance was observed in yellow color complex and after optimization acidic phthalate buffer pH 2.5 was selected for ion pair reaction. Yellow colored complex was shaken for 5 min with two portions (5, 3) of chloroform. The funnels were kept aside for 5 min to separate the aqueous and organic layers.
The optical characteristics such as Beer’s law limit, molar extinction coefficient, Sandel sensitivity, LOD and LOQ are summarized in Table 1. Accuracy and precision to be evaluated by performing replicate analyses in pure drug solution at three difference concentration levels, and by calculating the relative error (%) and relative standard deviation. The percentage of drug in the tablet was found to be in the range of 98.58-100.13 in replicate analysis (n=5). Recovery experiments were performed by adding known amount of standard drug to previously analyzed pharmaceutical dosage form. The results obtained by the proposed method were in good agreement with the labeled amounts.
| Data | Result |
|---|---|
| Linearity range | 1-30 μg/ml |
| Molar absorptivity | 2.9775 × 104 Lmol-1.cm-1 |
| Sandell sensitivity | 0.2264 × 10-2 μg/cm2/0.001 |
| Limit of detection (LoD) | 00524 μg/ml |
| Limit of quantitation (LoQ) | 0.1749 μg/ml |
| Regression equation | y = 0.0152 x + 0.0191 |
| Correlation coefficient (r) | 0.9998 |
The proposed method is simple, precise and reproducible and does not suffer from any interference due to common excipients usually present in the formulations. Due to high sensitivity and simple sample preparation, the method can be used for the analysis in quality control laboratories and as an experiment for undergraduate studies. Moreover spectrophotometric methods have obvious advantages over sophisticated instrumental analysis such as HPLC. Hence, simple and economical instrumental methods always have a role in pharmaceutical analysis.
Acknowledgements
The author are thankful to Torrent Research Center, Gandhinagar, for providing standard drug and to the Head, Pharmacy Department, Faculty of Technology and Engineering, M.S.University of Baroda for providing facilities to carry out the work.
References
- Budavari, S., Eds., In; The Merck Index, 13th Edn., Merck and Co. Inc., Whitehouse Station, NJ, 2001, 1626.
- Zhou, H., Khalilieh, S., Campestrine, J., Lachman, L. and Mcleod, J.,Gastroenterology., 2000, 118, 1206.
- Danneckar, R., Tynes, R., Heitz, F., Zollinger, M. and Fischer, V., J Pharmacol. Exp. Ther., 2001, 29, 1269.
- Bucheit, K. H., Gamse,fromR., Hoye, D., Klein, F., Kloppener, E.,Pfannkuche, H.J. and Mattes, H., J. Med. Chem., 1995, 38, 2331.
- Indian Pharmacopoeia, Vol. II, Govt. of India, Ministry of Health andFamily Welfare, New Delhi, 1996, A-144.
- Indian Pharmacopoeia, Vol. II, Govt. of India, Ministry of Health andFamily Welfare, New Delhi, 1996, A-202.