- Corresponding Author:
- Renu T. Jain
Bio-Evaluation Centre, Torrent Pharmaceuticals Ltd, Village Bhat, Gandhi Nagar-382 428, India
E-mail: dawn.foster@yale.edu
| Date of Submission | 24-Sept-2010 |
| Date of Revision | 27-Sept-2011 |
| Date of Acceptance | 29-Sept-2011 |
| Indian J Pharm Sci, 2011, 73 (5): 510-516 |
Abstract
Venlafaxine is a unique antidepressant approved for treatment of various depressive disorders. A single dose, crossover bioequivalence study was performed with two different formulations of venlafaxine 150 mg extended-release capsules in which the contents of capsule were mixed with applesauce and administered to healthy subjects under fed condition. A total of 24 healthy adult male subjects participated in this randomized, single-dose, non-blinded, two-way crossover study conducted at a single centre and 23 subjects completed the study as per the study protocol. After an overnight fast of 10 h, a high-fat and high-calorie breakfast was served 30 min before dosing. The subjects then received a single dose of either formulation administered with apple sauce followed by 240 ml of water as per randomized schedule in each period separated by a washout period of 7 days. A series of blood samples were collected upto 72 h for estimation of venlafaxine and its active metabolite, O-desmethylvenlafaxine. The quantification of venlafaxine and O-desmethylvenlafaxine was done by LC-MS/MS method and pharmacokinetic and statistical analysis by WinNonlin® 5.2 and SAS® 9.1.3. The results of the study demonstrated bioequivalence of two formulations as the 90% confidence interval for the intra-individual mean ratio of log-transformed Cmax, AUC0-t and AUC0-inf of the test to the reference formulation were found within the defined bioequivalence range of 80.00%-125.00%. Both the formulations were well tolerated. This alternative mode of administration may provide benefits to patients who have difficulty in swallowing the capsule as a whole.
Keywords
Applesauce, area under curve, bioequivalence, confidence interval, maximum plasma concentration, venlafaxine
Introduction
Venlafaxine, a bicyclic phenylethylamine derivative was first introduced in 1993 and its extended-release formulation was approved in 1997, by US FDA [1]. Venlafaxine and its active metabolite, O-demethylvenlafaxine (ODV) have unique pharmacological effect, which has a dual mechanism of action, a potent inhibitor of the reuptake of serotonin and norepinephrine at central nervous system nerve terminals and lacks significant affinity for muscarinic cholinergic, adrenergic and histaminergic receptors [2,3].
Venlafaxine is indicated in the treatment of major depressive disorder, generalized anxiety disorder, social anxiety disorder (social phobia) and panic disorder. The safety and efficacy of venlafaxine and ODV for the treatment of depression and anxiety have been well established in published clinical trials [4-9].
After oral administration, at least 92% of a single dose of venlafaxine is absorbed. The absolute bioavailability of venlafaxine is 45% [5]. Steadystate concentrations of venlafaxine and ODV in plasma are attained within 3 days of oral multiple dose therapy. Venlafaxine and ODV exhibited linear kinetics over the dose range of 75 to 450 mg/ day. Venlafaxine is extensively metabolized in the liver to active metabolite ODV and also to other metabolites. Metabolism of venlafaxine occurs primarily via O-demethylation, which is mediated by CYP2D6 (cytochrome P-450 isozyme), and, to a lesser extent, by N-demethylation, which is mediated by CYP3A4 [5]. When equal daily doses of venlafaxine were administered as either an immediate release tablet or the extended-release capsule, the exposure to both venlafaxine and ODV was similar for the two treatments, and the fluctuation in plasma concentrations was slightly lower with the venlafaxine extended-release (ER) capsule. Venlafaxine extended-release, therefore, provides a slower rate of absorption, but the same extent of absorption compared with the immediate release tablet. Food did not affect the bioavailability of venlafaxine or its active metabolite, ODV [4]. Time of administration (AM vs. PM) did not affect the pharmacokinetics of venlafaxine and ODV from the 75 mg venlafaxine extended-release capsule [4]. The Tmax with venlafaxine extended-release capsule is achieved at 5.5 h for venlafaxine and 9.0 h for ODV [5]. Renal elimination of venlafaxine and its metabolites is the primary route of excretion [5] with about 5% of an administered dose excreted in the urine as unchanged drug [5]. The steady-state half-lives of venlafaxine and ODV are approximately 5 h and 11 h, respectively [5].
Venlafaxine ER capsules are to be administered orally swallowing the capsule as a whole without crushing, dividing or chewing with food. Alternatively, for patients who have difficulty swallowing the capsule as a whole, the capsule may be opened and the entire contents be sprinkled on a spoonful of applesauce, swallowed immediately without chewing followed by a glass of water to ensure complete swallowing of the pellets.
This study was designed to assess bioavailability between test and reference formulation when the contents of venlafaxine 150 mg ER capsules were sprinkled over applesauce and administered to healthy subjects. Since administration of venlafaxine after a meal can decrease the nausea associated with this drug [10], this study was carried out under fed condition as recommended by the Office of Generic Drugs (OGD) [11], USA.
Materials And Methods
Subjects
The present bioequivalence study was conducted in 24 healthy adult male subjects aged between 18-45 years (both inclusive) with body mass index (BMI) of 18- 27 (both inclusive) kg/m2. All subjects were in good health as evidenced by medical histories, complete physical examination and routine laboratory tests performed within 28 days prior to the commencement of the study. None of the volunteers had history of an allergy to any component of the medication. All the participated subjects gave informed written consent prior to commencement of the study. Subjects did not receive any medication during two weeks period prior to the start of the study. The subjects were instructed during screening not to take any prescription and OTC medications subsequently until the completion of the study. All the subjects abstained from any xanthine-containing food or beverages or alcoholic products for 48 h prior to dosing and throughout the sampling schedule during each period. Smokers and subjects with positive breath alcohol test were excluded from the study.
Study protocol and study design
The study was carried out in accordance with the clinical research guidelines defined in the US 21 CFR part 312.20, Declaration of Helsinki by the World Medical Association (WMA) General Assembly, Tokyo (2004), Indian Council of Medical Research (ICMR) guidelines, applicable local regulatory guidelines and in-house standard operating procedures. The study protocol was approved by Institutional Ethics Committee before commencement of the study. The study was conducted at Bio-evaluation Centre of Torrent Research Centre, Ahmedabad.
The study was designed as a monocentric, nonblinded, randomized, two-period, two-treatment, two-sequence crossover single dose bioequivalence study under fed conditions. The test product was venlafaxine hydrochloride 150 mg ER capsule of Torrent Pharmaceuticals Ltd., India which was compared with Effexor XR® (containing venlafaxine 150 mg) of Wyeth Pharmaceuticals, USA (reference product). All subjects fasted for 10 h before breakfast and 4 h after study drug administration. A highcalorie, high-fat breakfast (approximately 949 kcal) was served to each subject 30 min before dosing. The test and reference capsules were administered as per SAS generated randomization schedule in each period. A wash out period of 7 days was kept between the two dosing to avoid any carry over effect. A series of blood samples were collected upto 72.0 h for estimation of Cmax and AUC for both venlafaxine and its active metabolite ODV. However, bioequivalence was proved based on the data of primary analyte venlafaxine as specified in the protocol.
To minimize bias in the study, the following precautions were taken during study execution: a) the treatments were given as per randomization schedule, b) volunteer number was assigned on first come first basis after fulfilling the study requirements, c) a cross over design was chosen in which each volunteer received both the treatments, d) analysts were blinded to the randomization code of the subjects.
Inclusion criteria for subjects
Subjects who met all of the following criteria were included in the study: (1) Sex: male (2) Age: 18-45 years. (3) Volunteer with BMI of 18-27 (inclusive both) kg/m2 with minimum of 50 kg weight (4) Healthy and willing to participate in the study. (5) Volunteer willing to adhere to the protocol requirements and to provide written informed consent. (6). Non-smokers or smoker who smokes less than 10 cigarettes a day.
Exclusion criteria for subjects
The subjects were excluded from the study who met any of the following exclusion criteria: (1) Clinically relevant abnormalities in the results of the laboratory screening evaluation. (2) Clinically significant abnormal ECG and/or Chest X-ray, (3) Systolic blood pressure less than 100 mm Hg or more than 140 mm Hg and diastolic blood pressure less than 60 mm Hg or more than 90 mm Hg, (4) Pulse rate less than 50/min or more than 100/ min. (5) Oral temperature less than 95°F or more than 98.6°F, (6) Respiratory rate less than 12/min or more than 20/min, (7) Addiction to alcohol or history of any drug abuse within the past 2 years, (8) Subjects found positive in breath alcohol test, (9) Recent History of kidney or liver dysfunction, (10) History of allergy to the test drug or any drug chemically similar to the drug under investigation, (11) Administration/ Intake of prescription for two weeks before the study, (12) Administration/ Intake of OTC medication for one week before the study, (13) Subjects suffering from any chronic illness such as arthritis, asthma, (14) HIV, HCV, HBsAg positive subjects, (15) Opioids, tetra hydrocannabinoids, amphetamine, barbiturates, benzodiazepine, Cocaine positive subjects based on urine test, (16) Subjects suffering from any psychiatric (acute or chronic) illness requiring medications, (17) Administration of any investigational products in the period 0 to 3 months before entry to the study, (18) Intake of barbiturates or any enzyme-inducing drug in last three months, (19) History of significant blood loss due to any reason, including blood donation in the past 12 weeks, (20) History of pre-existing bleeding disorder, (21) Existence of any surgical or medical condition, which, in the judgement of the Chief Investigator and/or clinical investigator, might interfere with the absorption, distribution, metabolism or excretion of the drug or likely to compromise the safety of Subjects, (22) Inability to communicate or co-operate due to language problem, poor mental development or impaired cerebral function.
Drug administration and study restrictions
A single oral dose of 150 mg of venlafaxine ER capsule of either test or reference study drug was administered by opening and sprinkling the entire contents of capsule over a spoon containing approximately 5 ml of applesauce just prior to ingestion followed by administration of 240 ml of water in each period, 30 min after standardized highcalorie, high-fat breakfast. The subject was advised not to chew the drug-food mixture but to swallow it completely. A thorough mouth check was done to ensure the drug-food mixture was swallowed by the subject completely. Subjects were seated upright for the first four h following administration of the study drug. They were prohibited from doing any sort of stressful physical activity during the in-house period. Subjects fasted for a period of atleast 4 h after dosing. Fluids were not permitted from 1 h before dosing to 2 h after dosing. Food and fluids were standardized with respect to content and time in both the periods of the study.
Blood sampling and processing
All blood samples were collected in pre-labeled polypropylene tubes containing 5 IU diluted heparin in normal saline for each ml of blood. Samples were collected through an indwelling cannula placed in a forearm vein. The cannula was kept in situ as long as possible by injecting about 0.5 ml of 5 IU/ml of heparin in normal saline solution to maintain the cannula patent. The blood samples were withdrawn at pre-dose and at 1.0, 2.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, 12.0, 14.0, 16.0, 18.0, 20.0, 24.0, 36.0, 48.0 and 72.0 h post-dose in each period. The allowed deviations for blood sampling were ±2 min for in-house sample (till 24.0 h) and ±1 h for ambulatory samples (36.0, 48.0 and 72.0 h). The blood samples were centrifuged at 2000 rpm for 10 min at 20°. The plasma was separated in pre-labeled polypropylene tubes, and subsequently stored at -70±20° until analysis. After completion of both the periods, the samples were transferred for bio-analysis. The samples received at analytical facility were frozen and in good condition.
Analysis of plasma
A LC-MS/MS method for estimation of Venlafaxine and ODV in human plasma was developed and validated. All samples of the same volunteer were measured in a single analytical run in order to eliminate the influence of the inter-assay variance on the assessment. Repeat assay was done wherever required and the same was documented. The analyst involved in assay of venlafaxine and ODV did not have access to randomization schedule.
Pharmacokinetic evaluation
Pharmacokinetic analysis was performed with the data of subjects who completed both the periods of the study using the WinNonlin® software version 5.2 [12]. All concentration values which were below limit of quantification (BLQ) at the initial absorption or at the terminal elimination phase were considered as zero. Missing values (i.e., Sample not submitted or analyzed, BLQ value between two-plasma concentrations, non-reportable value) were not included in the calculation of pharmacokinetic evaluation. The bioequivalence assessment was done with venlafaxine (parent drug), hence, pharmacokinetic parameters of ODV are not discussed.
The following pharmacokinetic parameters were estimated, Cmax: Maximum concentration of drug observed in plasma; AUC0-t: Area under the plasma concentration vs time curve from time zero to the last measurable concentration time t calculated using linear trapezoidal method; AUC0-inf: Area under the plasma concentration vs time curve from time zero to time infinity calculated as [AUC0-t + Ct/Lambda_z] where Ct is the last concentration of the drug; AUC_%Extrap: Extrapolated AUC percentage of total AUC calculated as (AUC0-inf - AUC0-t/AUC0- inf)*100; Tmax: Time required to reach maximum concentration of drug in plasma; Lambda_z (Kel): Elimination Rate constant; T1/2; Time taken by plasma concentration to reduce to 50% during the elimination phase. Non-compartmental method (Model 200 of WinNonlin® 5.2) was used to estimate pharmacokinetic parameters of venlafaxine and ODV.
Statistical analysis
Statistical analysis was carried out using SAS® (Version 9.1.3) [13]. Summary statistics was calculated for concentration-time data of test and reference products for all pharmacokinetic parameters which included arithmetic mean (mean), standard deviation (SD), coefficient of variation (CV%), minimum (min), maximum (max) and median. Analysis of Variance (ANOVA) was performed on the logtransformed data of Cmax, AUC0-t and AUC0-inf for Venlafaxine. The sequence, subject within sequence, period and formulation were considered as sources of variation. The sequence effect was tested using the subject within sequence effect as the error term. The formulation and period effects were tested against the residual mean square error. A probability (p) value was derived from Type III sum of squares. The Tmax for test and reference product was compared by a non- parametric test known as Wilcoxon Signed Rank Test.
The bioequivalence acceptance interval was set at 80.00 to 125.00%. The 90% confidence interval of Test/Reference mean ratio of venlafaxine was computed for the log-transformed pharmacokinetic parameters of Cmax, AUC0-t and AUC0 inf [11,14].
Results
A total of twenty four healthy Asian male subjects aged between 18 to 41 years with BMI in the range of 18.10 to 25.09 Kg/m2 were enrolled in the study, out of which twenty three subjects completed both the periods of the study. Hence, the final evaluation was carried out with data of twenty three subjects. The detailed demographic profiles of enrolled subjects are presented in Table 1.
| Parameters | Age (Years) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| Mean | 29.33 | 167.31 | 60.59 | 21.64 |
| SD | 6.60 | 5.75 | 6.80 | 2.09 |
| Max | 41 | 176.5 | 76 | 25.09 |
| Min | 18 | 155.5 | 50 | 18.10 |
| SD is standard deviation, min is minimum value, max is maximum value, N=total no. of volunteers enrolled for the study | ||||
Table 1: Overall demographic profiles of enrolled subjects (n=24)
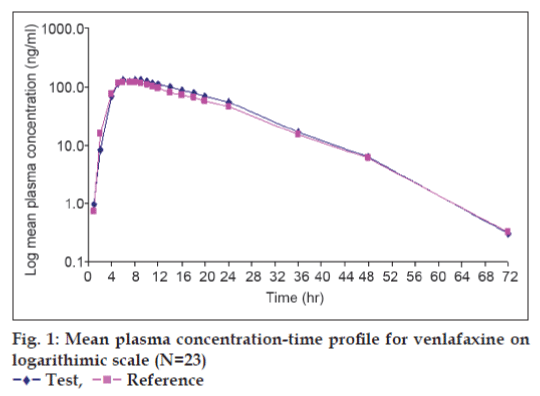
The Mean plasma concentration-time profile for test and reference product of venlafaxine on linear and semilog scale is shown in fig. 1.
The pharmacokinetic parameter of venlafaxine is given in Table 2. The mean±SD Cmax for test and reference product was 139.961±42.432 ng/ml and 129.7224±43.06998 ng/ml, respectively. The mean AUC0-t±SD for test product was 2590.307±1146.010 (h.ng/ml) and for reference product was 2291.412±1036.414 (h.ng/ml). The mean±SD half life (t1/2) for test and reference product was 7.5599 h and 7.8143 h, respectively. The median time to reach maximum plasma concentration was 8 h for test product and 7 h for reference product. The mean AUC% extrapolation was 2.5233 and 2.7762 for test and reference, respectively.
| Pharmacokinetic | Test/ | Mean | SD | Minimum | Median | Maximum | CV% |
|---|---|---|---|---|---|---|---|
| parameter | reference | ||||||
| Cmax (ng/ml) | Test | 139.961 | 42.432 | 83.898 | 134.394 | 267.851 | 30.3 |
| Reference | 129.7224 | 43.06998 | 67.6930 | 130.0810 | 292.4670 | 33.2 | |
| AUC0-t (h.ng/ml) | Test | 2590.307 | 1146.010 | 1075.684 | 2497.809 | 6008.221 | 44.2 |
| Reference | 2291.412 | 1036.414 | 784.528 | 2242.111 | 5858.804 | 45.2 | |
| AUC0-inf (h.ng/ml) | Test | 2658.482 | 1170.901 | 1100.765 | 2553.124 | 6062.48 | 44.0 |
| Reference | 2353.261 | 1047.764 | 816.512 | 2279.658 | 5923.555 | 44.5 | |
| AUC_% Extrap | Test | 2.5233 | 1.22642 | 0.8950 | 2.1983 | 5.6043 | 48.6 |
| Reference | 2.7762 | 1.15554 | 0.9342 | 2.7910 | 4.8897 | 41.6 | |
| Kel (h-1) | Test | 0.0956 | 0.02154 | 0.0665 | 0.0945 | 0.1510 | 22.5 |
| Reference | 0.0918 | 0.01787 | 0.0659 | 0.0876 | 0.1297 | 19.5 | |
| T1/2 (h) | Test | 7.5599 | 1.50748 | 4.5918 | 7.3345 | 10.4190 | 19.9 |
| Reference | 7.8143 | 1.4229 | 5.345 | 7.909 | 10.5135 | 18.2 | |
| Tmax (h) | Test | 7.217 | 1.6225 | 5.000 | 8.000 | 10.000 | 22.5 |
| Reference | 6.696 | 1.6078 | 5.000 | 7.000 | 10.000 | 24.0 | |
| %CV is percentage co-efficient of variation, N=Total no. of volunteers completed the study and analysed | |||||||
Table 2: Pharmacokinetic Parameters Of Venlafaxine (N=23)
Bioequivalence assessment
The bioequivalence assessment was done with 23 subjects who completed the study. Ratio analysis of untransformed and difference of log transformed primary pharmacokinetic parameters [Cmax, AUC0-t and AUC0-inf] for test and reference formulation were calculated for venlafaxine. The percentage geometric least square mean (LSM) ratio of test and reference values was expressed as point estimates of relative bioavailability. The Geometric LSM ratio and 90% confidence interval of venlafaxine are presented in Table 3.
| PK parameters | 90% confidence | Geometric LSM |
|---|---|---|
| [N=23] | interval | Ratio % (Test/ |
| Reference) | ||
| Ln(Cmax) | 101.38 to 115.10 | 108.03 |
| Ln(AUC0-t) | 106.07 to119.78 | 112.72 |
| Ln(AUC0-inf) | 105.82 to 119.44 | 112.42 |
Table 3: Geometric least squares mean (lsm) ratio and 90% confidence interval for Venlafaxine
Average bioequivalence was evaluated based on the 90% CI for the intra-individual mean ratio of logtransformed Cmax, AUC0-t and AUC0-inf of the test to the reference formulation. The 90% confidence interval for venlafaxine log transformed Cmax, AUC0-t and AUC0-inf was 101.38 to 115.10 with a ratio of 108.03%, 106.07 to 119.78 with a ratio of 112.72% and 105.82 to 119.44 with a ratio of 112.42% respectively. Hence, all pharmacokinetic parameters are within the accepted bioequivalence range 80.00-125.00% for Cmax, AUC0-t and AUC0-inf of venlafaxine.
The log-transformed pharmacokinetic parameters Cmax, AUC0-t and AUC0-inf of venlafaxine were subjected to analysis of variance (ANOVA) with the main effects of sequence, formulation and period at 5% level of significance (Table 4).
| PK Parameters | ANOVA results probability (P)-value | ||
|---|---|---|---|
| [N=23] | Sequence | Formulation | Period |
| Ln(Cmax) | 0.372 | 0.049 | 0.821 |
| Ln(AUC0-t) | 0.404 | 0.003 | 0.642 |
| Ln(AUC0-inf) | 0.385 | 0.003 | 0.631 |
| ANOVA is analysis of variance | |||
Table 4: Anova Results Of Venlafaxine
Safety evaluation
There were no serious adverse events reported in this study. Total 24 subjects were included in safety evaluations who were dosed at least once. Out of twenty four subjects dosed in both the periods, two adverse events (nausea and vomiting) were reported following the administration of test drug. Both the adverse events were mild in nature and resolved completely without any medication. There were no significant changes in pre-study and post-study laboratory evaluation in any of the volunteers.
Discussion
The FDA defines bioequivalence as the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study. To meet the regulatory criterion for bioequivalence, the 90% CIs for the ratio of the geometric LSM of Cmax, AUC0-t, and AUC0-inf between products should fall within the confidence-interval from 80.00 to 125.00% [14,15].
The reference formulation, Effexor XR® is launched in USA since 2007 by Wyeth Pharmaceuticals Inc., USA. It is recommended by the innovator that Effexor XR® capsule should be swallowed whole with fluid and not divided, crushed, chewed, or placed in water. Alternatively, it may be administered by carefully opening the capsule and sprinkling the entire contents on a spoonful of applesauce [5]. To provide such similar method of administration for test formulation, it is necessary to show both test and reference formulations are bioequivalent when administered with applesauce.
Although several studies have been published about venlafaxine pharmacokinetics, none of the published studies focused on the proof of bioequivalence between two products under similar administration method.
This present research work was planned and conducted as a non-blinded, randomized, single dose two-period, two-treatment, crossover bioequivalence study under fed conditions in healthy male subjects. Only male volunteers were enrolled to have a homogeneous population group. Prior consent was obtained from all subjects who participated in the study.
A total of 24 subjects were enrolled in the study out of which 23 subjects completed the study as per protocol. One volunteer was medically withdrawn from the study due to adverse event. Blood sampling was done up to 72.0 h after dosing which was adequate for profiling of pharmacokinetic parameters as evident from AUC. Wash out period of seven days between the two periods was sufficient for this study since there was no carryover effect observed in any subject. Plasma concentrations of venlafaxine and ODV were determined by a validated LC-MS/MS method in accordance with GLP requirements. As per regulatory requirement, only venlafaxine was taken for bioequivalence assessment.
The mean±SD values of Cmax, AUC0-t and AUC0-inf for test and reference formulations were comparable. The Tmax were compared for test and reference using non-parametric-Wilcoxon Signed Rank test for venlafaxine. The t1/2 of test (median 8 h) and reference formulation (median 7 h) was comparable. The extent of absorption (AUC) was covered more then 95% as evident from mean AUC% extrapolation (Table 2).
The 90% confidence interval of the mean ratios (test/reference) for log-transformed Cmax, AUC0-t and AUC0-inf were 101.38 to 115.10, 106.07 to119.78 and 105.82 to 119.44, respectively, which are falling within the prescribed bioequivalence range i.e., 80.00- 125.00%. The results of the present study clearly revealed that the two formulations are comparable with respect to the rate and extent of absorption when the capsule contents are administered by sprinkling over applesauce in healthy subjects under fed condition according to USFDA requirement. Further, both the formulations were well tolerated on single dose administration.
Since the generic formulation (test) of Torrent is bioequivalent with the innovator formulation (Effexor® 150 mg, reference) of Wyeth pharmaceuticals, USA, when administered with applesauce. Hence both the formulations can be interchanged without compromising therapeutic efficacy and safety. This alternative mode of administration will help to those populations who can not swallow the capsule as a whole.
Acknowledgements
This study was conducted at Torrent Research Centre, Ahmedabad, India.
References
- Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search. Drug Details. [Last accessed on 2012 Apr 15].
- Rudolph RL, Derivan AT. The safety and tolerability of venlafaxine hydrochloride: analysis of the clinical trials database. J ClinPsychopharmacol 1996;16(3 Suppl 2):54S-59S; discussion 59S-61S.
- Troy SM, Dilea C, Martin PT, Leister CA, Fruncillo RJ, Chiang ST. Pharmacokinetics of once-daily venlafaxine Extended release in healthy volunteers. CurrTher Res 1997;58:504-14.
- Holliday SM, Benfield P. Venlafaxine: A review of its pharmacology and therapeutic potential in depression. Drugs 1995;49:280-94.
- Effexor XR® [Venlafaxine Extended Release Capsules] Product Information. Philadelphia, PA 19101: Wyeth PharmaInc; Revised Sept 2003.
- Montgomery SA, Etnsuah R, Hackett D, Kunj NR, Rudolph RL. Venlafaxine versus placebo in the preventive treatment of recurrent major depression. J Clin Psychiatry 2004;65:328-36.
- Lenox-Smith AJ, Reynolds A. A double-blind, randomised, placebo controlled study of venlafaxine XL in patients with generalised anxiety disorder in primary care. Br J Gen Pract 2003;53:772-7.
- Allgulander C, Hackett D, Salinas E. Venlafaxine extended release (ER) in the treatment of generalized anxiety disorder: Twenty-four-week placebo-controlled dose-ranging study. Br J Psychiatry 2001;179:15-22.
- Liebowitz MR, Mangano RM, Bradwejn J, Asnis G. A randomized controlled trial of venlafaxine extended release in generalized social anxiety disorder. J Clin Psychiatry 2005;66:238-47.
- Troy SM, Parkar VP, Hicks DR, Pollack GM, Chiang ST. Pharmacokinetics and effect of food on the bioavailability of orally administered venlafaxine. J ClinPharmacol 1997;37:954-61.
- Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm091259.pdf. Draft Guidance on Venlafaxine Hydrochloride. [Last accessed on 2012 Apr 15].
- WinNonlin, Professional ed. version 5.2, Pharsight Corp., 800 West Elcamino Real, Suite 200, Mountain View CA 94040.
- SAS® software release. 9.1.3. Cary NC, USA: SAS Institute Inc.
- Centre for Drug Evaluation and Research, U.S. Department of Health and Human Services Food and Drug Administration: Guidance for Industry: Statistical Approaches to Establishing Bioequivalence-GeneralConsiderations. Available at http://www.fda.gov/downloads/Drugs/Guidance ComplianceRegulatoryInformation/Guidances/ucm070244.pdf [Last accessed on 2012 Apr 15]; 2001.
- Centre for Drug Evaluation and Research, U.S. Department of Health Human Services Food and Drug Administration: Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products-General Considerations. Rockville, MD; 2003.