- *Corresponding Author:
- A. R. Srividya
Department of Pharmaceutical Biotechnology, J. S. S College of Pharmacy, Ooty-643 001, India
E-mail: Pharmarsrividya@yahoo.com
| Date of Submission | 7 September 2007 |
| Date of Revision | 4 June 2008 |
| Date of Acceptance | 12 December 2008 |
| Indian J Pharm Sci, 2008, 70 (6): 812-815 |
Abstract
During the process of screening for a potent antimicrobial compound, a new strain was isolated from the soil sample of Thalaikunda village in Ooty, Tamil Nadu. That organism was name as NK 2 . It was found to be antagonistic to both bacterial and fungal test organisms. Production of antibiotic was more in a newly formulated broth. Antibiotic production reached maximum at the end of the 70 h of fermentation by stirred flask culture. The antimicrobial compound was extracted in n -butanol, ethyl acetate and methanol. Antimicrobial compound, which was produced by the soil isolate NK 2 did not showed cytotoxic activity on Vero cell lines.
Keywords
Antimicrobial compounds, fermentation, International Streptomycetes project (ISP), Streptomycetes spp, melanoid formation, milk coagulation, peptonisation
Over five thousand antibiotics have been identified from the culture of gram-positive, gram-negative and filamentous fungi but only hundred antibiotics have been commercially used to treat human, animal and plant disease [1]. A major feature of industrial antibiotic production is directed to screening programmes for new potent antibiotic producing organism either from natural sources or from established cultures. Screening for antibiotics producing microorganism, can be detected and isolated by the use of highly selective procedure which allows detection and isolation of only those microorganism of interest from a large population is possible. Soil is the largest source of microorganisms [2]. Majority of antibiotics so far isolated were produced from Streptomycetes, which are common inhabitants of the soil [3]. There are 23,000 known secondary metabolite, 42% of which are produced by Actinobacteria, 42% by fungi (Penicillium spp) and 16% by other bacteria [3] Streptomycetes spp. as the microorganisms become resistant after some time to a particular antibiotic, it is becoming necessary to find newer antibiotics to which the microorganism is sensitive [4]. In the present study, some bacteria and fungal strains were tested for antibiotic sensitivity. Soil isolate named as NK2 was found to be active on the selected microorganisms. Taxonomical studies were performed. The secondary metabolite of the soil isolate NK2 showed antimicrobial activity. In this paper isolation, characterization, bioprocessing and evaluation of product, obtained from the soil isolate, NK2 are described.
The cultures of tested microorganisms were obtained from National collection of industrial microorganisms, Pune. Vero cell lines were obtained from the Pasteur Institute of India, Coonoor. Media such as International Streptomycetes Project media (Internationally accepted Universal media for Streptomycetes), streptomycetes media (common media), nutrient agar media, Sabouraud dextrose agar and the ingredients which were used in the formulation of media were purchased from Hi-Media Laboratories, Mumbai. Various solvents used in this study were purchased from Ranbaxy Lab Ltd, SAS Nagar. Starch casein media was used for maintaining the culture NK2 [5].
The soil samples were collected from Thalaikunda village, Ooty, Tamil Nadu and screened for actinomycetes, which are capable of producing antibacterial substances. The NK2 isolate, showed antimicrobial activity against Escherichia coli, Pseudomonaous aeruginosa, Staphylococcus aureus, Bacillus subtilis, Aspergillus niger, Aspergillus fl avus, Candida albicans and Candida krusei using the supernatant form of broth of NK2 over nutrient agar medium by Agar cup plate techniques [6,7].
Fermentation media for NK2 was formulated based upon carbon utilization pattern [8]. A new fermentation medium consisting of soluble starch- 20 g, sucrose- 15 g, glucose- 5, soya bean meal- 20 g, yeast extract powder- 5 g, CaCO3- 3.2 g, MgSO4.7H2O- 2.5 g, K2HPO4- 5 g, MnCl2- 0.2 g, NaCl- 0.01 g, FeSO4.4 H2O- 0.002 g and silicone oil as an antifoaming agent- 0.3 ml/ l. The seeded medium consisted of glucose- 10 g, soluble starch- 10 g, yeast extract powder- 5 g, beef extract- 3 g, CaCO3- 2 g/l. A 250 ml conical flask containing 10 ml of seed medium was inoculated with a loopful growth of the selected strain grown on slants. The flask was incubated at 280 for 48 h. Ten millilitres of NK2 seed culture was transferred to a 1 l conical flask containing 100 ml of the same medium and then it was incubated at 28°, the second stage seed culture was used as the inoculum to initiate the fermentation in a 5 l containing 3 l of fermentation medium. The fermentation was carried out at 28° with sufficient aeration and agitation at 200 rpm until the pH reached neutrality. Culture growth was evaluated by centrifuging the fermented broth for 10 min at 5000 revolution per minute [9]. The percentage of packed cell volume, change in pH, antibiotic production was noted.
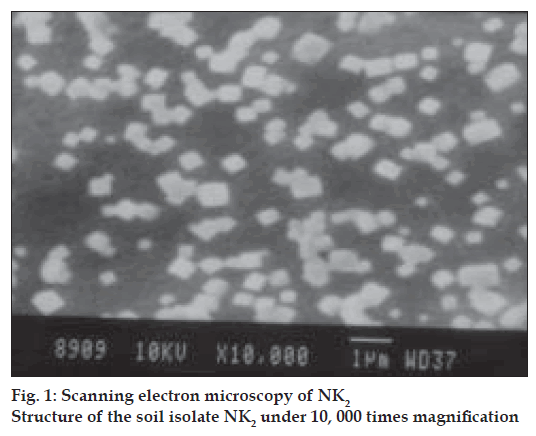
Soil isolate, NK2, was studied for its morphology and its characteristics by agar block method. The morphology was observed under scanning electron microscopy (fig. 1) Carbon utilization studies were performed and based on the new fermentation medium was formulated [9]. Development of melanin pigment in Wakesman No. 42 medium was evaluated by streaming the soil isolate on the slants followed by incubation at 28° and the results were observed at the interval of 12 h for 4 days and it was recorded [10-13].
Nitrate reducing property was evaluated by inoculating the isolate in organic nitrate broth and incubated at 28° for 5 days. From the 5th day, the cultures were observed for the nitrate reduction by using the reagents such as α-naphthal solution and sulphonilic acid. Development of the pink colour indicated the nitrate reducing property of the isolate [10-14]. Proteolytic activity of the isolate was evaluated by inoculating them in pasteurized milk and observing for the reduction of litmus paper, formation of white band, change in pH, formation of whey like brownish translucent band and gas formation up to 48 h [10-13]. Presence or proteolytic enzymes was determined by growing the soil isolate over the starch agar medium and incubated at 28° for 5-7 days. The development of clear zone indicated the hydrolysis of starch and it was flooded with Lugol’s iodine solution for confirmation [10-13].
The soil isolate was streaked and incubated for 4 days at 28° in a medium containing ferric ammonium citrate, dibasic potassium phosphate, Na2S2O4, yeast extract and agar [10-13]. The Nutrient gelatin medium was employed to grow the soil isolate. The protein gelatin is expected to be hydrolyzed by exoenzyme, if secreted by the isolate. The solid character of the medium depends on gelatin remained in the gel state [10-13]. The isolate was inoculated in glucose nutrient broth along with bromothymol blue as indicator and incubated at 28° for 15 days. At every 12 h of interval, change in colour was noted [10-13].
Inoculum (24 h old) was used to seed the flask at 10 % level. Fermentation was carried out for 5 d with 200 rpm at 28°. The active constituents were extracted from both filtrate and mycelia after separation by centrifugation from the fermented cultured broth. One part of the filtrate was extracted three times with equal volume of n-butanol and another part with ethyl acetate. The mycelia were extracted with methanol. All the three extracts were concentrated at 40° to obtain crude extracts. All the crude extracts of NK2 obtained from the fermented media were subjected to chromatographic analysis. Based on Rf values, crude antibiotic fractions were classified [14,15].
Test bacteria were grown on nutrient agar and fungi were grown on Sabouraud dextrose agar medium. The extracts were dissolved in corresponding solvents and 100 µl of the samples were placed in to the corresponding cups. Zone of inhibition was measured after 24 h incubation at 37° for bacteria and after 48 h incubation at 28° for fungi. The antimicrobial activity was estimated by measuring the diameter of the inhibitory zone [16]. All extracts obtained from broth culture of NK2 were tested for its cytotoxic activity on Vero cell lines using the Trypan Blue exclusion techniques [9]. The samples were tested at various concentrations between 125-500 µl/ml.
In the process of screening of soil actinomycetes, the isolate NK2 was found to be capable of producing antibiotic against bacteria and fungi. The soil isolate NK2 gave slightly positive results for the nitrate reduction and starch hydrolysis and it gave intense results for the acid production test. It does not have the capacity to produce H2S and Melanin pigments. NK2 showed white band and solid formation after 24 h for milk coagulation test and at 48 h it showed white band, more whey like brownish medium, solid formation and gas formation. NK2-isolate showed growth in medium containing lactose, good growth in medium containing sucrose, fructose and d(+) sorbitol. it showed good growth with fermentation in the medium containing glucose and maltose and good growth with no fermentation in the medium containing d(+)mannitol.
Scanning electron microscope revealed a rectangular shape with irregular grouping of the NK2 isolate fig. 1. The color of the aerial mycelium was cream in ISP-2 (YEME) yeast extract malt extract agar, ISP4 (inorganic salt starch agar), ISP6 (peptone yeast extract agar) ISP7 (tyrosine agar) and it was white in ISP-3 (oats meal agar and ISP 5 (glycerol asparagine agar). NK2 isolate showed brown colour soluble pigments in ISP–4 medium.
The production of antibiotic was carried out in stirred flask culture. The production began after inoculations, gradually reached the maximum at 70 h and slowly decreased. At 70 h the pH was 6.8 and there was slight increase to 7. Biomass reached the maximum at 94 h and then remained at the same level till 118th h. Fermentation parameters are listed in Table 1. After extraction with n-butanol, the fermented broth gave a cream colour powder with the percentage of yield 0.689%. Extraction with ethyl acetate yielded a brownish yellow powder with a yield of 0.0560% and methanol extract yielded a yellowish brown powder with a yield of 0.0548%. By trial and error method the optimal solvent system for TLC studies of NK2 was found to be butanol, acetic acid and water in the ratio of 9:0.5:0.5. The Rf (Retardation factor) values for the n-butanol, ethyl acetate and methanol was found to be 0.36, 0.08, 0.30, respectively.
| Time (h) | *Broth packed cell weight (g) | Packed cell volume (%) | pH |
|---|---|---|---|
| 14 | 0.02445 | 22 | 6.0 |
| 22 | 0.02489 | 25 | 6.5 |
| 38 | 0.02528 | 29 | 6.5 |
| 46 | 0.02576 | 32 | 6.0 |
| 62 | 0.02593 | 35 | 6.5 |
| 70 | 0.02634 | 39 | 6.8 |
| 86 | 0.02687 | 43 | 6.8 |
| 94 | 0.02719 | 46 | 7 |
| 110 | 0.02735 | 48 | 7 |
| 118 | 0.02745 | 49 | 7 |
*Weight of broth prior to fermentation = 0.02418 g / ml.
Table 1: Fermentation Parameter Of Soil Isolate, NK2
n-Butanol extract from the fermented media showed the antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis. Ethyl acetate extract showed the antimicrobial activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Candida albicans, Candida krusei E. coli, B. subtilis, S. aureus and Candida albicans Ca 27. The methanol extract showed activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Aspergillus niger, Candida albicans Ca 27. The antimicrobial activity of n-butanol, ethyl acetate and methanol from the stirred flask culture of NK2 is summarized in Table 2.
| Organisms | Zone of Inhibition (mm) | ||
|---|---|---|---|
| n- Butanol extract (100 µg/ml) | Ethyl acetate extract (100 µg/ml) | Methanol Extract (100 µg/ml) | |
| Escherichia coli | 33 | 25 | 24 |
| Pseudomonas aeruginosa | - | - | - |
| Bacillus subtilis | 32 | 14 | 14 |
| Staphylococcus aureus | 35 | 24 | 19 |
| Aspergillusniger | - | - | 22 |
| Aspergillusflavus | - | - | - |
| Candia albicans | - | 27 | 21 |
| Candida krusei | - | - | - |
Table 2: Microbial Sensitivity Of The Various Extracts Of NK2 Soil Isolates
The fractions that obtained from the isolate were found to be effective against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Candida albicans, Candida kruse, Aspergillus niger. Based up on the antimicrobial studies and taxonomical studies (Table 3), it concluded that compound obtained from the isolate was an antibiotic belongs to the Streptomycetes spp. Further work can be continued for the structural elucidation of the compounds obtained by the fermentation process and it can be compared with the standard antibiotics to prove its potency.
| Test performed | Results obtained | |||
|---|---|---|---|---|
| Biochemical tests | ||||
| Melanin formation | - | |||
| Nitrate reduction | + | |||
| H2S production | - | |||
| Starch hydrolysis | + | |||
| Gelatin liquiÞcation | - | |||
| Acid production | ++ | |||
| Milk coagulation and peptonisation | ||||
| After 24 hours | WB, S | |||
| After 48 hours | WB, More WLB, S, G | |||
| pH 7.0 | ||||
| Carbon utilization tests | ||||
| Sucrose | ++ | |||
| Lactose | + | |||
| Dextromannitol | ± ± | |||
| Dextro Sorbitol | + + | |||
| Glucose | + + + + | |||
| Maltose | + + + + | |||
| Fructose | + + | |||
| Culture characteristics | ||||
| ISP-2 media | G: Good | AM: Cream | R: Cream | Sp: Nil |
| ISP-3 media | G: Good | AM: White | R: Off white | Sp: Nil |
| ISP-4 media | G: Fair | AM: Cream | R: Cream | Sp: Brown |
| ISP-5 media | G: Fair | AM: White | R: White | Sp: Nil |
| ISP-6 media | G: Fair | AM: Cream | R: Yellow | Sp: Nil |
| ISP-7 media | G: Fair | AM: Cream | R: Cream | Sp: Nil |
For the biochemical tests: ++ denotes very intense, + denotes slightly formed and – denotes a negative result. Milk coagulation and peptonisation test results indicated by WB, white band; G, gas formed; WLB, whey like brownish medium and S, solid formation. For the litmus paper test, purple indicates alkalinity while pink indicates acidity. In the carbon utilization tests, + denotes growth, ++ denotes good growth, +++ indicate growth with fermentation, ++++ indicates good growth with fermentation, while ±± denotes growth with no fermentation and ±±± indicates good growth with no fermentation. Culture characteristics are describes as G for growth, AM for aerial mycelium, R for reverse colour and Sp for soluble pigment.
Table 3: Biochemical And Taxonomical Results For NK2 Soil Isolates
References
- Bulock JD, Kristiansen B. Basic Biotechnology, New York: Academic Press; 1997. p. 433.
- Waksman SA. ClassiÞ cation, identiÞ cation and description of genera and Species: The Actinomycetes, Vol. 2. Baltimore: Williams and Wilkins; 1961. p. 1-363.
- Kutzner KJ. The family Streptomycetaceae. In: A Hand Book on habitats, isolation and identification of bacteria, The Prokaryotes. Vol. 2. New York: Springer Verlag 1986. p. 2028-90.
- Lazzarini A, Cavaletti L, Toppo G, Marinelli. F. Rare Genera of Actinomycetes as Potential Producers of New Antibiotics. Antonie Van Leeuwenhoek 2000;78:399-405.
- Kuster E, Williams ST. Selection media for isolation of Streptomycetes. Nature 1964;202:928-9.
- Waksman SA. The Actinomycetes: Their Nature Ocurrence, Activites and Importance. 1st ed. Waltham, Mass: Chronica Botanica Co; 1950. p. 17
- Casida LE. Industrial Microbiology, New Delhi: Wiley Eastern Ltd. 1968. p. 210.
- Pridham TG, Gottlieb D. An Antimicrobial Substance Produced streptomyces roseolilacines J Bacteriol 1948; 56:107-14.
- Hara M, Mokundai T, Kobayashi E. Distribution of Actinomycetes in Soil. J Antibiol 1990;12:1513-8.
- Bergy DH, Breed RS. Bergey.s Manual of Determinative Bacteriology, 7th ed. Baltimore: Williams and Wilkins; 1957. p. 612-789.
- Davis BD, Dulbecco R, Eisen HN, Ginsberg HS. Microbiology. 4th ed. Baltimore: Lippincott Williams and Wilkins 1990. p. 667.
- Egorov NS. Antibiotics: A Scientific Approach. Moscow: Mir Publishers; 1985. p. 60
- Vandamme EJ. Biotechnology of Industrial Antibiotics, Drugs and Pharmaceutical Scinces. Vol. 22, New York: Marcel Dekker; 1984. p. 62.
- Aszalos A, Davis S, Frost D. The Characterization of the New Viridomycin. J Chromatogr 1968;37:487-98.
- Funayama S, Tshibashi M. Metabolic Product of Microorganism 2341 Urdamycins, New Angucycling Antibiotic from Streptomyces Fradiae. J Antibio 1989;12:1734-40.
- Karanagh F. Dilution Methods of Antibiotic Assay. Analytical Microbiology. Indianapolis: Academic Press; 1985. p. 207.