- Corresponding Author:
- M. M. Abukhader
Faculty of Pharmacy, Applied Science University, Amman-11931, Jordan
Present address: Department of Pharmacy, Oman Medical College, P.O. 620, P.C. 130, Muscat, Sultanate of Oman
E-mail: majed.abukhader@gmail.com
| Date of Submission | December 09, 2011 |
| Date of Revision | April 16, 2012 |
| Date of Acceptance | April 24, 2012 |
| Indian J Pharm Sci, 2012, 74 (3): 195-200 |
Abstract
The maximum tolerated dose for intraperitoneal injection and oral ingestion of thymoquinone was determined in male and female Wistar rats. A range of dose levels of thymoquinone: 20, 30 and 40 mg/kg body weight for intraperitoneal injection and 200, 300 and 500 mg/kg body weight for oral ingestion were tested for acute toxicity in rats. The results showed that the maximum tolerated dose for intraperitoneal injection was 22.5 mg/kg in male rats and 15 mg/kg in females, whereas for oral ingestion it was 250 mg/kg in both male and female rats. There were different signs of toxicity shown in rats which received intraperitoneal injection from those that received oral ingestion of thymoquinone. Rats which received intraperitoneal injection of thymoquinone showed toxicity signs which were related to acute pancreatitis. Meanwhile, rats which received oral ingestion of thymoquinone showed transient toxicity signs. Two deaths were reported at dose of 500 mg/kg as a result of bowel obstruction complications. The data presented in this study indicate that the route of administration of thymoquinone could have an influence on thymoquinone toxicity outcome in both genders.
Keywords
Acute pancreatitis, acute toxicity, adynamic ileus, thymoquinone
Thymoquinone (TQ) is a promising bioactive phytochemical compound which is found in the seeds of Nigella sativa plant [1]. It has recently attracted significant scientific attention due to its potent in vitro and in vivo anticancer and effective antioxidant properties [2,3]. Although it has a wide range of therapeutic benefits, there is lack of clinical evaluation of TQ in humans and the need for clinical studies to assess its health benefits on human is required [1,2,4]. Therefore, toxicity studies are essential and a step to the right direction to provide a solid starting point for further preclinical and clinical evaluation of such an important phytochemical compound. In literature, there is few reported research work describing TQ toxicity signs and symptoms. These include peritonitis and abdominal muscle contractions when TQ was given intraperitoneally (IP) [5,6] and difficulty in respiration (dyspnoea) when TQ was given orally [6,7] at different dose frequency exposure (acute, subacute and subchronic) in rats and mice. It was noticed that in this reported research work, the maximum tolerated dose (MTD) of TQ, which is defined as the highest dose that is safe to administer to animal models in the absence of intolerable adverse effects, was not addressed. Furthermore, there was no attempt to explain a possible mechanism of TQ toxicity with IP and oral routes and the effect of gender (male and female) on TQ toxicity was overlooked.
The aim of this study was to find the MTD value for acute exposure of TQ and address the possible mechanism of TQ toxicity using IP and oral routes. In preclinical and clinical toxicology, the gender issue is becoming an important investigation aspect [8,9], therefore, the effect of gender on TQ toxicity will be an investigation point in our study. Anatomical and biochemical analysis were implemented to achieve the aim set for our study.
Materials and Methods
TQ (2-isopropyl-5-methyl-1,4-benzoquinone) was obtained from Sigma USA. TQ solution was prepared by dissolving in 0.5% dimethyl sulphoxide (DMSO) followed by the addition of olive oil. The solution was diluted using olive oil at different concentrations to allow doses of 20, 30 and 40 mg/kg for IP administration and 200, 300 and 500 mg/kg for oral administration of TQ.
Male and female Wistar albino rats (11-13 weeks old, 170-220 g) were obtained from the Jordan University of Science and Technology (Irbid, Jordan) and housed in the animal house which was properly equipped for animal experiments. Animals were housed at constant humidity and temperature (25±5°) with 12 h light/ dark cycle. Tap water and commercial pellet diet were provided ad libitum. All procedures were carried out under the guidelines of the Animal Ethics Committee of Applied Science University, Amman, Jordan.
Experimental design
Sixty four male rats and an equal number of female rats were acclimatised for a 5-day period. The test subjects were individually weighed and marked prior to treatment. The reported TQ LD50 in rats after IP injection was 57.5 mg/kg and after oral ingestion was 794.3 mg/kg [6], a dose escalation study was initiated on test subjects in accordance with a standard procedure to determine the MTD [10,11]. The rats were divided into two groups: The first group received acute dose of TQ intraperitoneally and the second group received acute dose of TQ orally. Each group was further subdivided according to the dose level under investigation; 0, 20, 30, 40 mg/kg body weight for IP injection of TQ and 0, 200, 300 and 500 mg/kg body weight for oral ingestion of TQ. Control group was given only 0.5% DMSO and olive oil. Eight male and eight female rats were used in each dose level experiment. Once treatment commenced, the animals were observed for 5 days at 24 h intervals, with necropsies performed at the end of the experiment. Dose levels to be tested were not applied in parallel but were given with 2-4 days between each dose level.
Blood sample collection
Control and treated rats were sacrificed by cervical dislocation on day five of the experiment and blood was obtained by heart puncture and collected in plain blood sampling tubes. Blood serum samples were snap-frozen in liquid nitrogen for subsequent biochemical analysis.
Glucose level determination
The glucose level was determined in the whole blood of the control and the treated rats using Glucolab autocoding device (Infopia Co. Ltd., Korea) provided with blood glucose test strips. After 24 h of IP injection or oral ingestion of TQ, rats were kept without food (fasting) for 2 h and by using a needle puncture of the tail, a blood drop was taken to measure blood glucose with the help of Glucolab autocoding device. The values were expressed as units of milligram (mg) per decilitre (dl).
Serum α-amylase levels determination
The serum α-amylase levels were measured using a commercial kit (Linear Chemicals, Barcelona, Spain). The values were expressed as units of enzyme (U) per litre (l). The method was based on the hydrolysis of the synthetic specific substrate 2-chloro-ρ-nitrophenyl- α-D-maltotrioside (CNP-G3) by α-amylase generating the 2-chloro-ρ-nitrophenol (CNP) the absorbance of which was detected at 405 nm.
Serum triglyceride levels determination
The serum triglyceride levels were measured using a commercial kit (Biosystems S. A., Barcelona, Spain). The values were expressed as milligram per litre (mg/l) of triglyceride in serum. The method was based on coupled reactions which involved: Lipase, glycerol kinase, glycerol-3-phosphate oxidase, peroxidase, adenosine triphosphate (ATP), 4-chlorophenol and 4-amino antipyrine in piperazine- N,N′-bis(2-ethanesulfonic acid) (PIPES buffer). The reaction ended with the formation of the coloured complex quinoneimine the absorbance of which was detected at 500 nm.
Data analysis
Statistical analysis was performed using Prism v. 4.01 (GraphPad Software, CA, USA). Glucose data were analysed using Student’s t-test, where as ANOVA was used for the analysis of triglyceride and amylase data. Comparisons of toxicity between male and female groups were made according to Chi-Square test. Data were expressed as mean±SEM. P<0.05 was considered statistically significant.
Results
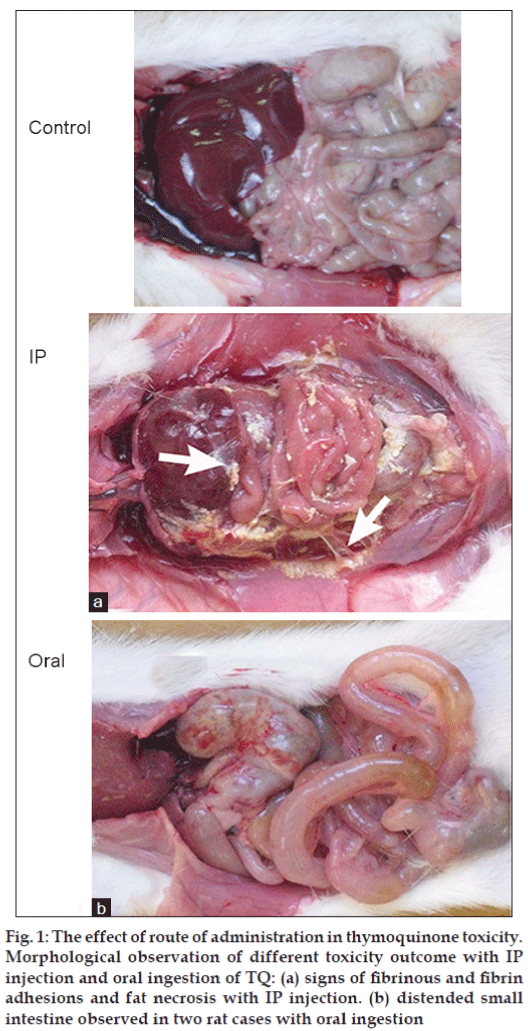
Single IP injection of TQ dose of 20, 30 and 40 mg/kg was given to male and female rats and were observed for 5 days. The majority (26 rats; 81.25%) of male and female rats which received TQ dose of 30 and 40 mg/kg showed TQ-related toxicity signs which appeared within 72 h postdose such as: irritability, lethargy, piloeriction and slight abdominal swelling. Moreover, rats which suffered from toxicity also showed a drop in body weight of about 17.3±4.6 g on the 3rd and 4th day of the experiment. On the 5th day of the experiment, 74% of these rats gained weight of about 5 g and 26% of the rats continued to lose weight. Regarding TQ dose of 20 mg/kg, 25% of female rats which received the dose showed TQ-related toxicity signs mentioned above whereas none of the male rats showed these signs (Table 1). Therefore, according to the guidelines for determining MTD [10-12], the TQ dose of 22.5 mg/ kg is MTD for IP injection in male rats and the TQ dose of 15 mg/kg is MTD for IP injection in female rats. All control and treated male and female rats of all dose levels were sacrificed in the morning of day five of the experiment and macroscopic examination of body organs was carried out. More than 50% of the treated rats showed numerous fibrous and fibrinous adhesions between various organs as well as adhesions between organs and the body wall. Fat necrosis was observed in the transverse mesocolon or in the mesentery (fig. 1a). Moreover, the peritoneal cavity contained a large volume of blood stained fluid.
| Dose (%) | Female | Male | Control | ||||
|---|---|---|---|---|---|---|---|
| 20 mg/kg | 30 mg/kg | 40 mg/kg | 20 mg/kg | 30 mg/kg | 40 mg/kg | ||
| Live rats with no toxicity | 6 (75) | 2 (25) | None | 8 (100) | 3 (37.5) | 1 (12.5) | All |
| Live rats with toxicity | 2 (25) | 6 (75) | 8 (100) | None | 5 (62.5) | 7 (87.5) | None |
Value in parenthesis represents percentage, n=8/dose level
Table 1: Toxicity data recorded for male and female rats which received different dose levels of tq intraperitoneally
Figure 1: The effect of route of administration in thymoquinone toxicity. Morphological observation of different toxicity outcome with IP injection and oral ingestion of TQ: (a) signs of fibrinous and fibrin adhesions and fat necrosis with IP injection. (b) distended small intestine observed in two rat cases with oral ingestion
Single oral ingestion of TQ dose of 200, 300 and 500 mg/kg was given to male and female rats and were observed for 5 days. Signs of weight loss, diarrhoea, hypoactivity, slight abdominal distention and shortage of breath (dyspnoea) were observed in 34% of the rats which received 300 and 500 mg/kg within the 48 h postdose. Thereafter, rats regained weight and the toxicity signs started to disappear until the 5th day of the experiment. It was noticed that there was a difference in the incident of these TQ-related toxicity signs between male and female rats (Table 2). This could be due to drug disposition differences between both genders [13]. One male and one female rat at a dose of 500 mg/kg showed remarkable signs of discomfort, hypoactivity, irritability, abdominal distention and dyspnoea within 48 h postdose and died soon afterwards. Luckily this incident happened under close observation and in an attempt to find an explanation, both rats were quickly laparotomised and it was found that the bowel (mainly the small intestine) was dilated and the other vital organs were normal (fig. 1b). Regarding rats which received 200 mg/kg, male and female rats showed no signs of toxicity. It can be concluded that the MTD for the oral ingestion of TQ in both male and female rats was 250 mg/kg. On day five of the experiment, all sixty two male and female rats of all dose levels were sacrificed and macroscopic examination of body organs was carried out. The vital visceral organs (heart, lungs, liver and kidneys) and the peritoneum were found to be normal without any apparent sign of damage or necrosis.
| Dose (%) | Female | Male | Control | ||||
|---|---|---|---|---|---|---|---|
| 200 mg/kg | 300 mg/kg | 500 mg/kg | 200 mg/kg | 300 mg/kg | 500 mg/kg | ||
| Number of rats suffered from toxicity within 48 h | None | 3 (37.5) | 5 (62.5) | None | 1 (12.5) | 2 (25) | None |
| Recovery at day 5 of the experiment (% recovery) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Deaths recorded during the experiment | None | None | 1 (12.5) | None | None | 1 (12.5) | None |
Value in parenthesis represents percentage, n=8/dose level
Table 2: Toxicity data recorded for male and female rats which received different dose levels of tq orally
Determination of blood glucose level and biochemical analysis of serum amylase and triglycerides were carried out and results are as follows:
Glucose level: IP injection of TQ caused a significant reduction in blood glucose level (hypoglycaemia) in one-half (50%) of the male and female rats which were found to suffer from toxicity (Table 3) and insignificant reduction in the other 50% when compared with the control. In case of oral ingestion of TQ, all treated male and female rats of all dose levels showed no blood glucose reduction when compared with the control.
| Dose | Female | Male‡ | Control | |||
|---|---|---|---|---|---|---|
| Glucose | ||||||
| Number of rats with ↓ glucose | 1 | 2 | 5 | 2 | 4 | None |
| Glucose level | 58 | 54 ± 1.4* | 46 ± 9.4* | 65 ± 1.4* | 61.3 ± 6.4* | 108.5 ± 6.9 |
| Triglyceride | ||||||
| Number of rats with ↑ triglyceride | 2 | 6 | 8 | 5 | 7 | None |
| Triglyceride level | 97.2 ± 4.7* | 104.8 ± 11.5*† | 96.1 ± 4.3* | 103.6 ± 11.5*† | 98.5 ± 6.3* | 78.1 ± 7.9 |
| Amylase | ||||||
| Number of rats with ↑ amylase | 2 | 6 | 8 | 5 | 7 | None |
| Amylase level | 716.5 ± 24.8* | 697.3 ± 47.3* | 792.3 ± 70.8* | 685.3 ± 48.7* | 746.4 ± 67.9* | 572.3 ± 50.3 |
* P<0.05 when compared with control. †Insignificant (P>0.05) when triglyceride level compared with other treated groups. ‡No toxicity observed with male rats at TQ dose of 20 mg/kg (refer Table 1)
Table 3: Level of measured blood glucoste (mg/dl), serum triglyceride (mg/l) and serum amylase (u/l) for ip treated male and female rats which showed toxicity (n=28) in all dose levels
Triglyceride level: In all IP-treated male and female rats which were found to suffer from toxicity, there was a significant elevation in triglyceride levels which indicated hyperlipidaemia (Table 3). Normal triglyceride level was recorded with rats which received TQ orally. Amylase level: In all IP-treated male and female rats which were found to suffer from toxicity, there was a significant elevation in amylase levels which indicated pancreatitis (Table 3). Normal amylase level was recorded with rats which received TQ orally.
Discussion
The toxicity signs observed in rats which received IP injection of TQ were body weight loss and signs of diffuse inflammation such as fibrin deposition and the adhesiveness of visceral organs as well as the presence of blood stained fluids in peritoneal cavity (peritoneal effusion). These toxicity signs are indicators of generalised peritonitis [14]. Leakage of pancreatic enzymes into the peritoneal cavity is one of the major causes of the generalised peritonitis [14]. Therefore, male and female rats which showed signs of toxicity first developed acute pancreatitis followed by generalised peritonitis. This was confirmed by a significant increase in serum amylase level measured and also fat necrosis which was observed in the transverse mesocolon or in the mesentery. The link between the IP injection of TQ and the development of acute pancreatitis has to be clarified. There are many published research reporting the hypoglycaemic effect of TQ [15,16]. Accordingly, blood glucose level was measured for male and female rats which showed signs of toxicity and a significant reduction (P<0.05) in blood glucose was observed in one-half (50%) of these rats after 24 h postdose. This could lead to the assumption that all male and female rats which were found to suffer from toxicity experienced reduction in blood glucose level sometimes during the 24 h postdose. Subsequently, one-half (50%) of rats recovered with an insignificant blood glucose level and the other half stayed hypoglycaemic. TQ effect on blood glucose was explained to involve many proposed mechanisms one of which suggests that TQ enhances and stimulates glucose uptake and utilization by peripheral tissues suggesting an extrapancreatic effect in reducing glucose levels in blood circulation [17,18]. Low blood glucose level for a long period of time could induce lipolysis [19,20]. Furthermore, Al-Naqeeb and Ismail [21] reported that TQ was effective in regulating Apolipoprotein A-1 and Apolipoprotein B100 gene expression in human hepatic cell lines. These physiological effects of TQ has explain the elevated triglyceride levels (hyperlipedimia) detected in rats which showed signs of toxicity (Table 3). In literature, the relationship between hyperlipidaemia and acute pancreatitis was reported [22,23]. This has lead us to conclude that acute pancreatitis which was observed in rats which showed toxicity was a result of hyperlipedaemia induced by the IP injection of TQ. It is worth mentioning that no death occurred during the 5-day period of the experiment which could indicate that rats suffered from interstitial acute pancreatitis which is a mild nonlife threatening form of pancreatitis [24].
With respect to rats which received oral ingestion of TQ, data collected from experimental observations and analysis suggest that single oral ingestion of TQ in doses lower than 500 mg/kg body weight could be nonlethal. Signs of transient toxicity observed with 300 and 500 mg/kg such as weight loss, slight abdominal distention and dyspnoea could be due to an indirect effect of oral ingestion of TQ on the bowel and lungs. One of the possible explanations for the abdominal distention observed is TQ like other quinone derivatives, is considered as a redox-cycler which is metabolised in vivo to hydroquinone or semiquinone radicals by enzymatic or nonenzymatic reactions leading to the generation of superoxide anion radicals [25,26]. It is documented that free radicals could be involved in inflammation [27]. Superoxide and hydrogen peroxide increases mucosal and vascular permeability and are involved in the recruitment and activation of neutrophils [27]. This could cause lymphoid hyperplasia involving the Peyer’s patches which are usually found in ileum, the lowest portion of the small intestine. The enlargement of Peyer’s patches could disturb the myenteric plexus nerve function which is responsible for gastrointestinal tract motility causing loss of the intestinal peristaltic movement (adynamic ileus) and bowel obstruction. This leads to the accumulation of gas and fluids inside the lumen of the intestine causing intestinal dilatation. This progressive accumulation of gas and fluids inside the lumen leads to third spacing of fluid, electrolytes and proteins resulting in dehydration and hypovolemia due to volume depletion and electrolyte disturbances [28]. Moreover, the abdominal distention could cause increased abdominal pressure on the diaphragm leading to compromised ventilation and dyspnoea. Therefore, it can be assumed that rats which suffered from toxicity due to single oral TQ dose of 300 and 500 mg/kg developed adynamic ileus within 48 h and recovered until the 5th day of the experiment. Regarding male and female rats which died earlier on the 3rd day of the experiment, they could not tolerate the complications of adynamic ileus represented by either hypotension and shock or dyspnoea.
TQ was pharmacologically tested against many disease models in vivo in which oral ingestion of TQ was the main route of administration [29]. Therefore, the determination of MTD for the oral ingestion of TQ was required and it was 250 mg/kg for the acute dose of TQ. Regarding subacute and subchronic dose administration of TQ, it was noticed that a large number of studies used oral TQ dose in the range of 10-100 mg/kg body weight without any reported toxicity or deaths [7,30-36]. Presumably, the MTD level would be 100 mg/kg which is in agreement with initial observations (data not published) which suggest MTD around the above dose level.
The relationship between gender difference and TQ toxicity was statistically assessed in this study. Although the toxicity incidence in both IP and oral administration seemed to be higher among female rats than male rats, this difference was not statistically significant. In this regard, the data presented can be considered as a preliminary evaluation of potential relationship between gender difference and TQ toxicity and further experimentation is required to unequivocally establish this relationship.
The present study discusses valuable information about TQ toxicity in Wistar rats which could add up to what is published in literature: (1) Determine the maximum toxic dose for single IP injection and oral ingestion of TQ in male and female rats. (2) Provide possible mechanism of TQ toxicity with IP injection and oral ingestion of TQ which requires further histopathological evaluation. The toxicity response differs in both IP injection and oral ingestion of TQ which can be explained by the fact that IP injection results in complete absorption of TQ into systemic circulation, whereas with oral ingestion, TQ is biotransformed in gastrointestinal tract or metabolised in the liver.
Acknowledgements
The author would like to acknowledge the help of Mrs. Sana Al-Ebbini in biochemical analysis and Mr. Salem Al-Shawabkeh in animal experiments. The author also would like to thank Dr. Feras El-Hajji and Samar Khater for their valuable discussion. The author much appreciates the help of Ruthmae I. Brown for critical reading of the manuscript. The author is grateful to Applied Science University, Amman, Jordan, for the financial support granted to this research work.
References
- Shimizu Y, Takahiko S, El-Mahkoudy A, Hideki N, Tadashi T. Mechanism of the pharmacological actions of a plant-derived active compound, thymoquinone. Vet Biochem 2004;41:15-21.
- Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: A promising anticancer drug from natural sources. Int J Biochem Cell Biol 2006;38:1249-53.
- Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug ChemToxicol 2003;26:87-98.
- Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R. Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 2009;5:1-11.
- Hawsawi ZA, Ali BA, Bamosa AO. Effect of Nigella sativa (Black Seed) and thymoquinone on blood glucose in albino rats. Ann Saudi Med 2001;21:242-4.
- Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigellasativa, in mice and rats. J Ayub Med Coll Abbottabad 2008;20:25-7.
- Badary O, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Almazar MM. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res 1998;44:56-61.
- Nicolson TJ, Mellor HR, Roberts RR. Gender differences in drug toxicity. Trends PharmacolSci 2010;31:108-14.
- Branch DR. Gender-selective toxicity of thimerosal. ExpToxicolPathol 2009;61:133-6.
- Cutler N, Sramek J. Investigators perspective on the MTD. J ClinPharmacol 2000;40:1184-7.
- Collins J. Regulators perspective on MTD: Is there a role for surrogate markers. J ClinPharmacol 2000;40:1199-201.
- Tobaccodocuments.org [internet]. Maryland: BorristonLabratories, Inc.; 2001 Available from: http://tobaccodocuments.org/lor/00953611.html. [Last updated 2001 Dec 6; accessed 2011 Oct 20]
- Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol 2011;2011:187103.
- Withrow SJ, Black AP. Generalized peritonitis in small animals. Vet Clin North Am Small AnimPract 1979;9:363-79.
- AbuKhader MM. Thymoquinone: A promising antidiabetic agent. Int J Diabetes DevCtries 2012;32:65-68.
- Mathur M, Gaura J, Sharmaa R, Haldiyaa KR. Antidiabetic properties of a spice plant Nigella sativa. J EndocrinolMetab 2011;1:1-8.
- Fararh KM, Shimizu Y, Shiina T, Nikami H, Ghanem MM, Takewaki T. Thymoquinone reduces hepatic glucose production in diabetic hamsters. Res Vet Sci 2005;79:219-23.
- El-Dakhakhny M, Mady N, Lembert N, Ammon HP. The hypoglycemic effect of Nigella sativa oil is mediated by extrapancreatic actions. Planta Med 2002;68:465-6.
- Caprio S, Gelfand RA, Tamborlane WV, Sherwin RS. Oxidative fuel metabolism during mild hypoglycemia: Critical role of free fatty acids. Am J Physiol 1989;256:E413-9.
- Battezzati A, Benedini S, Fattorini A, PiceniSereni L, Luzi L. Effect of hypoglycemia on amino acid and protein metabolism in healthy humans. Diabetes 2000;49:1543-51.
- Al-Naqeeb G, Ismail M. Regulation of apolipoprotien A-1 and apolipoprotien B100 genes by thymoquinone rich fraction and thymoquinone in HepG2 cells. J Food Lipids 2009;16:245-58.
- Gumaste VV. Hyperlipidemia and pancreatitis: The chicken or the egg? Am J Gastroenterol 1996;91:1275-6.
- Gianfrate L, Ferraris L. Acute pancreatitis, hyperlipidemia, and diabetic ketoacidosis: Who comes first? Am J Gastroenterol 1998;93:1393-4.
- Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: Abdominal complications and fate of fluid collections. Am J Roentgenol 2008;190:643-9.
- Koka PS, Mondal D, Schultz M, Abdel-Mageed AB, Agrawal KC. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: Role of reactive oxygen species. ExpBiol Med (Maywood) 2010;235:751-60.
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol 2000;13:135-60.
- Simmonds, N. Free radicals in gastrointestinal and hepatic disease. In: Blake D, Winyard P, editors. Immunopharmacology of Free Radical Species. London: Academic Press; 1995. p. 143.
- Kulaylat M, Doerr R. Small bowel obstruction. In: Holzheimer RG, Mannick JA, editors. Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: ZuckschwerdtVerlagGmBH; 2001. Available from: http://www.ncbi.nlm.nih.gov/books/NBK6873/. [Last accessed 2011 Oct 20].
- Salem ML. Immunomodulatory and therapeutic properties of the Nigellasativa L. seed. IntImmunopharmacol 2005;5:1749-70.
- Al-Majed AA, Al-Omar FA, Nagi MN. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol 2006;543:40-7.
- Al-Enazi MM. Effect of thymoquinone on malformations and oxidative stress-induced diabetic mice. Pak J BiolSci 2007;10:3115-9.
- Fararh KM, Ibrahim AK, Elsonosy YA. Thymoquinone enhances the activities of enzymes related to energy metabolism in peripheral leukocytes of diabetic rats. Res Vet Sci 2010;88:400-4.
- Kanter M. Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochem Res 2008;33:579-88.
- Kanter M. Thymoquinone attenuates lung injury induced by chronic toluene exposure in rats. ToxicolInd Health 2011;27:387-95.
- El-Saleh SC, Al-Sagair OA, Al-Khalaf MI. Thymoquinone and Nigellasativa oil protection against methionine-induced hyperhomocysteinemiain rats. Int J Cardiol 2004;93:19-23.
- Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: A possible mechanism of action. Cell BiochemFunct 2002;20:143-51.