- *Corresponding Author:
- M. K. Das
Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh-786 004,India
E-mail: du_mkd@yahoo.co.in
| Date of Submission | 28 September 2004 |
| Date of Revision | 27 May 2005 |
| Date of Acceptance | 7 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1): 41-46 |
Abstract
Trazodone hydrochloride has not been previously investigated and reported for potential administration through transdermal route. Therefore, the present investigation was carried out to study the effect of polymeric composition, drug content and plasticizer on the permeation of trazodone hydrochloride across the mouse epidermis for the development of transdermal therapeutic system. The pseudolatex films with different combination of polymers, plasticizer and drug were prepared from aqueous colloidal polymer dispersions. The polymers used were Eudragit RL100 and RS100. Triethylcitrate was used as plasticizer. The in vitro release and skin permeation through mouse epidermis from the prepared films were studied using Keshary-Chien diffusion cell. The in vitro drug release increased with increasing amount of Eudragit RL100 in the film. It was observed that the maximum skin permeability was attained at a loading dose of 10% w/w in the film. The in vitro flux decreased gradually at higher concentration up to 13% w/w. The present study has demonstrated the potential of the fabricated pseudolatex transdermal films for sustained release of trazodone hydrochloride. The concentration of triethylcitrate in the film markedly affected the skin permeation properties of trazodone hydrochloride.
Transdermal delivery has many advantages over conventional modes of drug administration; it thus avoids hepatic first-pass metabolism, potentially decreases side effects and improves patient compliance [1]. Trazodone hydrochloride (TZN) is an oral antidepressant drug indicated in the symptomatic treatment of moderate to severe depression. It acts by inhibiting serotonin uptake in the brain. In spite of good bioavailability (81±29%) after oral administration, contraindicative manifestations are associated with TZN therapy. The peak plasma concentrations are achieved after 0.5 to 4 h in fasting subjects. The plasma half-life is about 6 h, which makes frequent dosing necessary to maintain the therapeutic blood level of the drug for a long-term treatment. The prolonged and controlled release transdermal delivery system providing therapeutic level of TZN in schizophrenic patients in a convenient low dose form may avoid some of the complications of higher dose oral therapy [2-4].
Pseudolatex consists of colloidal polymer particles dispersed in water [5]. It can be described as a twocompartment system, one compartment being a hydrophilic polymer forming a three-dimensional network, and the other compartment being water. These are widely studied biomaterials for the preparation of sustained release drug dosage form. Their high biocompatibility in a number of applications makes them promising candidates for preventing the side effects. It provides more reliable and steady release of drugs [6]. These latexes have been used extensively to formulate oral controlled release drug delivery systems in the form of coated small particles, beads, or tablets [7-8]. Drugcontaining latex particles for topical [9], ophthalmic [10], or parenteral formulations [11-12] prepared by dissolving the drug in the polymer solution before emulsification and pseudolatex formation have been reported. The present work was undertaken to study the effect of polymer concentration, drug content and plasticizer concentration in pseudolatex films on the release and permeation across mouse epidermis of TZN.
Materials and Methods
Trazodone HCl was purchased from ICN Biomedical, USA. Eudragit NE30D, Eudragit RL100 and RS100 were gift samples from Rohm Pharma, Darmstadt, Germany. Triethylcitrate (TEC) was purchased from E. Merck, India. All other chemicals used in this study were of analytical grade and were used as received without further purification. Double-distilled water from all-glass still was used in all experiments.
Preparation of backing membrane
Eudragit NE30D suspension was dried in air. The films thus obtained were crushed into pieces. Two hundred and fifty milligrams (250 mg) of the polymer was dissolved in 5 ml of acetone and was poured over an aluminium dish and dried overnight. Dryness of the cast films was monitored by judging the degree of transparency.
Preparation of drug containing films
The films were prepared using the compositions shown in Table 1. Acetone solution (5 ml) of the Eudragit RL100 and RS100 at different ratios was prepared by dissolving the polymer to make a 10% w/v solution. The polymer solution was then dispersed in phosphate buffer solution of pH 7.0 (2 ml) containing appropriate amount of dissolved TZN under mild agitation. The drug containing latex (7 ml) was cast on backing membrane into aluminium Petri dishes (13 cm2). The organic solvent was subsequently eliminated to leave stable Eudragit RS/RL pseudolatex films.
| F.N.Code | Compositions | |||
|---|---|---|---|---|
| Polymer Ratios | Plasticizer (TEC) %w/w | Drug % w/w | ||
| RL100 | RS100 | |||
| F1 | 4 | 1 | — | 5 |
| F2 | 1 | 1 | — | 5 |
| F3 | 1 | 2 | — | 5 |
| F4 | 4 | 1 | — | 10 |
| F5 | 4 | 1 | — | 15 |
| F6 | 4 | 1 | 6 | 10 |
| F7 | 4 | 1 | 13 | 10 |
| F8 | 4 | 1 | 20 | 10 |
Table 1: Composition Of Pseudolatex Films
Preparation of mice abdominal skin
The male albino mice were sacrificed by excess chloroform inhalation and hair on the abdominal skin was removed with an electric clipper taking extreme precaution not to damage skin. The shaved skin was then excised from the animal; subcutaneous tissue was surgically removed [13]. The skin surface was observed under microscope for existence of cuts and wounds. The full-thickness skin thus prepared was soaked in distilled water at 60° for 60 s, followed by careful removal of the epidermis with intact stratum corneum [14]. The epidermis was washed with distilled water, dried in desiccator at approximately 25% RH, wrapped in aluminium foil and stored at 4±1° [15]. At the time of use, the epidermis was rehydrated by immersion in water for 1 h at room temperature.
In vitro drug release study
The fabricated film was placed in between the donor and receptor compartment of a Keshary-Chien diffusion cell so as to keep the drug releasing surface towards the receptor compartment, which was filled with saline phosphate buffer (pH 7.4) at 37±1°. The elution medium was stirred magnetically. The aliquots (1 ml) withdrawn over a period of 8 h were passed through membrane filter (pore size 0.22 μ) and drug content were estimated spectophotometrically at 246 nm after proper dilution with saline phosphate buffer (pH 7.4).
In vitro skin permeation study
The mouse epidermis was mounted onto a Keshary- Chien diffusion cell in such a way that stratum corneum side of the skin continuously remained in an intimate contact with the transdermal film in the donor compartment and the dermis side was in constant contact with the receptor solution. The receptor chamber was filled with saline phosphate buffer, pH 7.4 as elution medium, at 37±1°, being stirred magnetically. The aliquots (1 ml) withdrawn over a period of 24 h were filtered through membrane filter and the drug content was assayed at 246 nm spectrophotometrically after proper dilution with the elution medium.
Scanning electron microscopy (SEM)
The surface morphologies of the films were investigated by using a Hitachi S-415A Scanning electron microscope at 25 kV. Prior to examination, samples were gold-coated to render them electrically conductive.
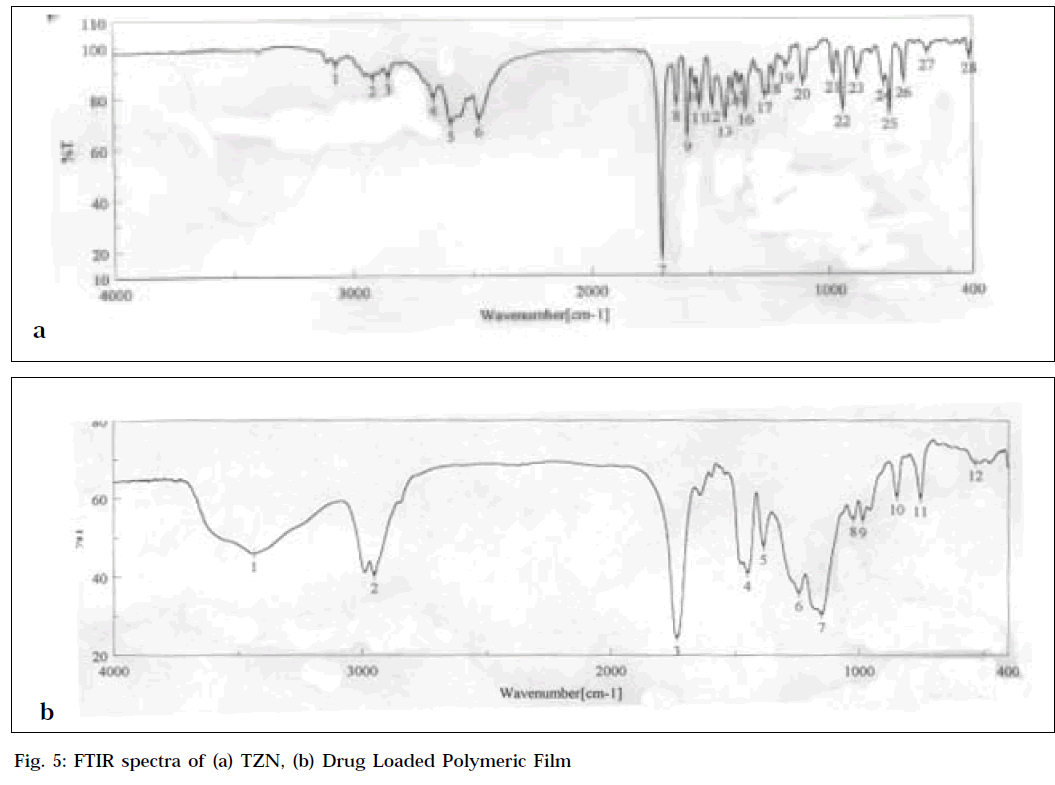
Fourier transforms infrared spectroscopy (FTIR)
Drug-polymer interactions were studied by FTIR spectroscopy. The spectra were recorded for TZN, blank polymeric film and drug-loaded polymeric film using FTIR-spectrophotometer (FTIR-410, Jasco, Japan) from KBr pellets. The scanning range was 400-4000 cm-1 and the resolution was 2 cm-1.
Data and statistical analysis
The TZN concentration was corrected for sampling effects according to the following equation [16]:C1 n = Cn (VT/ VT-VS) (C1 n-1/Cn-1), where, C1n is the corrected concentration of the nth sample, Cn is the measured concentration of TZN in the nth sample, Cn-1 is the measured concentration of the TZN in the (n-1)th sample, VT is the total volume of the receiver fluid, and VS is the volume of the sample drawn.
The cumulative amounts of TZN released and permeated per unit area (μg/cm2) were plotted against time (h), and the slope of the linear portion of the plot was estimated [17] as steady-state flux (μg/cm2/h).
Data are expressed as mean±SEM (n=3). Statistical evaluation was performed by one-way analysis of variance (ANOVA). When a statistically significant difference (P<0.05) was obtained, student’s t-test was performed to evaluate statistical differences between the individual means.
Results and Discussion
The two polymers were mixed in various proportions (formulations F1 to F3) to study the influence of polymeric compositions on the drug release from mixed films as function of Eudragit RL/RS ratio. The drug release increased with increasing amount of the more hydrophilic Eudragit RL100 [18]. As the Eudragit RL100 content increased, the release rate constant increased from 306.8±6.37 μg/cm2/h for formulation F2 to 360.7±25.76 μg/cm2/h for formulation F1. This increase in release rate was significantly different (P<0.05). In contrast, as the Eudragit RS100 content increased, the release rate constant decreased to 200.9±32.14 μg/cm2/h for formulation F3. This decrease in release rate compared to F2 was also significantly different (P<0.05).
Eudragit RL100 and RS100 are copolymers of acrylic and methacrylic acid esters with a low content (2.5-5%) of quaternary ammonium groups. The ammonium groups are responsible for the permeability and swelling of these water-insoluble films. The higher proportion of quaternary ammonium groups in Eudragit RL100 resulted in rapid hydration and drug release, whereas the lower proportion of ammonium groups in Eudragit RS100 is responsible for controlling the release of TZN. A suitable proportion of RS100 and RL100 may be used to achieve prolonged release of the drug.
Initial rapid release was observed, gradually approaching constant values for the rest of time, thus conforming to the controlled release behaviour of the formulations. The initial quick release (burst effect) would help to achieve the therapeutic plasma concentration of the drug in the minimum time, and the constant release later on would then provide a prolonged and controlled release of drug. Burst effect may be due to the initial migration of the drug towards the surface of the films.
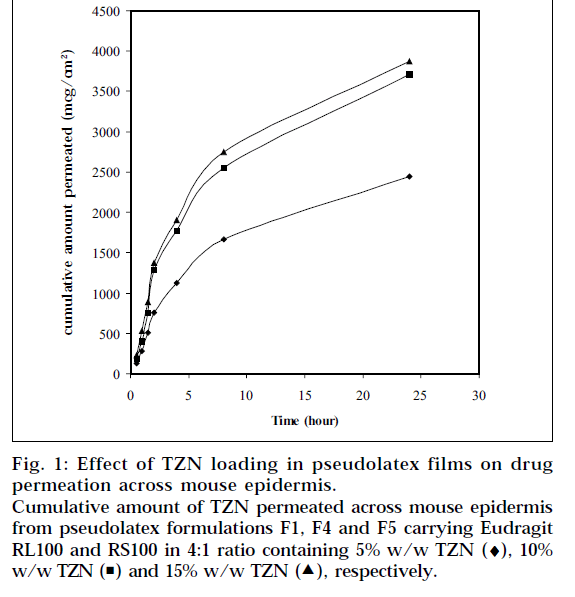
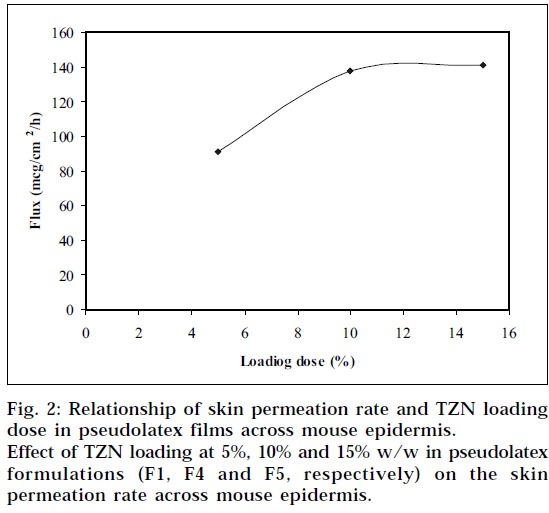
Formulation F1 was selected for in vitro skin permeation study, since it provides highest release of TZN from the pseudolatex film and loaded with different level of TZN. Fig. 1 depicts the influence of TZN levels on in vitro skin permeation of the drug through mouse epidermis. The drug permeation rate increased with increasing amount of TZN in the formulations. When 5%, 10%, 15% (based on polymer weight) TZN was incorporated into the formulation F1, F4, F5, respectively, the drug permeation rates were calculated to be 91.3±3.79, 137.8±5.02 and 140.9±0.46 μg/cm2/h respectively. Higher level of TZN corresponding to lower level of the polymer in the formulation resulted in an increase in the drug release rate. As more drugs are released, more channels are produced in the film matrix, which might have been the cause for faster drug release rates. This enhancement of drug release rate from the films produced a higher drug concentration gradient between the skin and the elution medium, which acts as the driving force for higher drug permeation. The skin permeation of TZN took place at a constant rate over a period of 8 h, after which the rate slightly reduced but was found to be steady.
Figure 1: Effect of TZN loading in pseudolatex films on drug permeation across mouse epidermis. Cumulative amount of TZN permeated across mouse epidermis from pseudolatex formulations F1, F4 and F5 carrying Eudragit RL100 and RS100 in 4:1 ratio containing 5% w/w TZN (♦), 10% w/w TZN ( ) and 15% w/w TZN (
) and 15% w/w TZN ( ), respectively.
), respectively.
The permeation rate of TZN through the mouse epidermis, as found by the magnitude of the slope, was observed to increase steadily as the loading dose of TZN in the pseudolatex film was increased from 5 to 10% w/w. Loading the film with 15% w/w drug did not show any significant increase in the flux. The formulation containing 5% w/w drug was significantly different statistically (P<0.05) from formulation containing 10% w/w and 15% w/w drug, whereas no statistically significant difference (P>0.05) was observed between the formulation containing 10% w/w and 15% w/w drug. The results therefore showed that with increased loading dose up to 10% w/w, both the release and corresponding permeation rates increased simultaneously. This indicates that both the device and skin are controlling the steadystate permeation of TZN through the mouse epidermis. If the skin were the only controlling factor in the steadystate permeation rate, there would be no change in the permeation rate with an increase in the release rate. Therefore, the results suggest that increasing the drug release rate from the device can increase the skin permeation of TZN. Increasing the loading dose beyond 10% w/w showed no significant change in the permeation rate. This is due probably to the skin permeation of TZN reaching plateau level at 10% w/w loading dose (Fig. 2) in the film, indicating the attainment of equilibrium concentration of TZN on the skin surface, thereby producing a reservoir effect of the drug.
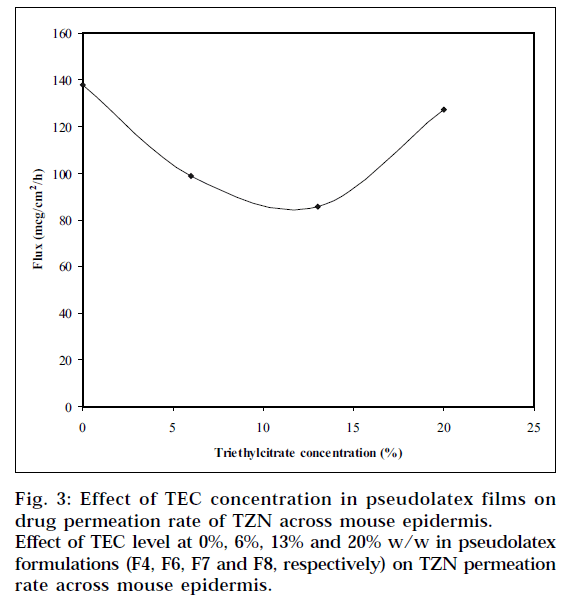
Eudragit RL100 and RS100 require the addition of plasticizers to reduce the minimum film formation temperature. Triethylcitrate, a water-soluble plasticizer, was chosen in the study. The plasticizer diffuses into and softens the polymer particles. This softening promotes latex coalescence and film formation. The effect of TEC concentration on TZN permeation rate across mouse epidermis is shown in Fig. 3. It is indicated from the plot that the drug permeation rate decreased gradually as the amount of TEC in the pseudolatex film was increased from 0 to 10% w/w, then went through a minimum level up to 13% w/w. This plateau may be due to complete coalescence of the latex particles resulting in more continuous film. When 20% w/w TEC was incorporated in the film, the drug permeation rate was increased. There was a significant difference (P<0.05) in skin permeation rate as the TEC concentration increased from 0 to 13% w/w; however, statistically no significant difference (P>0.05) was observed between the films containing 0 and 20% w/w TEC.
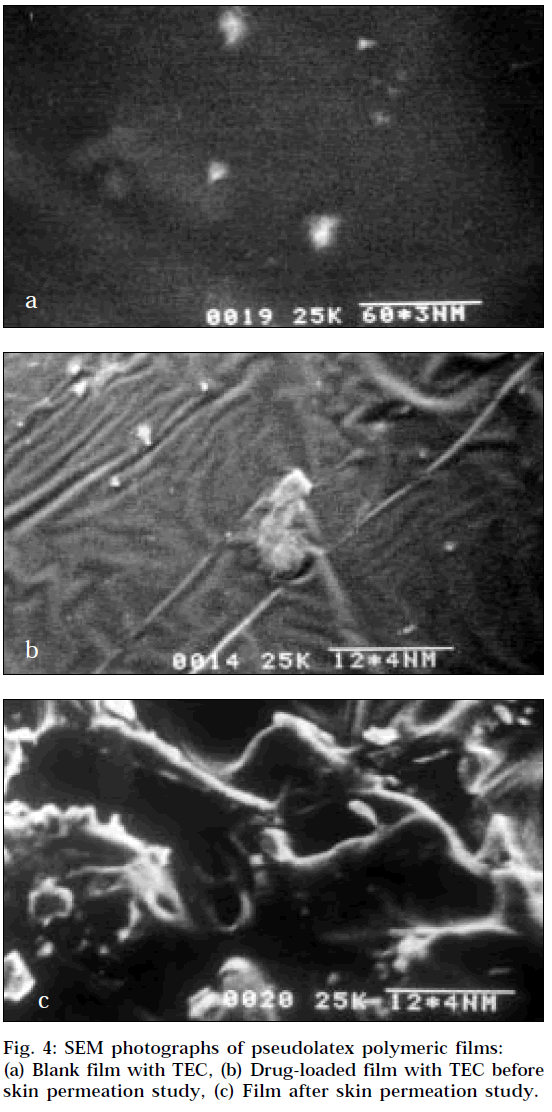
The surface morphologies of the films are displayed in Fig. 4, which exhibit uniformity. The SEM of a film prepared without drug and TEC exhibited brittleness. The polymer particles resisted deformation, and water evaporated through the channels between uncoalesced polymer particles without particle fusion resulting in brittle films. It may be the reason for the faster drug release across mouse epidermis from the film containing no TEC. The Fig. 4a represents the continuous film containing 13% w/w TEC. At low TEC concentrations (<13%), the latex particles were insufficiently plasticized but partially fused. This partial fusion of polymer particles may explain the slower release across mouse epidermis at low TEC concentrations when compared to the film containing no TEC. The Fig. 4b exhibits the SEM photograph of films containing drug and TEC before permeation study and exhibit uniformity. At high TEC concentration (>13%), the increase in permeation rate is probably due to the leaching of the water-soluble plasticizer resulting in formation of pores in the film shown in Fig. 4c. The diffusion of TEC into aqueous media from cast films prepared from aqueous colloidal polymer dispersions has previously been reported [19].
The FTIR spectra of drug-loaded polymeric film and of pure drug are shown in Fig. 5. The characteristics aliphatic CH stretching vibration, C=0 stretching vibration and aromatic C=C stretching vibration of the drug (Fig. 5a) appeared in the spectra of drug-loaded polymeric film (Fig. 5b). These studies revealed that there was no significant interaction between drug and polymer.
The fabricated pseudolatex films containing TZN showed good skin permeation profile of the drug, and the effect prolonged over a period of 24 h. The advantage of single topical administration prolonging the drug effect steadily at least over a period of 24 h in case of schizophrenic patients is obvious. The interesting finding in this work is that the concentration of the plasticizer triethylcitrate in the films significantly affects the permeation of the drug through the skin.
Acknowledgements
The authors wish to acknowledge the facilities provided by the Department of Pharmaceutical Technology, Jadavpur University, and the Department of Pharmaceutical Sciences, Dibrugarh University, to carry out the research work.
References
- Chien, Y.W., In; Robinson, J.R. and Lee, V.H., Eds., Controlled Drug Delivery: Fundamentals and Applications, 2nd Edn., Marcel Dekker Inc., New York, 1987, 523.
- Gorecki, D.K.J. and Verbeck, R.K., In; Florey, K., Eds., Analytical Profile of Drug Substances, Vol. 16, Academic Press Inc., London, 1987, 693.
- Baldessarini, R.J., In; Goodman Gilman, A., Rall, T.W., Nies, A.S. and Taylor, P., Eds., The Pharmacological Basis of Therapeutics, 8th Edn., Vol. 1, Pergamon Press, Maxwell Macmillan Publishing Corporation, New York, 1985, 383.
- Gummans, R.E., Mackenthum, A.V. and Russell, J.W., Brit. J. Clin.Pharmacol., 1984, 18 431.
- Bodmeier, R. and Paerakakul, O., Int. J. Pharm., 1990, 59, 197.
- Panigrahi, L. and Ghosal, S.K., Indian J. Pharm. Sci., 2002, 64, 79.
- Goodhart, F.W., Harris, M.R., Murthy, K.S. and Nesbitt, R.U., Pharm. Tech., 1984, 4, 64.
- Gumowski, F., Doelker, E. and Gurny, R., Pharm. Tech., 1987, 2, 27.
- Biiyiikayaylaci, S., Joshi, Y.M., Peck, G.E. and Banker, G.S., In; Anderson, J.M. and Kim, S.W., Eds., Recent Advances in Drug Delivery Systems, Plenum, New York, 1984, 291.
- Gurny, R., Boye, T. and Houssam, I., J. Control. Release, 1985, 2, 353.
- Gurny, R., Peppas, N.A., Harrington, D.D. and Banker, G.S., DrugDevelop. Ind. Pharm., 1981, 7, 1.
- Krause, H.J., Schwarz, A. and Rohdewald, P., Int. J. Pharm., 1985,27, 145.
- Thomas, N.S. and Panchaguula, R., Euro. J. Pharm. Sci., 2003,18, 71.
- Zaho, K. and Singh, J., J. Control. Release, 1998, 55, 253.
- Swarbrick, J. and Bom, J., J. Invests. Dermatol., 1982, 78, 63.
- Hayton, W.L. and Chen, T., J. Pharm. Sci., 1982, 71, 820.
- Julraht, K., Keith, A.P. and James, A.W., Drug Develop. Ind.Pharm., 1995, 21, 1377.
- Jambhekar, S.S., Green, P.J. and Rojanasakul, Y., Drug Develop.Ind. Pharm., 1987, 13, 2789.
- Bodmeier, R. and Paeratakul, O., Drug Develop. Ind. Pharm1992, 18, 1865.